Characteristics and Treatment Methods of Medical Waste Incinerator Fly Ash: A Review
Abstract
:1. Introduction
2. Special Characteristics of MWIFA
2.1. High Content of Chlorides
2.2. High Content of Dioxins
2.3. High Content of Carbon Constituents
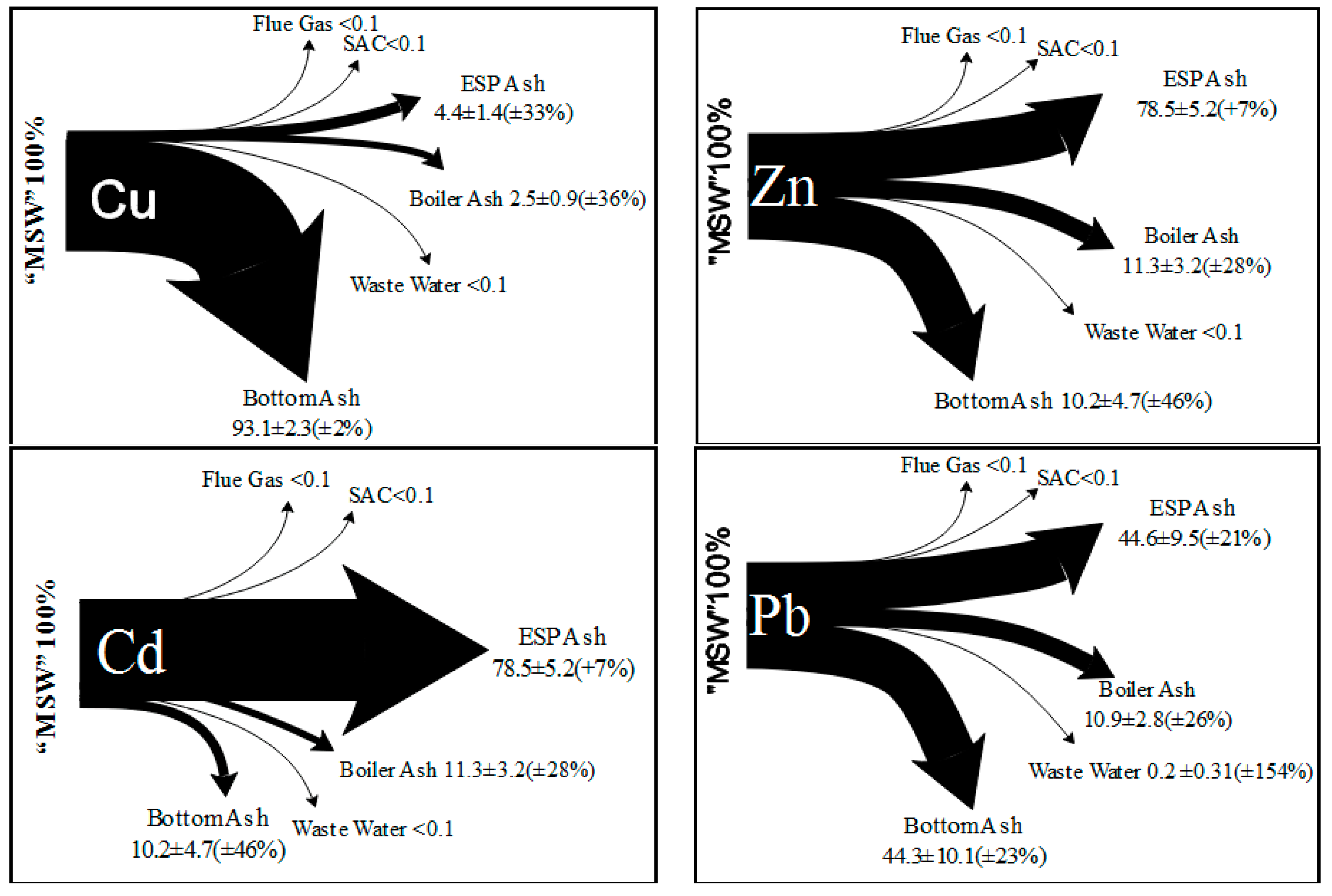
2.4. High Content of Heavy Metals
3. Available Techniques for MWIFA Treatment
3.1. Water Washing Pretreatment (WWP)
3.2. Acid Leaching Pretreatment (ALP)
3.3. Cement Solidification Technology (CST)
3.4. Melting Technique (MT)
3.5. Roasting
3.6. Low Temperature Reatment (LTTT)
3.7. Catalytic Hydro-Dechlorination (CHD)
3.8. Supercritical Water Oxidation (SCWO)
3.9. Hydrothermal Treatment (HTT)
3.10. Mechanochemical Technique (MCT)
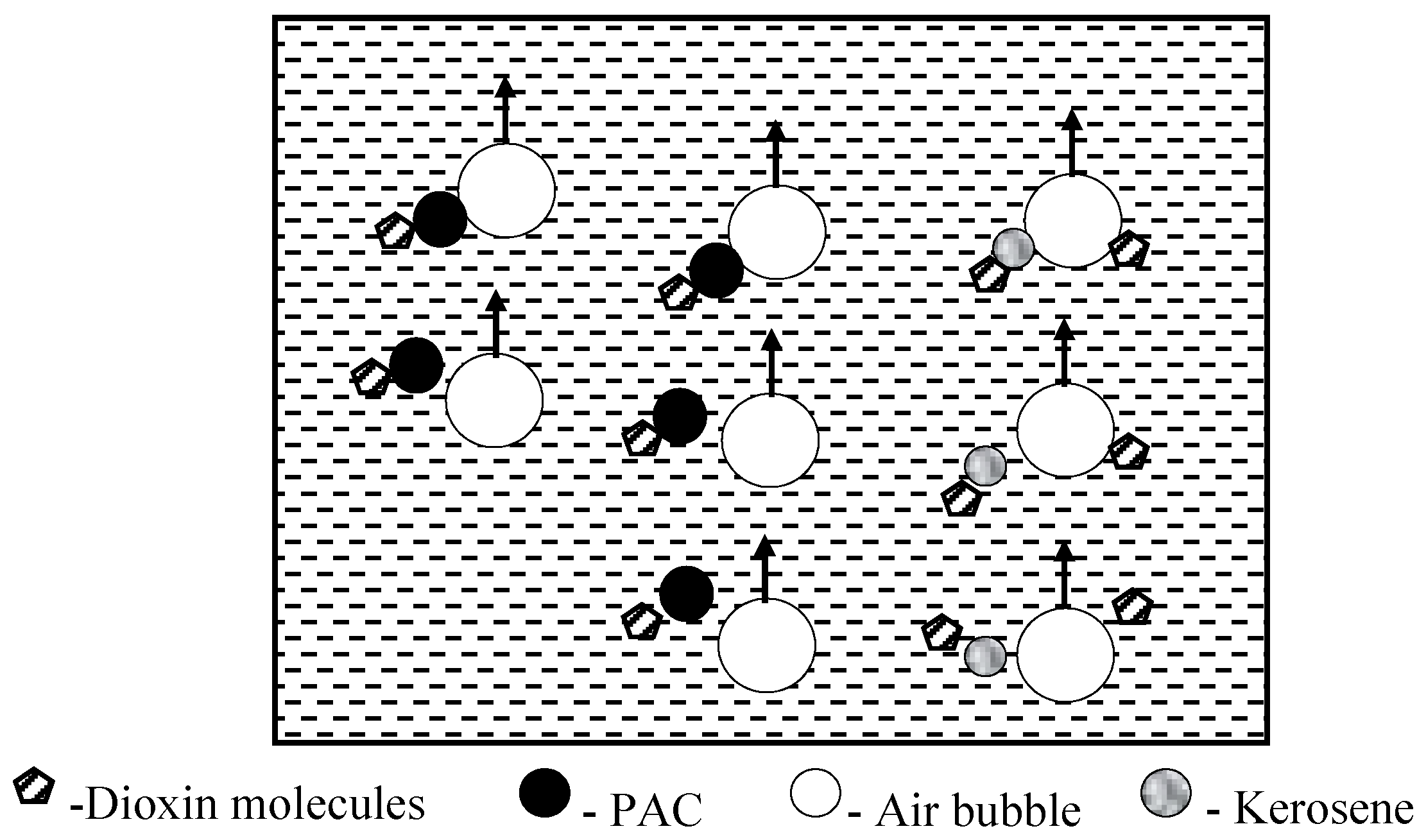
3.11. Flotation Treatment
3.12. Microwave Treatment (MWT)
4. Concluding Remarks and Future Work
- The pretreatment methods of acid leaching and water washing are applied to remove chlorine and heavy metals from fly ash, respectively. Both are worth promoting for MWIFA treatment by collaborating with other post-treatment methods. HCl and H2SO4 are the most efficient lixiviants at present. However, insoluble chlorides are difficult to remove with the water washing process and wastewater generated must also be treated. These drawbacks should be overcome.
