Comparison of Membrane Chromatography and Monolith Chromatography for Lactoferrin and Bovine Serum Albumin Separation
Abstract
:1. Introduction
2. Materials and Methods
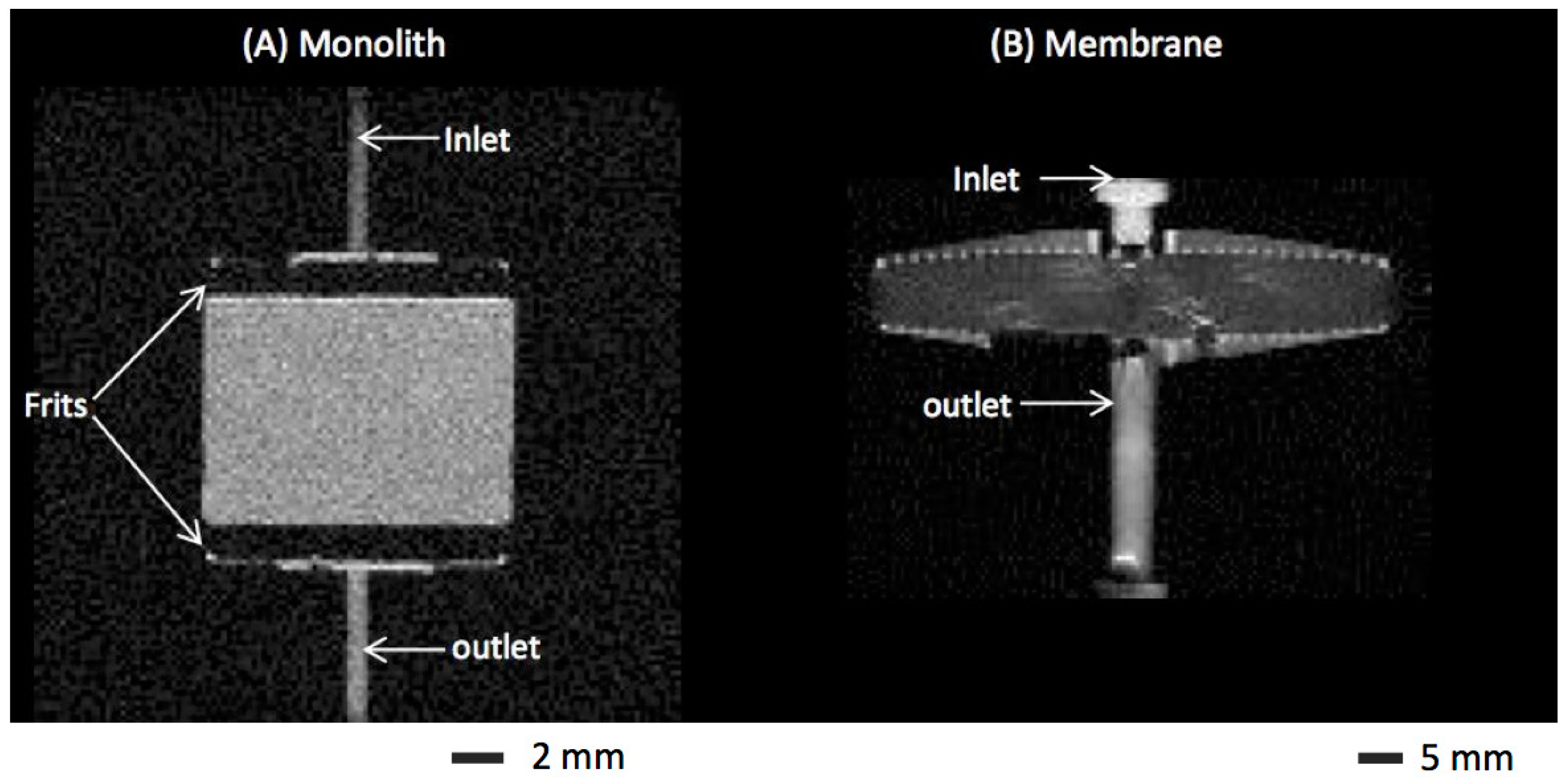
2.1. Chromatographic Media and Devices
2.2. Protein Solutions
2.3. BSA-LF Mixture Separation
3. Results
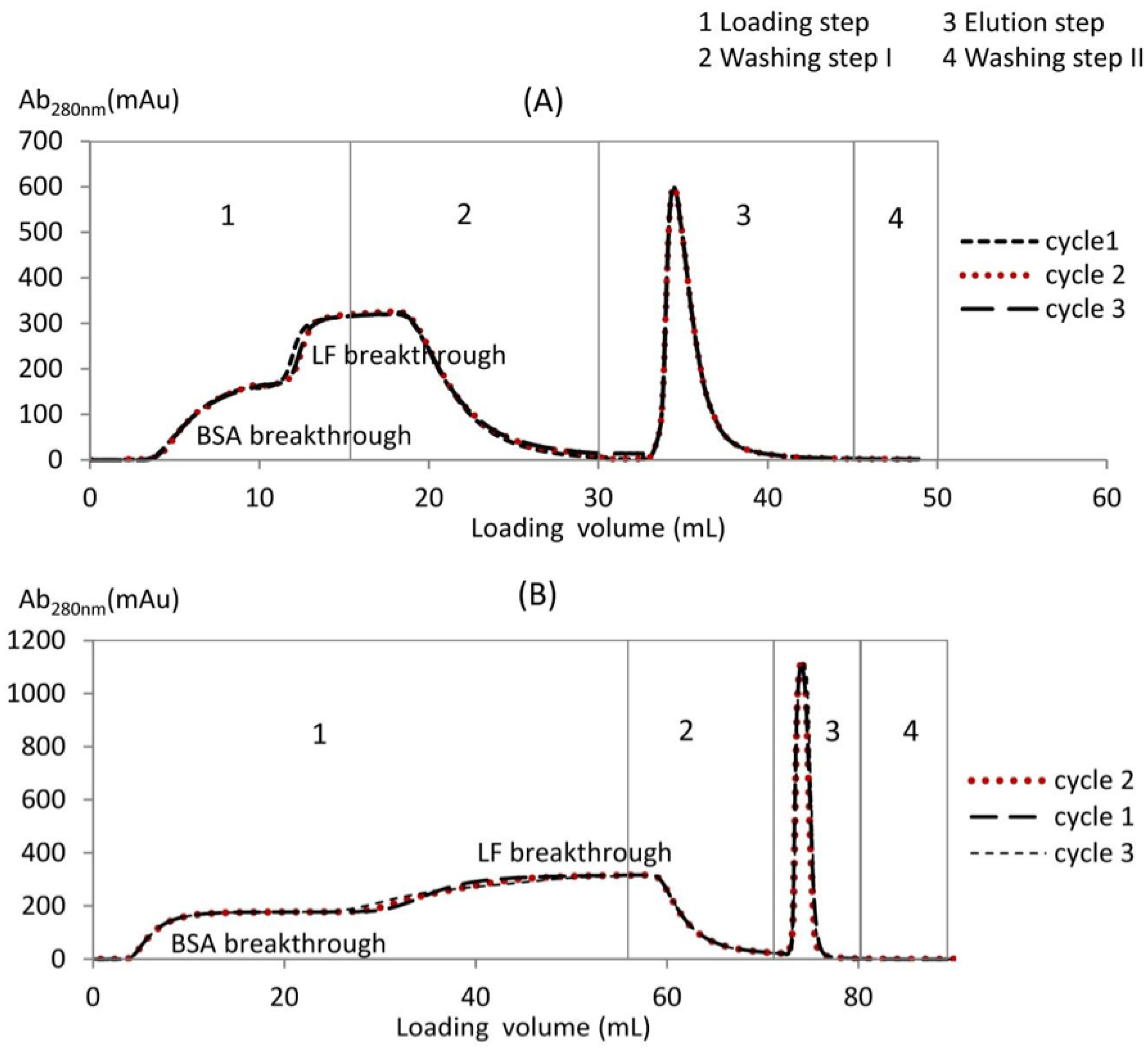
3.1. BSA-LF Mixture Separation
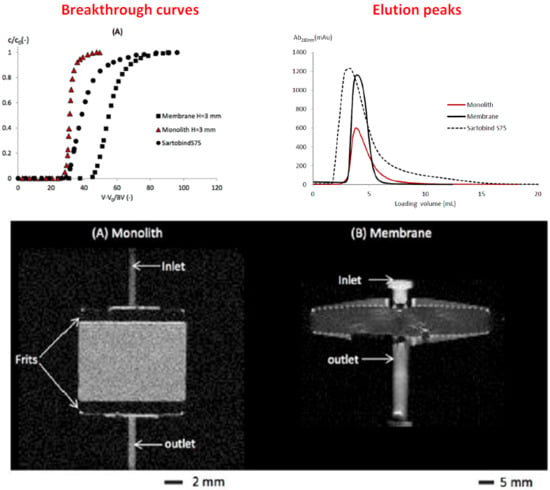
3.2. BSA Non-Binding Breakthrough Curves
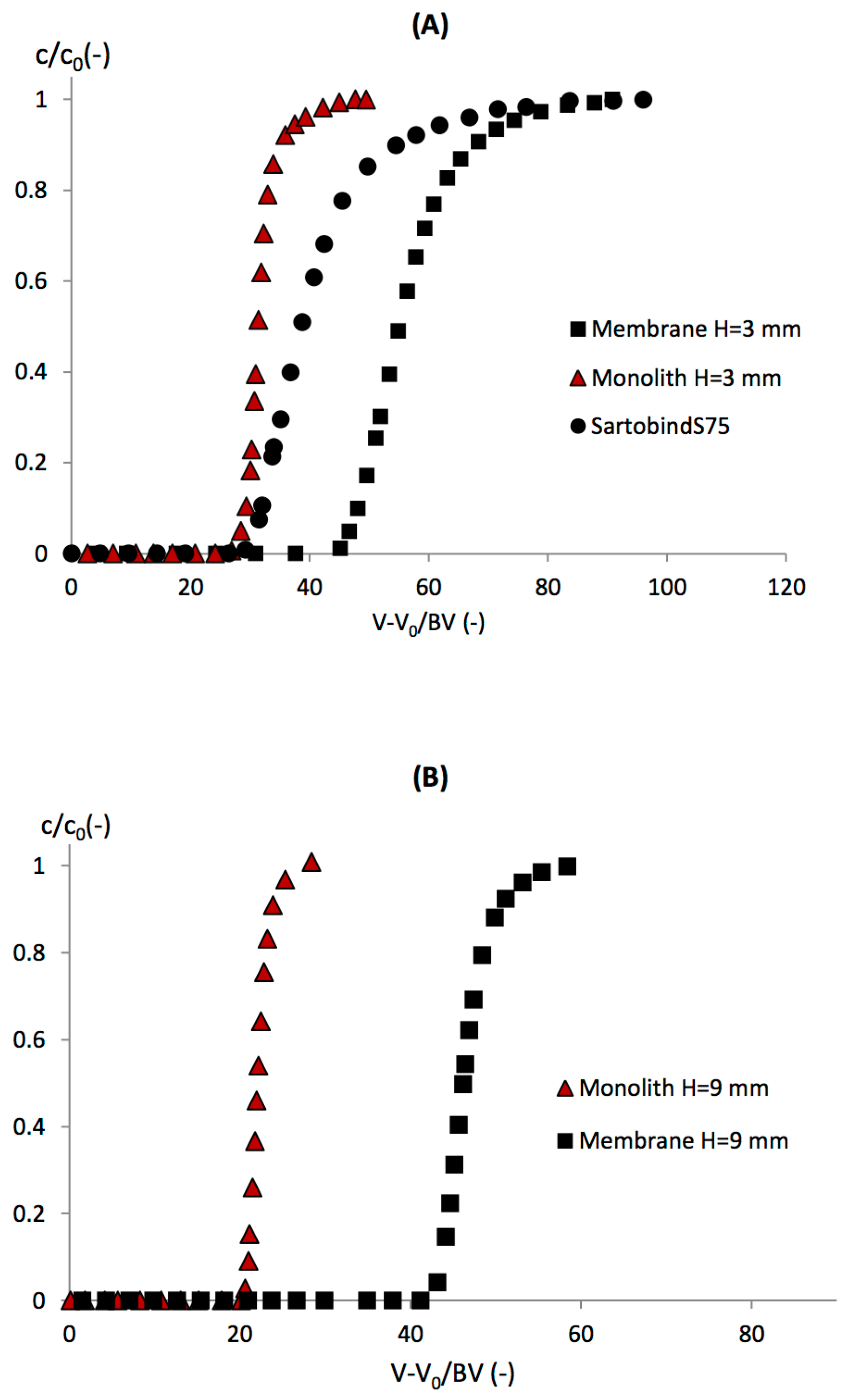
3.3. LF Binding Breakthrough Curves
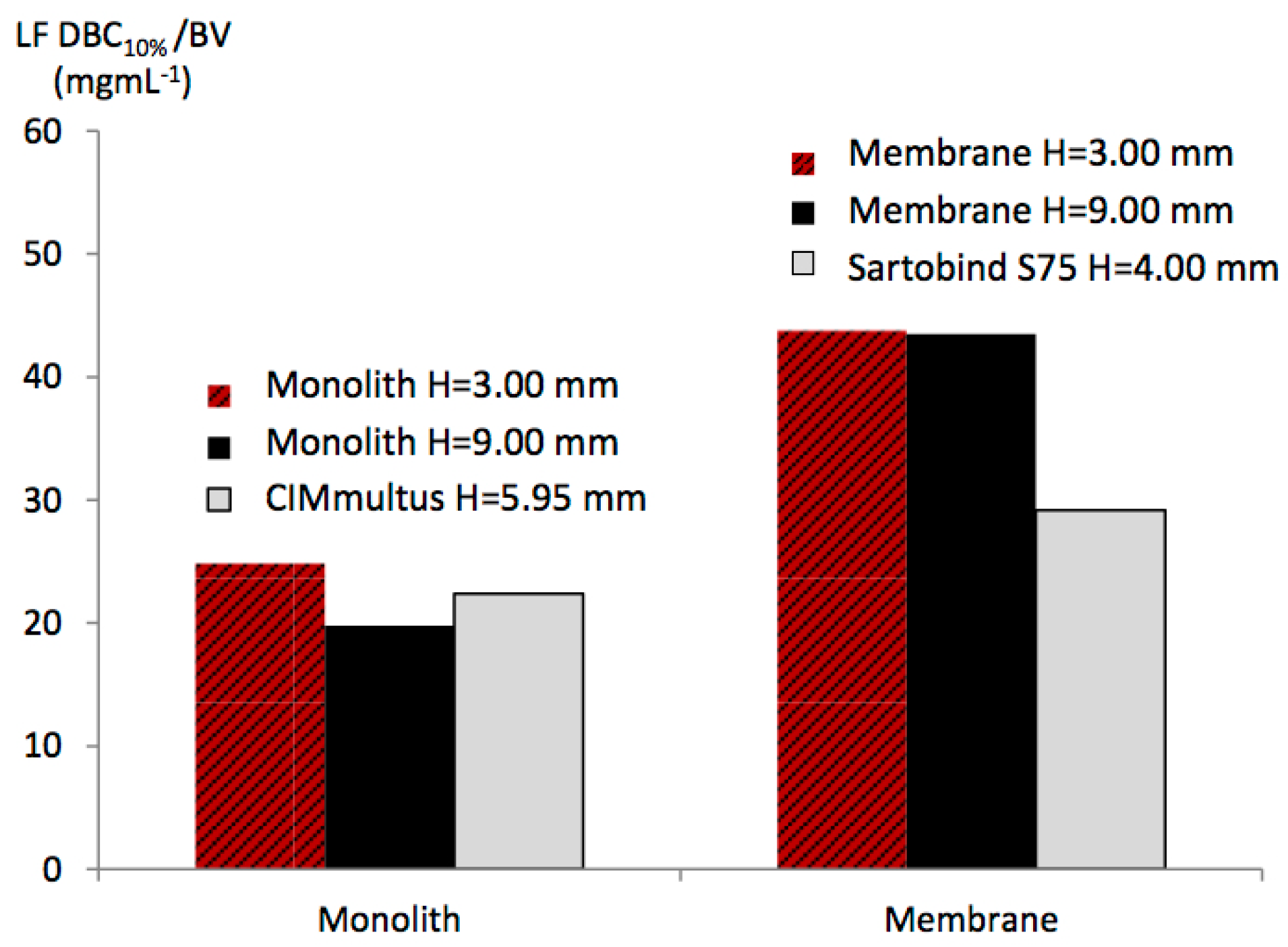
3.4. Effect of the Bed Height
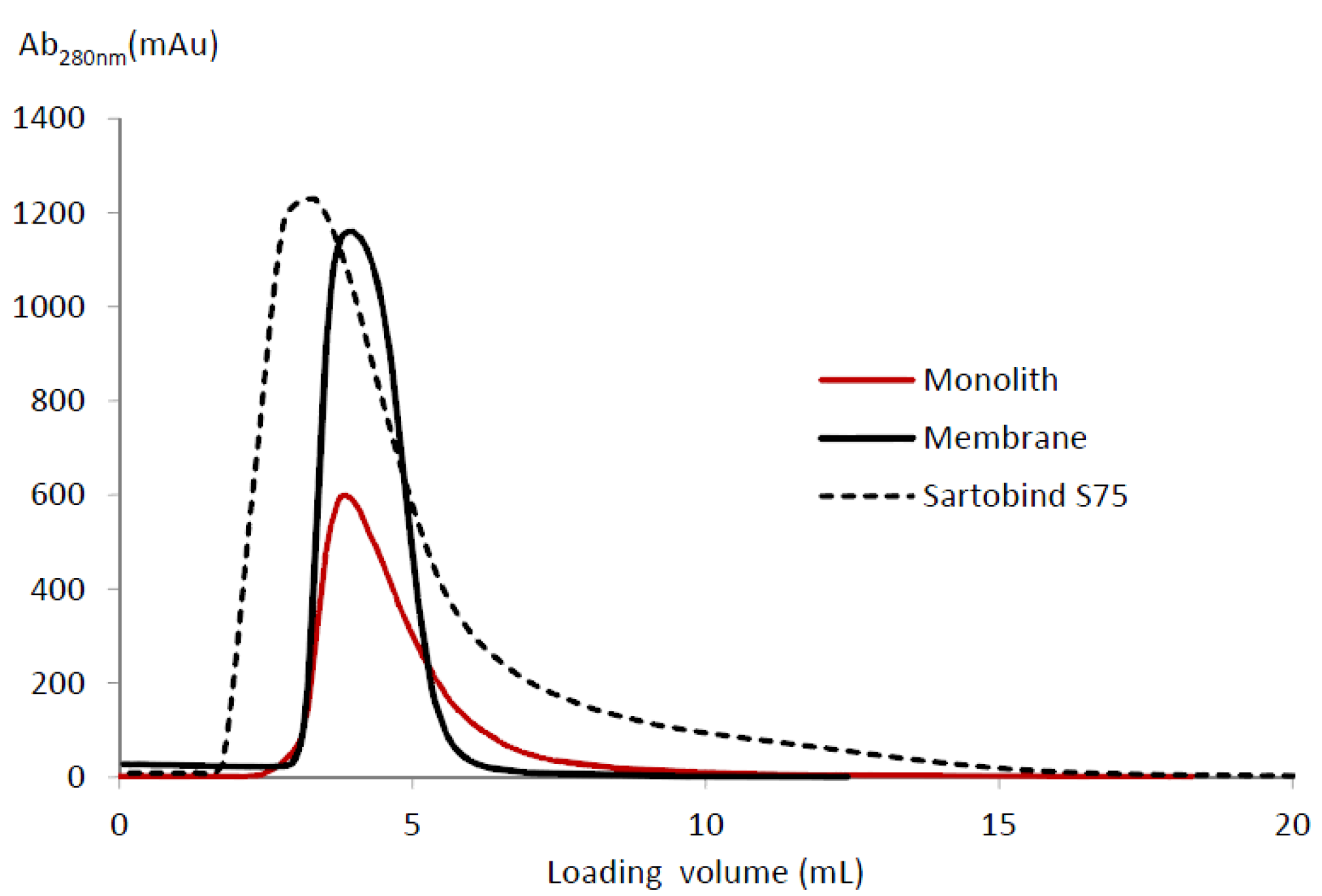