- The post-treatment methods, such as roasting, residual carbon melting, the mechanochemical technique, flotation, and microwave treatment are recommended for the treatment of MWIFA after overall consideration of the special characteristics of MWIFA, and thorough consideration and balancing of environmental, technological, economical information.
- Cement solidification can prevent heavy metals leaching out but detoxification of dioxins is unable to realize. Furthermore, the characteristics of high chloride and carbon contents for MWIFA tend to weaken the effect of cement solidification. Thus, a future study is needed to focus on eliminating the influence of chlorides and carbons or thoroughly removing both substances.
- Melting can efficiently destroy dioxins and stabilize heavy metals; however, its high energy consumption and investment cost restrain its widespread application. This technology is theoretically unsuitable for the treatment of MWIFA at a small scale, except for residual carbon melting furnaces.
- Roasting treatment is suitable for the treatment of MWIFA and can decompose dioxins and recover heavy metals, which fully combines the characteristics of high chlorines and heavy metals in MWIFA.
- Intermediate treatment methods such as low-temperature thermal treatment, catalytic hydro-dechlorination, SCWO, and hydrothermal treatment, are effective in the decomposition of dioxins in MWIFA. However, there is limitation in the treatment of heavy metals in MWIFA. In addition, these methods remain in the laboratory testing stage, and some operations may cause severe secondary pollution or dioxin regeneration.
- The mechanochemical technique is effective at decomposing dioxins and stabilizing/removing heavy metals. It is low-cost, environmental friendly, and has no tail gas production. However, mechanochemical techniques remain in the development stage. Unknown difficulties must be overcome for future application.
- Flotation technology is recommended for handling MWIFA containing a high content of carbons, dioxins, chlorines, and heavy metals. This technology can efficiently remove carbon constituents, dioxins, and heavy metals from MWIFA. It is helpful to evaluate the harmlessness, reduction, and resource recovery of MWIFA. A combination of flotation and reburning treatment may be an especially promising method to resolve difficulties of MWIFA treatment.
- The microwave method makes full use of the specific characteristics of MWIFA through a strong microwave-absorbing medium, PAC. After microwave treatment, efficient decomposition of dioxins and solidification of heavy metals can be achieved. Meanwhile, a combination of flotation and microwave treatment can achieve both heavy metal removal and dioxin decomposition. Most successful cases have been demonstrated on the lab scale; however full-scale application is still in development. Comprehensive comparison of treatment techniques of MWIFA is given in Table 10.
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, H.Q.; Liu, F.; Wei, G.X.; Zhang, R.; Zang, D.D. Two-Step flotation treatment for removal of toxic matter from hospital solid waste incinerator fly ash. Aerosol Air Qual. Res. 2017, 17, 1329–1340. [Google Scholar] [CrossRef]
- Weber, R. Relevance of PCDD/PCDF formation for the evaluation of POPs destruction technologies—Review on current status and assessment gaps. Chemosphere 2007, 67, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.H.; Peng, Z.; Lu, S.Y.; Li, X.D.; Ni, M.J. Degradation of PCDD/Fs by mechanochemical treatment of fly ash from medical waste incineration. J. Hazard. Mater. 2007, 147, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Lee, W.J.; Lee, W.S.; Chang-Chien, G.P.; Tsai, P.J. Effect of chlorine content in feeding wastes of incineration on the emission of polychlorinated dibenzo-p-dioxins/dibenzofurans. Sci. Total Environ. 2003, 302, 185–198. [Google Scholar] [CrossRef]
- Margarida, J.Q.; João, C.B.; Rosa, M.Q.F. Treatment and use of air pollution control residues from MSW incineration, an overview. Waste Manag. 2008, 28, 2097–2121. [Google Scholar]
- Zhou, X.; Zhou, M.; Wu, X.; Han, Y.; Geng, J. Reductive solidification/stabilization of chromate in municipal solid waste incinerator fly ash by ascorbic acid and blast furnace slag. Chemosphere 2017, 182, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Wang, L.C.; Hsieh, L.T.; Hsieh, L.T.; Li, H.W.; Jiang, H.C. Effect of temperature and CaO addition on the removal of polychlorinated dibenzo-p-dioxins and dibenzofurans in fly ash from a medical waste incinerator. Aerosol Air Qual. Res. 2012, 12, 191–199. [Google Scholar] [CrossRef]
- Liao, W.T.; Wang, Y.F.; Tsai, C.H.; Tsai, Y.I.; Wu, Z.L.; MKuo, Y.M. Polychlorinated dibenz-p-dioxin and Dibenzofuran (PCDD/F) emission behavior during incineration of laboratory wastes. Part 2, PCDD/F profiles and characteristics of output materials. Aerosol Air Qual. Res. 2014, 14, 1206–1214. [Google Scholar] [CrossRef]
- Sukandar, S.; Yasuda, K.; Tanaka, M.; Aoyama, I. Metals leachability from medical waste incinerator fly ash, a case study on particle size comparison. Environ. Pollut. 2006, 144, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.W.; Zhang, S.K.; Hai, J.; Lei, M. Status and perspectives of municipal solid waste incineration in China, A comparison with developed regions. Waste Manag. 2017, 69, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Tzanakos, K.; Mimilidou, A.; Anastasiadou, K.; Stratakis, A.; Gidarakos, E. Solidification/stabilization of ash from medical waste incineration into geopolymers. Waste Manag. 2014, 34, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Wei, G.X.; Zhang, R. Removal of carbon constituents from hospital solid waste incinerator fly ash by column flotation. Waste Manag. 2013, 33, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Chen, G.F.; Zhu, Y.C.; Yao, D.; Wang, W.F.; Wang, L.J. Assessment of leaching behavior and human bioaccessibility of rare earth elements in typical hospital waste incineration ash in China. Front. Environ. Sci. Eng. 2017, 11, 5. [Google Scholar] [CrossRef]
- Aylin, A.; Esra, T.K.; Aylin, Y. Compressive strength and heavy metal leaching of concrete containing medical waste incineration ash. Constr. Build. Mater. 2017, 138, 326–332. [Google Scholar]
- Hanif, A.; Parthasarathy, P.; Ma, H.Y.; Fan, T.Y.; Li, Z.J. Properties improvement of fly ash cenosphere modified cement pastes using nano silica. Cement. Concr. Compos. 2017, 81, 35–48. [Google Scholar] [CrossRef]
- Singh, H.; Khosla, H. Comparative evaluation of performance of cold sprayed and bare N06601 superalloy in medical waste incinerator at 900 °C. Mater. Chem. Phys. 2017, 200, 57–69. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, A.E.M.; Salem, H.A. Effect of cement addition; solution resting time and curing characteristics on fly ash based geopolymer concrete performance. Constr. Build. Mater. 2016, 123, 581–593. [Google Scholar] [CrossRef]
- Yasuhara, A.; Katami, T. Leaching behavior of polychlorinated dibenzo-p-dioxins and furans from the fly ash and bottom ash of a municipal solid waste incinerator. Waste Manag. 2007, 27, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.C.; Lee, C.; Yoon, O.S.; Kim, H. Medical waste management in Korea. J. Environ Manag. 2006, 80, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.; Lee, W.J.; Huang, K.L.; Chang-Chien, G.P. Effect of raw materials on emissions of polychlorinated dibenzo-p-dioxins and dibenzofurans from the stack flue gases of secondary aluminum smelters. J. Hazard. Mater. 2007, 147, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.; Lee, W.J.; Tsai, P.J.; Mou, J.L.; Chang-Chien, G.P.; Yang, K.T. A novel method to enhance polychlorinated dibenzo-p-dioxins and dibenzofurans removal by adding bio-solution in EAF dust treatment plant. J. Hazard. Mater. 2008, 150, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, R.Z.; Xue, J.; Li, J.H. Generation and distribution of PAHs in the process of medical waste incineration. Waste Manag. 2013, 33, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Gunes, G.; Saral, A.; Yıldız, S.; Kuzu, S.L. Determination of optimum dose of adsorbent for PCDD/F removal in the flue gas of a medical waste incineration plant. Chem. Eng. Res. Des. 2015, 104, 695–702. [Google Scholar] [CrossRef]
- Yoon, Y.W.; Jeon, T.W.; Son, J.K.; Kim, K.Y.; Kwon, E.H. Characteristics of PCDDs/PCDFs in stack gas from medical waste incinerators. Chemosphere 2017, 188, 478–485. [Google Scholar] [CrossRef] [PubMed]
- McKay, G. Dioxin characterisation; formation and minimisation during municipal solid waste (MSW) incineration, review. Chem. Eng. J. 2002, 86, 343–368. [Google Scholar] [CrossRef]
- Hsu, W.T.; Liu, M.C.; Hung, P.C.; Chang, S.H.; Chang, M.B. PAH emissions from coal combustion and waste incineration. J. Hazard. Mater. 2016, 318, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Meng, A.H.; Long, Y.Q.; Li, Q.H.; Zhang, Y.G. A review of dioxin-related substances during municipal solid waste incineration. Waste Manag. 2015, 36, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, A.; Watanabe, S.; Iwakiri, R.; Honda, K. Removal of dioxins and dioxin-like PCBs from fish oil by countercurrent supercritical CO2 extraction and activated carbon treatment. Chemosphere 2009, 75, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Van Caneghem, J.; Block, C.; Vandecasteele, C. Destruction and formation of dioxin-like PCBs in dedicated full scale waste incinerators. Chemosphere 2014, 94, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Takaoka, M.; Takeda, N.; Matsumoto, T.; Oshita, K. Application of microwave-assisted extraction to the analysis of PCBs and CBzs in fly ash from municipal solid waste incinerators. J. Hazard. Mater. 2006, 137, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, H.J.; Chen, J.P. Vapor-phase sorption of hexachlorobenzene on typical municipal solid waste (MSW) incinerator fly ashes; clay minerals and activated carbon. Chemosphere 2010, 81, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yang, L.B.; Zhou, J.Z.; Liu, L.Y.; Qian, G.R.; Nobutoshi, O.; Mamoru, M.; Kokyo, O.; Shigeo, H. Characteristics of dioxins content in fly ash from municipal solid waste incinerators in China. Chemosphere 2013, 92, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.C.; Yan, J.H.; Xie, Z.M. Detoxifying PCDD/Fs and heavy metals in fly ashes from medical waste incinerators with a DC double arcs plasma torch. J. Environ. Sci. 2013, 25, 1362–1367. [Google Scholar] [CrossRef]
- Chang, Y.M.; Hung, C.Y.; Chen, J.H.; Chang, C.T.; Chen, C.H. Minimum feeding rate of activated carbon to control dioxin emissions from a large-scale municipal solid waste incinerator. J. Hazard. Mater. 2009, 161, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yan, J.H.; Lu, S.Y.; Li, X.D.; Gu, Y.L.; Dai, H.F.; Ni, M.J.; Cen, K.F. Characteristic of polychlorinated dibenzo-p-dioxins and dibenzofurans in fly ash from incinerators in China. J. Hazard. Mater. 2008, 150, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.X.; Liu, H.Q.; Zhang, R.; Zhu, Y.W.; Xu, X.; Zang, D.D. Application of microwave energy in the destruction of dioxins in the froth product after flotation of hospital solid waste incinerator fly ash. J. Hazard. Mater. 2017, 325, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.W.; Lin, T.C.; Wang, L.C.; Chang-Chien, G.P. The PCDD/F removal efficiency of a medical waste incinerator dual-bag filter system. Aerosol Air Qual. Res. 2014, 14, 1223–1231. [Google Scholar] [CrossRef]
- Chen, J.; Chen, T.; Wang, Q.; Wang, C.; Guo, Y.; Lu, S.Y.; Li, X.D. PCDD/Fs emission levels of hazardous and medical waste incineration in China. Acta Sci. Circumstantiae 2014, 34, 973–979. [Google Scholar]
- Shibayama, A.; Kim, Y.H.; Harjanto, S.; Harjanto, S.J.; Sugai, Y.C.; Okada, K.; Fujita, T. Remediation of contaminated soil by fly ash containing dioxins from incineration by using flotation. Mater. Trans. 2005, 46, 990–995. [Google Scholar] [CrossRef]
- Grzegorz, W. The reduction of dioxin emissions from the processes of heat and power generation. Air Waste Manag. Assoc. 2011, 61, 511–526. [Google Scholar]
- Hung, P.C.; Chen, Q.H.; Chang, M.B. Pyrolysis of MWIFA - Effect on dioxin-like congeners. Chemosphere 2013, 92, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.X.; Liu, H.Q.; Liu, F.; Zhang, R.; Zhu, Y.W.; Gao, S.Y. Reburning treatment of the froths obtained after the flotation of incinerator fly ash. Aerosol Air Qual. Res. 2017, 17, 1084–1096. [Google Scholar] [CrossRef]
- Lu, S.Y.; Ji, Y.; Buekens, A.; Ma, Z.Y.; Jin, Y.Q.; Li, X.D.; Yan, J.H. Activated carbon treatment of municipal solid waste incineration flue gas. Waste Manag. Res. 2013, 31, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Lin, C.; Chang-Chien, G.P. Characteristics of PCDD/Fs in a particles filtration device with activated carbon injection. Aerosol Air Qual. Res. 2009, 9, 317–322. [Google Scholar] [CrossRef]