3.5. Elution Peaks
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gebauer, K.H.; Thömmes, J.; Kula, M.-R. Plasma protein fractionation with advanced membrane adsorbents. Biotechnol. Bioeng. 1997, 54, 181–189. [Google Scholar] [CrossRef]
- Pabby, A.K.; Rizvi, S.S.H.; Requena, A.M.S. Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Ghosh, R. Protein separation using membrane chromatography: Opportunities and challenges. J. Chromatogr. A 2002, 952, 13–27. [Google Scholar] [CrossRef]
- Hunter, A.K.; Carta, G. Protein adsorption on novel acrylamido-based polymeric ion-exchangers: IV. Effects of protein size on adsorption capacity and rate. J. Chromatogr. A 2002, 971, 105–116. [Google Scholar] [CrossRef]
- Jungbauer, A.; Hahn, R. Polymethacrylate monoliths for preparative and industrial separation of biomolecular assemblies. J. Chromatogr. A 2008, 1184, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Stickel, J.J.; Fotopoulos, A. Pressure-flow relationships for packed beds of compressible chromatography media at laboratory and production scale. Biotechnol. Prog. 2001, 17, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Charcosset, C. Membrane Processes in Biotechnology and Pharmaceutics; Elsevier: Oxford, UK, 2012. [Google Scholar]