- Wang, C.F.; Zhu, N.M.; Wang, Y.M.; Zhang, F.S. Co-detoxification of transformer oil-contained PCBs and heavy metals in medical waste incinerator fly ash under sub- and supercritical water. Environ. Sci. Technol. 2012, 46, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Buekens, A.; Cen, K. Waste incineration; PVC; and dioxins. J. Mater. Cycles Waste Manag. 2011, 13, 190–197. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Heavy metal removal from sediments by biosurfactants. J. Hazard. Mater. 2001, 85, 111–125. [Google Scholar] [CrossRef]
- Zhao, L.J.; Zhang, F.S.; Wang, K.S.; Zhu, J.X. Chemical properties of heavy metals in typical hospital waste incinerator ashes in China. Waste Manag. 2008, 29, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.J.; Zhang, F.S.; Hao, Z.P.; Wang, H.L. Levels of polycyclic aromatic hydrocarbons in different types of hospital waste incinerator ashes. Sci. Total Environ. 2008, 397, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, H.B.; Huang, Q.F.; Wang, Q.; Zhou, B.Y.; Li, J.H. Hazardous waste generation and management in China, A review. J. Hazard. Mater. 2008, 158, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Li, B.H.; Deng, Z.Y.; Wang, W.X.; Fang, H.S.; Zhou, H.B.; Deng, F.X.; Huang, L.; Li, H.Y. Degradation characteristics of dioxin in the fly ash by washing and ball-milling treatment. J. Hazard. Mater. 2017, 339, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Fedje, K.K.; Ekberg, C.; Skarnemark, G.; Steenari, B.M. Removal of hazardous metals from MSW fly ash—An evaluation of ash leaching methods. J. Hazard. Mater. 2010, 173, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Lin, C.L.; Zeng, W.Y. Effect of waste incineration and gasification processes on heavy metal distribution. Fuel Process. Technol. 2014, 125, 67–72. [Google Scholar] [CrossRef]
- Liu, H.Q.; Wei, G.X.; Zhang, R.; Liu, F.G. Simultaneous Removal of Heavy Metals and PCDD/Fs from Hospital Waste Incinerator Fly Ash by Flotation Assisted with Hydrochloric Acid. Sep. Sci. Technol. 2014, 49, 1019–1028. [Google Scholar] [CrossRef]
- Wang, F.H.; Zhang, F.; Chen, Y.J.; Gao, J.; Zhao, B. A comparative study on the heavy metal solidification/stabilization performance of four chemical solidifying agents in municipal solid waste incinerator fly ash. J. Hazard. Mater. 2015, 300, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Charles, H.K.L.; Alvin, W.M.; John, P.B.; McKay, G. Use of Incineration MSW Ash, A Review. Sustainability 2010, 2, 1943–1968. [Google Scholar]
- Xie, Y.J.; Zhu, J.X. Leaching toxicity and heavy metal bioavailability of medical waste incinerator fly ash. J. Mater. Cycles Waste Manag. 2013, 15, 440–448. [Google Scholar] [CrossRef]
- Tang, J.F.; Steenari, B.M. Leaching optimization of municipal solid waste incineration ash for resource recovery, A case study of Cu; Zn; Pb and Cd. Waste Manag. 2016, 48, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Vavva, C.; Voutsas, E.; Magoulas, K. Process development for chemical stabilization of fly ash from municipal solid waste incineration. Chem. Eng. Res. Des. 2017, 125, 57–71. [Google Scholar] [CrossRef]
- Liu, G.R.; Zhan, J.Y.; Zheng, M.H.; Li, L.; Li, C.P.; Jiang, X.X.; Wang, M.; Zhao, Y.Y.; Jin, R. Field pilot study on emissions; formations and distributions of PCDD/Fs from cement kiln co-processing fly ash from municipal solid waste incinerations. J. Hazard. Mater. 2015, 299, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Saffarzadeh, A.; Shimaoka, T.; Kawano, T. Existence of Cl in municipal solid waste incineration bottom ash and dechlorination effect of thermal treatment. J. Hazard. Mater. 2014, 267, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Dong, Z.L.; Yang, E.H. Strain hardening cementitious composites incorporating high volumes of municipal solid waste incinerator fly ash. Constr. Build. Mater. 2017, 146, 183–191. [Google Scholar] [CrossRef]
- Mangialardi, T. Effects of a washing pre-treatment of municipal solid waste incinerator fly ash in the hydration behavior and properties of ash-Portland cement mixtures. Adv. Cem. Res. 2004, 16, 45–54. [Google Scholar] [CrossRef]
- Huang, K.; Inoue, K.; Harada, H.; Kawakita, H.; Ohto, K. Leaching behavior of heavy metals with hydrochloric acid from fly ash generated in municipal waste incineration plants. Trans. Nonferrous Metals Soc. China 2011, 21, 1422–1427. [Google Scholar] [CrossRef]
- Funari, V.; Mäkinen, J.; Salminen, J.; Revitzer, H. Metal removal from municipal solid waste incinerator fly ash, a comparison between chemical leaching and bioleaching. Waste Manag. 2017, 60, 39–406. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, Y. Leaching heavy metals in municipal solid waste incinerator fly ash with chelator/biosurfactant mixed solution. Waste Manag. Res. 2015, 33, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Ecke, H.; Sakanakura, H.; Matsuto, T.; Tanaka, N.; Lagerkvist, A. State-of-the-art treatment processes for municipal solid waste incineration residues in Japan. Waste Manag. Res. 2000, 18, 41–51. [Google Scholar] [CrossRef]
- Poona, C.S.; Qiao, X.C.; Lin, Z.S. Pozzolanic properties of reject fly ash in blended cement pastes. Cem. Concr. Res. 2003, 33, 1857–1865. [Google Scholar] [CrossRef]
- Aubert, J.E.; Husson, B.; Sarramone, N. Utilization of municipal solid waste incineration (MSWI) fly ash in blended cement part 1, processing and characterization of MSWIFA. J. Hazard. Mater. 2006, 136, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Lampris, C.; Stegemann, J.A.; Cheeseman, C.R. Solidification/stabilisation of air pollution control residues using Portland cement, Physical properties and chloride leaching. Waste Manag. 2009, 29, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.N.; Li, J.M.; Huo, B.Q.; Ji, B.W. Application of Sulfoaluminate cement for solidification/stabilization of fly ash from municipal solid waste incinerators. Appl. Mech. Mater. 2012, 178–181, 795–798. [Google Scholar] [CrossRef]
- Bie, R.S.; Chen, P.; Song, X.F.; Ji, X.Y. Characteristics of municipal solid waste incinerator fly ash with cement solidification treatment. J. Energy Inst. 2016, 89, 704–712. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, A. Geo-environmental application of municipal solid waste incinerator ash stabilized with cement. J. Rock Mech. Geotech. Eng. 2017, 9, 370–375. [Google Scholar] [CrossRef]
- Bo, D.; Zhang, F.S.; Zhao, L.J. Influence of supercritical water treatment on heavy metals in medical waste incinerator fly ash. J. Hazard. Mater. 2009, 170, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.Q.; Wei, G.X.; Zhang, R. Effect of water washing pre-treatment on the properties of glass-ceramics from incinerator fly ash using electronic arc furnace. J. Wuhan Univ. Technol. Mater. Sci. 2013, 28, 62–68. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Montangnaro, F.; Santoro, L. Soluble salt removal from MSWIFA and its stabilization for safer disposal and recovery as road basement material. Waste Manag. 2012, 32, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Chuieh, P.T. Life cycle assessment of reusing fly ash from municipal solid waste incineration. Procedia Eng. 2015, 118, 984–991. [Google Scholar] [CrossRef]
- Sakai, S.; Hiraoka, M. Municipal solid waste incinerator residue recycling by thermal processes. Waste Manag. 2000, 20, 249–258. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.H.; Tu, X.; Chi, Y.; Li, X.D.; Lu, S.Y.; Cen, K.F. Thermal treatment of municipal solid waste incinerator fly ash using DC double arc argon plasma. Fuel 2009, 88, 955–958. [Google Scholar] [CrossRef]
- Liu, H.Q.; Wei, G.X.; Liang, Y.; Dong, F.Y. Glass-ceramics made from arc-melting slag of waste incinerator fly ash. J. Cent. South Univ. Technol. 2011, 18, 1945–1952. [Google Scholar] [CrossRef]
- Kim, K.H.; Seo, Y.C.; Nam, H.; Joung, H.T.; You, J.C.; Kim, D.J.; Seo, Y.C. Characteristics of major dioxin/furan congeners in melted slag of ash from municipal solid waste incinerators. Microchem. J. 2005, 80, 171–181. [Google Scholar] [CrossRef]
- Vehlow, J.; Bergfeldt, B.; Hunsinger, H. PCDD/F and related compounds in solid residues from municipal solid waste incineration—A literature review. Waste Manag. Res. 2006, 24, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gao, X.B.; Zheng, L.; Lan, Y.X. Reductive dechlorination of polychlorinated dibenzo-p-dioxins and dibenzofurans in MSWIFA by sodium hypophosphite. Sep. Sci. Technol. 2006, 52, 186–190. [Google Scholar]
- Jiang, Y.H.; Xi, B.D.; Li, X.J.; Zhang, L.; Wei, Z.M. Effect of water-extraction on characteristics of melting and solidification of fly ash from municipal solid waste incinerator. J. Hazard. Mater. 2009, 161, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Chiu, W.T.; Wang, T.M.; Chen, C.T.; Tzeng, C.C. Porous materials produced from incineration ash using thermal plasma technology. Waste Manag. 2014, 34, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.C.C.; Chuang, T.N.; Huang, C.W. Achieving zero waste of municipal incinerator fly ash by melting in electric arc furnaces while steel making. Waste Manag. 2017, 62, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Vehlow, J. Reduction of dioxin emissions from thermal waste treatment plants, a brief survey. Rev. Environ. Sci. Biotechnol. 2012, 11, 393–405. [Google Scholar] [CrossRef]
- Jakob, A.; Stucki, S.; Rpwj, S. Complete heavy metal removal from fly ash heat treatment, influence of chlorides on evaporation rates. Environ. Sci. Technol. 1996, 30, 3275–3283. [Google Scholar] [CrossRef]
- Nowak, B.; Rocha, S.F.; Aschenbrenner, P.; Rechberger, H.; Winter, F. Heavy metal removal from MSW fly ash by means of chlorination and thermal treatment, influence of the chloride type. Chem. Eng. J. 2012, 179, 178–185. [Google Scholar] [CrossRef]
- Chen, W.S.; Chang, F.C.; Shen, Y.H.; Chen, W.S.; Chang, F.C.; Shen, Y.H.; Tsai, M.S.; Ko, C.H. Removal of chloride from MSWI fly ash. J. Hazard. Mater. 2012, 237–238, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Chris, C.Y.C.; Donald, W.K. Behaviour of metals under the conditions of roasting MSW incinerator fly ash with chlorinating agents. J. Hazard. Mater. 1999, 64, 75–89. [Google Scholar]
- Donald, W.K.; Chris, C.Y.C.; Hilary, M. Chromium behavior during thermal treatment of MSW fly ash. J. Hazard. Mater. 2002, 90, 39–49. [Google Scholar]
- Lundin, L.; Aurell, J.; Marklund, S. The behavior of PCDD and PCDF during thermal treatment of waste incineration ash. Chemosphere 2011, 84, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, H.; Masui, M.; Namiki, N.; Kim, J.C.; Otani, Y. Suppression of solidification of calcium-rich incinerator fly ash during thermal treatment for decomposition/detoxification of dioxins. Adv. Powder Technol. 2007, 18, 143–154. [Google Scholar] [CrossRef]
- Wu, H.L.; Lu, S.Y.; Yan, J.H.; Li, X.D.; Chen, T. Thermal removal of PCDD/Fs from medical waste incinerator fly ash—Effect of temperature and nitrogen flow rate. Chemosphere 2011, 84, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, Y.; Matsuto, T.; Tanaka, N.; Masuda, T. Influence of residual carbon on the decomposition process of PCDD/Fs in MSWIFA. Chemosphere 2005, 58, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Chen, Y.M.; Wan, P.Y.; Fan, M.; Yang, X.J. Chemical degradation of drinking water disinfection byproducts by millimeter-sized particles of iron-silicon and magnesium-aluminum alloys. J. Am. Chem. Soc. 2010, 132, 2500–2501. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, Z.Y.; Wan, H.Q.; Zheng, J.Z.; Yin, D.Q.; Zheng, S.R. Aqueous bromate reduction by catalytic hydrogenation over Pd/Al2O3 catalysts. Appl. Catal. B Environ. 2010, 96, 307–313. [Google Scholar] [CrossRef]
- Bahri, A.; Calvo, L.; Polo, A.M.; Gilarranz, M.A.; Mohedano, A.F.; Rodriguez, J.J. Identification of by-products and toxicity assessment in aqueous-phase hydrodechlorination of diuron with palladium on activated carbon catalysts. Chemosphere 2013, 91, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sainero, L.M.; Seoane, X.L.; Fierro, J.L.G.; Arcoya, A. Liquid-phase hydrodechlorination of CCl4 to CHCl3 on Pd/carbon catalysts, nature and role of Pd active species. J. Catal. 2002, 209, 279–288. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, J.P.; Zhang, H.J.; Ni, Y.W.; Zhang, Q. Dechlorination of dioxins with Pd/C in ethanol–water solution under mild conditions. Sep. Purif. Technol. 2008, 59, 164–168. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, Z.M.; Ma, Z.; Bian, S.W.; Song, W.G. Highly active and stable material for catalytic hydrodechlorination using ammonia-treated carbon nanofibers as Pd supports. J. Phys. Chem. C 2008, 112, 1199–1203. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, K.; Wang, W.J.; Xu, Z.; Wan, H. Pd supported on boron-doped mesoporous carbon as highly active catalyst for liquid phase catalytic hydrodechlorination of 2; 4-dichlorophenol. Appl. Catal. A Gen. 2014, 470, 336–343. [Google Scholar] [CrossRef]