- Orr, V.; Zhong, L.; Moo-Young, M.; Chou, C.P. Recent advances in bioprocessing application of membrane chromatography. Biotechnol. Adv. 2013, 31, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Thömmes, J.; Kula, M.-R. Membrane chromatography—An integrative concept in the downstream processing of proteins. Biotechnol. Prog. 1995, 11, 357–367. [Google Scholar] [CrossRef]
- Chang, C.-S.; Ni, H.-S.; Suen, S.-Y.; Tseng, W.-C.; Chiu, H.-C.; Chou, C.P. Preparation of inorganic-organic anion-exchange membranes and their application in plasmid DNA and RNA separation. J. Membr. Sci. 2008, 311, 336–348. [Google Scholar] [CrossRef]
- Huang, S.; Roy, S.; Hou, K.; Tsao, G. Scaling-up of affinity-chromatography by radial-flow cartridges. Biotechnol. Prog. 1988, 4, 159–165. [Google Scholar] [CrossRef]
- Orr, V.; Scharer, J.; Moo-Young, M.; Honeyman, C.H.; Fenner, D.; Crossley, L.; Suen, S.-Y.; Chou, C.P. Simultaneous clarification of Escherichia coli culture and purification of extracellularly produced penicillin G acylase using tangential flow filtration and anion-exchange membrane chromatography (TFF-AEMC). J. Chromatogr. B 2012, 900, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Madadkar, P.; Wu, Q.; Ghosh, R. A laterally-fed membrane chromatography module. J. Membr. Sci. 2015, 487, 173–179. [Google Scholar] [CrossRef]
- Josic, D.; Buchacher, A.; Jungbauer, A. Monoliths as stationary phases for separation of proteins and polynucleotides and enzymatic conversion. J. Chromatogr. B: Biomed. Sci. Appl. 2001, 752, 191–205. [Google Scholar] [CrossRef]
- Strancar, A.; Podgornik, A.; Barut, M.; Necina, R. Short Monolithic Columns as Stationary Phases for Biochromatography; Freitag, P.D.R., Ed.; Modern Advances in Chromatography; Springer: Heidelberg, Germany, 2002; pp. 49–85. Availiable online: http://link.springer.com/chapter/10.1007/3-540-45345-8_2 (accessed on 2 October 2013).