- Yang, Z.J.; Xia, C.H.; Zhang, Q.; Chen, J.P.; Liang, X.M. Catalytic detoxification of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans in fly ash. Waste Manag. 2007, 27, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.; Wang, Y.; Li, Y.M. Preparation, structure, and electrochemical properties of reduced grapheme sheet films. Adv. Funct. Mater. 2009, 19, 2782–2789. [Google Scholar] [CrossRef]
- Kim, Y.; Noh, Y.; Lim, E.J.; Lee, S.; Choi, S.M.; Kim, W.B. Star-shaped Pd/Pt core-shell catalysts supported on reduced grapheme oxide with superior electrocatalytic performance. J. Mater. Chem. A 2014, 2, 6976–6986. [Google Scholar] [CrossRef]
- Chin, Y.T.; Lin, C.; Chang-Chien, G.P.; Wang, Y.M. PCDD/F Formation Catalyzed by the Metal Chlorides and Chlorinated Aromatic Compounds in Fly Ash. Aerosol Air Qual. Res. 2012, 12, 228–236. [Google Scholar] [CrossRef]
- Okajima, I.; Kawasaki, S.I.; Noguchi, H.; Sako, T. Decomposition of dioxins and PCBs with supercritical water. J. Adv. Technol. Phys. 2012, 3, 021212. [Google Scholar]
- Zou, D.A.; Chi, Y.; Fu, C.; Dong, J.; Wang, F.; Ni, M.J. Co-destruction of organic pollutants in municipal solid waste leachate and dioxins in fly ash under supercritical water using H2O2 as oxidant. J. Hazard. Mater. 2013, 248–249, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, K.C.; Mahiko, T.; Sekizawa, K.; Izumizaki, Y. Supercritical water oxidation of polychlorinated biphenyls based on the redox reactions promoted by nitrate and nitrite salts. J. Supercrit. Fluids 2006, 39, 54–62. [Google Scholar] [CrossRef]
- Kim, K.; Son, S.H.; Kim, K.S.; Kim, K.; Kim, Y.C. Environmental effects of supercritical water oxidation (SCWO) process for treating transformer oil contaminated with polychlorinated biphenyls (PCBs). Chem. Eng. J. 2010, 165, 170–174. [Google Scholar] [CrossRef]
- Bermejo, M.D.; Cocero, M.J. Destruction of an industrial wastewater by supercritical water oxidation in a transpiring wall reactor. J. Hazard. Mater. 2006, 137, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.C.; Diono, W.; Sasaki, M.; Goto, M. Reduction of total acid number (TAN) of naphthenic acid (NA) using supercritical water for reducing corrosion problems of oil refineries. Fuel 2012, 94, 620–623. [Google Scholar] [CrossRef]
- Wang, S.Z.; Guo, Y.; Wang, L.A.; Wang, Y.Z.; Xu, D.H.; Ma, H.H. Supercritical water oxidation of coal, Investigation of operating parameters effects reaction kinetics and mechanism. Fuel Process. Technol. 2011, 92, 291–297. [Google Scholar] [CrossRef]
- Zhang, G.H.; Liu, X.S.; Thomas, J.K. Radiation induced physical and chemical processes in zeolite materials. Radiat. Phys. Chem. 1998, 51, 135–152. [Google Scholar] [CrossRef]
- Somerset, V.S.; Petrik, L.F.; White, R.A.; Klink, M.J.; Key, D.; Iwuoha, E.I. Alkaline hydrothermal zeolites synthesized from high SiC > 2 and AI2O3 co-disposal fly ash filtrates. Fuel 2005, 76, 793–799. [Google Scholar]
- Murayama, N.; Takahashi, T.; Shuku, K.; Lee, H.H.; Shibata, J. Effect of reaction temperature on hydrothermal syntheses of potassium type zeolites from coal fly ash. Int. J. Miner. Process. 2008, 158, 616–622. [Google Scholar] [CrossRef]
- Yang, G.C.C.; Yang, T. Synthesis of zeolites from municipal incinerator fly ash. J. Hazard. Mater. 1998, 58, 103–120. [Google Scholar] [CrossRef]
- Bayuseno, A.P.; Schmahl, W.W.; Mullejans, T. Hydrothermal processing of MSWI Fly ash-towards new stable minerals and fixation of heavy metals. J. Hazard. Mater. 2009, 167, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Brunner, G. Near critical and supercritical water. Part I. Hydrolytic and hydrothermal processes. J. Supercrit. Fluids 2009, 47, 373–381. [Google Scholar] [CrossRef]
- Brunner, G. Near and supercritical water. Part II, Oxidative processes. J. Supercrit. Fluids 2009, 47, 382–390. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Zhang, P.F.; Chen, D.Z.; Zhou, B.; Li, J.Y.; Li, X.W. Hydrothermal treatment of municipal solid waste incinerator fly ash for dioxin decomposition. J. Hazard. Mater. 2012, 207–208, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Shibuya, E.; Kanamaru, Y.; Uyama, K.; Nishioka, M. Hydrothermal decomposition of PCDDs/PCDFs in MSWI fly ash. Chemosphere 1996, 32, 203–208. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Tardio, J.; Prasad, J.; Föger, K.; Akolekar, D.B. Wet Oxidation and Catalytic Wet Oxidation. Ind. Eng. Chem. Res. 2006, 45, 1221–1258. [Google Scholar] [CrossRef]
- Zhang, F.S.; Itoh, H. Extraction of metals from municipal solid waste incinerator fly ash by hydrothermal process. J. Hazard. Mater. 2006, 136, 663–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.S.; Kan, L.L. Leaching behavior of heavy metals from municipal solid wastes incineration (MSWI) fly ash used in concrete. J. Hazard. Mater. 2009, 164, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Li, X.; Yong, C.; Yan, J. Co-disposal of heavy metals containing waste water and medical waste incinerator fly ash by hydrothermal process with addition of sodium carbonate, a case study on Cu(II) removal. Water Air Soil Pollut. 2010, 209, 391–400. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Zhang, P.F.; Li, J.Y.; Chen, D.Z. Stabilization and separation of heavy metals in incinerator fly ash during the hydrothermal treatment process. J. Hazard. Mater. 2015, 299, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.Z.; Zhang, C.; Zhang, J.L.; Li, P.F.; Wei, Y.M. Seed-assisted hydrothermal treatment with composite silicon−aluminum additive for solidification of heavy metals in municipal solid waste incinerator fly ash. Energy Fuels 2016, 30, 10661–10670. [Google Scholar] [CrossRef]
- Fukasawa, T.; Horigome, A.; Tsu, T. Utilization of incinerator fly ash from biomass power plants for zeolite synthesis from coal fly ash by hydrothermal treatment. Fuel Process Technol. 2017, 16, 792–798. [Google Scholar]
- Shi, D.Z.; Hu, C.Y.; Zhang, J.L.; Li, P.F.; Zhang, C.; Wang, X.M.; Ma, H. Silicon-aluminum additives assisted hydrothermal process for stabilization of heavy metals in fly ash from MSW incineration. Fuel Process. Technol. 2017, 165, 44–53. [Google Scholar] [CrossRef]
- Mallampati, S.R.; Simion, C.; Mitoma, Y. Novel separation and immobilization of heavy metals in municipal solid waste fly ash by grinding with nano-Fe/Ca/CaO/[PO4] mixture. Environ. Prog. Sustain. Energy 2016, 35, 1693–1698. [Google Scholar] [CrossRef]
- Birke, V.; Mattik, J.; Runne, D.; Benning, H.; Zlatovic, D. Dechlorination of recalcitrant polychlorinated contaminants using ball milling. In Ecological Risks Associated with the Destruction of Chemical Weapons; Springer: Dordrecht, The Netherlands, 2006; pp. 111–127. [Google Scholar]
- Nah, I.W.; Hwang, K.Y.; Shul, Y.G. Effect of metal and glycol on mechanochemical dechlorination of polychlorinated biphenyls (PCBs). Chemosphere 2008, 73, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Cagnetta, G.; Hassan, M.M.; Huang, J.; Yu, G.; Weber, R. Dioxins reformation and destruction in secondary copper smelting fly ash under ball milling. Sci. Rep. 2016, 6, 22925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasser, A.; Sposito, G.; Cheney, M.A. Mechanochemical degradation of 2;4-adsorbed on synthetic birnessite. Colloids Surf. A 2000, 163, 117–123. [Google Scholar] [CrossRef]
- Maria, D.R.P.; Annalisa, N.; Matteo, S. Mechanochemical removal of organo-chlorinated compounds by inorganic components of soil. Chemosphere 2004, 55, 1485–1492. [Google Scholar]
- Mallampati, S.R.; Mitoma, Y.; Okuda, T.; Sakita, S.; Simion, C. Simultaneous decontamination of cross-polluted soils with heavy metals and PCBs using a nano-metallic Ca/CaO dispersion mixture. Environ. Sci. Pollut. Res. 2014, 21, 9270–9277. [Google Scholar] [CrossRef] [PubMed]
- Cagnetta, G.; Robertson, J.; Huang, J.; Zhang, K.L.; Yu, G. Mechanochemical destruction of halogenated organic pollutants, A critical review. J. Hazard. Mater. 2016, 313, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Lv, Z.W.; Du, L. Emission characteristic of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/Fs) from medical waste incinerators (MWIs) in China in 2016, A comparison between higher emission levels of MWIs and lower emission levels of MWIs. Environ. Pollut. 2017, 221, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Mao, Q.J.; Lu, S.Y.; Buekens, A.; Xu, S. Dioxins degradation and reformation during mechanochemical treatment. Chemosphere 2017, 180, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Prakash, A.; Falkner, J.C. Low-field magnetic separation of monodisperse Fe3O4 nano crystals. Science 2006, 314, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Ding, Q.; Sun, Y.Z.; Jiang, C.; Gao, X.H.; Yan, J.H. Characterization of mechanochemical treated fly ash from a medical waste incinerator. J. Environ. Sci. 2010, 22, 1643–1648. [Google Scholar] [CrossRef]
- Mao, Q.J.; Lu, S.Y.; Wei, Y.L. Mechanochemical decomposition of polychlorinated biphenyls contaminated soil using a horizontal ball mill. Environ. Chem. 2016, 35, 607–614. [Google Scholar]
- Mitoma, Y.; Miyata, H.; Egashira, N.; Simion, A.M.; Kakeda, M. Mechanochemical degradation of chlorinated contaminants in fly ash with a calcium-based degradation reagent. Chemosphere 2011, 83, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Marulanda, V.; Bolãnos, G. Supercritical water oxidation of a heavily PCB-contaminated mineral transformer oil, Laboratory-scale data and economic assessmen. J. Supercrit. Fluids 2010, 54, 258–265. [Google Scholar] [CrossRef]
- Mouton, J.; Mercier, G.; Blais, J.F. Amphoteric Surfactants for PAH and Lead Polluted-Soil Treatment Using Flotation. Water Air Soil Pollut. 2009, 197, 381–393. [Google Scholar] [CrossRef]
- Harvey, P.A.; Nguyen, A.V.; Evans, G.M. Influence of Electrical Double-Layer Interaction on Coal Flotation. J. Colloid Interface Sci. 2002, 250, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Takaoka, M.; Takeda, N.; Oshita, K. Polychlorinated biphenyls removal from weathered municipal solid waste incinerator fly ash by collector-assisted column flotation. J. Hazard. Mater. 2003, 100, 259–270. [Google Scholar] [CrossRef]
- Huang, Y.; Takaoka, M.; Nobuo Takeda, A. Chlorobenzenes removal from municipal solid waste incinerator fly ash by surfactant-assisted column flotation. Chemosphere 2003, 52, 735–743. [Google Scholar] [CrossRef]
- Huang, Y.; Takaoka, M.; Nobuo Takeda, A.; Oshita, K. Partial removal of PCDD/Fs; coplanar PCBs; and PCBs from municipal solid waste incinerator fly ash by a column flotation process. Environ. Sci. Technol. 2007, 41, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.X.; Liu, H.Q.; Zhang, R.; Zhu, Y.W.; Xu, X. Mass concentrations of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) and heavy metals in different size fractions of hospital solid waste incinerator fly ash particles. Aerosol Air Qual. Res. 2016, 16, 1569–1578. [Google Scholar] [CrossRef]
- Liu, H.Q.; Liu, F.; Wei, G.X.; Zhang, R.; Zhu, Y.W. Effects of surfactants on the removal of carbonaceous matter and dioxins from weathered incinerator fly ash. Aerosol Air Qual. Res. 2017, 17, 2338–2347. [Google Scholar] [CrossRef]
- Nagano, S.; Tamon, H.; Adzumi, T.; Nakagawa, K.; Suzuki, T. Activated carbon from municipal waste. Carbon 2000, 38, 915–920. [Google Scholar] [CrossRef]
- Wang, S.L.; Mulligan, C.N. An evaluation of surfactant foam technology in remediation of contaminated soil. Chemosphere 2004, 57, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.Y.; Lo, S.L.; Hsieh, C.H.; Chen, C.L. Sintering of MSWI fly ash by microwave energy. J. Hazard. Mater. 2009, 163, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.H.; Li, W.; Peng, J.H.; Niu, H.; Huang, M. Microwave-absorbing characteristics of mixtures of different carbonaceous reducing agents and oxidized ilmenite. Int. J. Miner. Process. 2009, 93, 289–293. [Google Scholar] [CrossRef]
- Chou, S.Y.; Lo, S.L. Effects of microwave-absorbing additives on heavy metal immobilization. Environ. Eng. Sci. 2013, 30, 317–323. [Google Scholar] [CrossRef]
- Lin, Z.R.; Zhao, L.; Dong, Y.H. Application of microwave-irradiated manganese dioxide in the removal of polychlorinated biphenyls from soil contaminated by capacitor oil. Environ. Technol. 2013, 5, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.K.; Lo, S.L. Microwave irradiation, A sustainable way for sludge treatment and resource recovery. Renew. Sustain. Energy Rev. 2013, 18, 288–305. [Google Scholar] [CrossRef]