- Svec, F.; Fréchet, J.M.J. Modified poly(glycidyl metharylate-co-ethylene dimethacrylate) continuous rod columns for preparative-scale ion-exchange chromatography of proteins. J. Chromatogr. A 1995, 702, 89–95. [Google Scholar] [CrossRef]
- Jungbauer, A.; Hahn, R. Monoliths for fast bioseparation and bioconversion and their applications in biotechnology. J. Sep. Sci. 2004, 27, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, P. Technology trends in antibody purification. J. Chromatogr. A 2012, 1221, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, P.; Richieri, R.; Zaidi, S. A comparison of microparticulate, membrane, and monolithic anion exchangers for polishing applications in the purification of monoclonal antibodies. In Proceedings of the IBC International Conference and Exposition, Boston, MA, USA, 1–4 October 2007.
- Cramer, S.M.; Holstein, M.A. Downstream bioprocessing: Recent advances and future promise. Curr. Opin. Chem. Eng. 2011, 1, 27–37. [Google Scholar] [CrossRef]
- Hormann, K.; Müllner, T.; Bruns, S.; Höltzel, A.; Tallarek, U. Morphology and separation efficiency of a new generation of analytical silica monoliths. J. Chromatogr. A 2012, 1222, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Podgornik, A.; Barut, M.; Strancar, A.; Josić, D.; Koloini, T. Construction of large-volume monolithic columns. Anal. Chem. 2000, 72, 5693–5699. [Google Scholar] [CrossRef] [PubMed]
- Podgornik, A.; Strancar, A. Convective Interaction Media (CIM)—Short layer monolithic chromatographic stationary phases. Biotechnol. Annu. Rev. 2005, 11, 281–333. [Google Scholar] [CrossRef] [PubMed]
- Podgornik, A.; Jancar, J.; Merhar, M.; Kozamernik, S.; Glover, D.; Cucek, K.; Barut, M.; Strancar, A. Large-scale methacrylate monolithic columns: Design and properties. J. Biochem. Biophys. Methods 2004, 60, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.K.; Etzel, M.R. Fractionation of lactoperoxidase and lactoferrin from bovine whey using a cation exchange membrane. J. Food Sci. 1997, 62, 996–1000. [Google Scholar] [CrossRef]
- Valiño, V.; San Román, M.F.; Ibañez, R.; Ortiz, I. Improved separation of bovine serum albumin and lactoferrin mixtures using charged ultrafiltration membranes. Sep. Purif. Technol. 2014, 125, 163–169. [Google Scholar] [CrossRef]
- Mihelič, I.; Podgornik, A.; Koloini, T. Temperature influence on the dynamic binding capacity of a monolithic ion-exchange column. J. Chromatogr. A 2003, 7, 159–168. [Google Scholar] [CrossRef]
- Tatárová, I.; Fáber, R.; Denoyel, R.; Polakovic, M. Characterization of pore structure of a strong anion-exchange membrane adsorbent under different buffer and salt concentration conditions. J. Chromatogr. A 2009, 1216, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Vahedipour, K.; Lin, M.; Vogel, J.H.; Haynes, C.; von Lieres, E. Computational fluid dynamic simulation of axial and radial flow membrane chromatography: Mechanisms of non-ideality and validation of the zonal rate model. J. Chromatogr. A 2013, 1305, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Teepakorn, C.; Fiaty, K.; Charcosset, C. Effect of geometry and scale for axial and radial flow membrane chromatography—Experimental study of bovin serum albumin adsorption. J. Chromatogr. A 2015, 1403, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Teepakorn, C.; Fiaty, K.; Charcosset, C. Optimization of lactoferrin and bovine serum albumin separation using ion-exchange membrane chromatography. Sep. Purif. Technol. 2015, 151, 292–302. [Google Scholar] [CrossRef]
- Van Beijeren, P.; Kreis, P.; Zeiner, T. Ion exchange membrane adsorption of bovine serum albumin—Impact of operating and buffer conditions on breakthrough curves. J. Membr. Sci. 2012, 415–416, 568–576. [Google Scholar] [CrossRef]
- Hahn, R.; Tscheliessnig, A.; Bauerhansl, P.; Jungbauer, A. Dispersion effects in preparative polymethacrylate monoliths operated in radial-flow columns. J. Biochem. Biophys. Methods 2007, 70, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, S.C.; David, V. Essentials in Modern HPLC Separations, 1st ed.; Elsevier: Oxford, UK, 2012; Availiable online: http://store.elsevier.com/Essentials-in-Modern-HPLC-Separations/Serban-Moldoveanu/isbn-9780123850133/ (accessed on 17 September 2015).

| Ion exchange chromatography device | Membrane H = 3.0 mm | Membrane H = 9.0 mm | Sartobind S75 | Monolith H = 3.0 mm | Monolith H = 9.0 mm | CIMmultus |
|---|---|---|---|---|---|---|
| Support matrix | Stabilized reinforced cellulose | Poly(glycidyl methacrylate-co-ethylene dimethacrylate) | ||||
| Average pore diameter (μm) | 3.0–5.0 | 1.35 (CIM disc) | 2.1 (CIMmultus) | |||
| Porosity | 0.78 [28] | 0.60 (CIM disc) [27] | ||||
| Permeability (m2) | 10−13 [29] | 1.11 × 10−14 [27] | ||||
| Functionalized group | Sulfonic acid | Sulfonyl group | ||||
| Housing | CIM housing | CIM housing | Already packed | CIM housing | CIM housing | Already packed |
| Bed height H (mm) | 3.00 | 9.00 | 4.00 | 3.00 | 9.00 | 5.95 |
| Diameter Dm (mm) | 16.00 | 16.00 | 25.00 | 12.00 (16.0 with the fitting ring) | 12.00 (16.0 with the fitting ring) | Outer: 18.60 mm Inner: 6.70 mm Tube length: 4.20 mm |
| Number of discs | 11 | 33 | 15 | 1 | 3 | - |
| Bed volume BV (mL) | 0.6 | 1.8 | 2.1 | 0.34 | 1.02 | 1 |
| Flow rate (BV·min−1) | 12.0 | 18.0 | 24.0 | |
|---|---|---|---|---|
| LF DBC10% per BV of chromatographic support (mg·mL−1) | Monolith H = 3.0 mm | 24.74 ± 0.43 | 24.50 ± 0.02 | 24.29 ± 0.25 |
| Membrane H = 3.0 mm | 43.80 ± 0.81 | 42.25 ± 2.12 | 41.84 ± 2.25 | |
| Sartobind S75 | 29.15 ± 0.38 | 27.44 ± 0.14 | 28.89 ± 0.47 | |
| Elution Properties | Monolith H = 3.00 mm | Membrane H = 3.00 mm | Sartobind S75 |
|---|---|---|---|
| LF eluted mass/BV (mg·mL−1) | 19.33 | 32.58 | 14.05 |
| Asymmetry ratio | 3.37 | 2.10 | 3.61 |
| Tailing factor | 2.68 | 1.73 | 3.00 |
| Peak width at half-height (mL) | 1.65 | 1.32 | 2.64 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teepakorn, C.; Fiaty, K.; Charcosset, C. Comparison of Membrane Chromatography and Monolith Chromatography for Lactoferrin and Bovine Serum Albumin Separation. Processes 2016, 4, 31. https://doi.org/10.3390/pr4030031
Teepakorn C, Fiaty K, Charcosset C. Comparison of Membrane Chromatography and Monolith Chromatography for Lactoferrin and Bovine Serum Albumin Separation. Processes. 2016; 4(3):31. https://doi.org/10.3390/pr4030031
Chicago/Turabian StyleTeepakorn, Chalore, Koffi Fiaty, and Catherine Charcosset. 2016. "Comparison of Membrane Chromatography and Monolith Chromatography for Lactoferrin and Bovine Serum Albumin Separation" Processes 4, no. 3: 31. https://doi.org/10.3390/pr4030031
APA StyleTeepakorn, C., Fiaty, K., & Charcosset, C. (2016). Comparison of Membrane Chromatography and Monolith Chromatography for Lactoferrin and Bovine Serum Albumin Separation. Processes, 4(3), 31. https://doi.org/10.3390/pr4030031