- Liu, X.L.; Quan, X.; Bo, L.L.; Chen, S.; Zhao, Y.Z.; Chang, M. Temperature measurement of GAC and decomposition of PCP loaded on GAC and GAC-supported copper catalyst in microwave irradiation. Appl. Catal. A Gen. 2004, 264, 53–58. [Google Scholar] [CrossRef]
- Jou, C.J.G.; Wu, C.R.; Lee, C.L. Application of Microwave Energy to Treat Granular Activated Carbon Contaminated with Chlorobenzene. Environ. Prog. Sustain. 2009, 29, 272–277. [Google Scholar] [CrossRef]
- Chen, C.L.; Lo, S.L.; Kuan, W.H. A case study of the microwave sintering for the stabilization of MSWI fly ash. Sustain. Environ. Res. 2010, 20, 381–385. [Google Scholar]
- Yuen, F.K.; Hameed, B.H. Recent developments in the preparation and regeneration of activated carbons by microwaves. Adv. Colloid Interface Sci. 2009, 149, 19–27. [Google Scholar] [CrossRef] [PubMed]



| SiO2 | CaO | Al2O3 | Fe2O3 | MgO | K2O | Na2O | SO3 | Cl | TiO2 | F | LOI | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MWIFA | 17.13 | 24.42 | 2.85 | 1.78 | 1.80 | 2.80 | 15.20 | 6.37 | 20.43 | 1.34 | 2.59 | 11.10 | [12] |
| MWIFA | 9.06 | 5.37 | 10.11 | 1.49 | 3.48 | 1.64 | 22.05 | 1.03 | 17.07 | - | 0.75 | - | [13] |
| MWIFA | 8.00 | 38.50 | 6.90 | 1.10 | 2.30 | 3.30 | 1.60 | 1.60 | 30.70 | 3.20 | - | - | [14] |
| MSWIFA | 73.10 | 1.06 | 16.70 | 1.96 | - | 3.94 | 2.42 | - | - | 0.35 | - | - | [15] |
| MSWIFA | 55.37 | 19.39 | 9.20 | 4.93 | 0.41 | 0.43 | 0.24 | 1.53 | 0.44 | - | - | - | [16] |
| MSWIFA | 62.30 | 0.50 | 28.10 | 2.10 | 1.00 | 1.00 | 0.50 | 0.40 | - | - | - | 2.50 | [17] |
| Treatment Object | Total Numbers of Incinerators | Treatment Capacity | Types of Incinerators | Air Pollution Control Device | Concentrations of Dioxins (ng/g) | References |
|---|---|---|---|---|---|---|
| MSW | 15 | 300~1500 t/d | Grate-type or fluidized bed incinerator | SS + AC + BF | 2.8–190 | [33] |
| MSW | 16 | 300~1800 t/d | mass-burning | SD + AC + BF; SD | 9.07–46.68, average 23.53 | [34] |
| MSW | 5 | 200~350 t/d 400~700 t/d | Grate-type or fluidized bed incinerator | SS + BF | 19.2–236 | [35] |
| HSW | 1 | 10 t/d | rotary kiln + circulated fluidized bed; | SS + BF | 2918 | |
| HSW | 15 t/d | rotary kiln | SS + AC + bag filter | 78.79 | [36] | |
| HSW | 1 | 10 t/d | rotary kiln fluidized bed multi-stage pyrolysis | SS + AC + bag filter | 67.83, 125.3 | [3] |
| HSW | 1 | 20 t/d | Rotary kiln + second combustion chamber | SS + AC + BF | [37,38] |
| Heavy Metals (mg/g) | MWIFA | MSWIFA | ||
|---|---|---|---|---|
| Range | Average | Range | Average | |
| Cr | 0.02–0.26 | 0.06 | 0.07–0.86 | 0.21 |
| Ni | 0.12–0.18 | 0.04 | 0.02–0.12 | 0.06 |
| Zn | 8.29–121.41 | 78.69 | 0.40–25.80 | 7.66 |
| Cd | 0.12–0.64 | 0.23 | 0.03–0.47 | 0.13 |
| Ba | 1.21–2.90 | NF | 0.54–4.30 | NF |
| Pb | 1.90–5.40 | 3.54 | 0.20–10.60 | 2.83 |
| Cu | 0.80–2.91 | 1.70 | 0.19–1.30 | 0.68 |
| As | 0.07–0.24 | 0.17 | 0.09–0.24 | 0.05 |
| Objectives | Optimal Parameters | Findings | Reference |
|---|---|---|---|
| 90% Washed ash + 10% Portland cement solidification. | liquid/solid = 0.88, washing time = 15 min, setting time = 3.30 h or 5.30 h. | 50–80% of chlorides were washed out. Washing shortens the setting time of solidified body. The expense reduced by approximately 50 to 63%. After solidified body was stored for seven days at 20 °C, leachabilities of Cd, Cr, Cu, and Pb were lower than the limited values of Italy. | [66] |
| Cementitious stabilization | Cement proportion = 13–40 wt.%. water content = 20–30 wt.% | Cementitious body is used for transportation or landfilling. And heavy metals can be stabilized. Besides, the solid waste mass is increased by up to 40 wt.%. The cost was about 240 CNY/t. | [67] |
| Activators + cement stabilization | Ash/cement/Ca(OH)2/Na2SO4 (K2SO4 or CaCl2) = 100/25/20/5.80. Water/(fly ash + cement) = 0.35. | The addition of Ca(OH)2 together with either Na2SO4, CaCl2, or K2SO4 improves the hydration reaction of carbon enriched fly ash. After curving for 90 days, compressive strength reaches to 35 MPa under optimal conditions. | [68] |
| Four-stage waste washing pretreatment + stabilization | No found. | Four-step washing pretreatment can optimize the stability and compressive strength of fly ash and produce usable concrete aggregates. | [63] |
| Sand additives + blended cement Solidification. | sand/mixture = 3; water/mixture = 0.47 or 0.50. cement/fly ash = 3 | Cement and furnace slag can produce modified ash, which can substitute cement in dispose of the pretreated fly ash harmlessly; but it presented a poor immobilization for Cr. | [69] |
| Portland cement Solidification | Fly ash/cement < 50 wt.%. L/s (w/s) = 0.40. | Fly ash contained with 16 wt.% of chlorides cannot be effectively immobilized when cement content is lower than 50 wt.%. Compressive strengths were low after 28 days. | [70] |
| Sulfoaluminate cement Solidification. | fly ash amount = 50%; water/binder = 0.30. curving time = 28 days. | Under the optimal conditions, compressive strength was 32.60 MPa. Leaching concentration of Zn, Pb, and Cu meet the threshold values of China. Sulfoaluminate cement was proved to be better than Portland cement in solidification. | [71] |
| Water washing pretreatment + Portland cement stabilization | Washing conditions: L/s = 5:1, 0.50 h; binder: sand: water = 2:6:1. Fly ash: cement = 1:1. | Washing process can remove >80% of Cl− and SO3− in the MSWIFA. Compressive strength was 11.52 MPa when mixing with 50% fly ash and curving for 28 days. Final products were used as base construction/decorative material, backfill, or patios. | [72] |
| Portland cement solidification to produce fly ash based geopolymer concrete. | aggregate:sand = 1:1, ash = 400 kg, cement = 15 wt.%, resting time = 30 min, temperature = 70 °C. | Compressive strength was higher than 25 MPa when curving for 7 to 28 days under optimal conditions. A 30 min of resting time was found to be more effective than 24 h. | [17] |
| Fly ash cenosphere modified cement pastes with nano silica (NS). | Water/binder = 0.30. Cement/ash = 9:1. nano silica = 1 wt.%. | Application of fly ash cenosphere can decrease the density of the final products without greater loss for strength (about 75 MPa). An excellent compact microstructure was obtained after the addition of nano silica. | [15] |
| Cement solidification of MSWIFA from India. | Cement content < 6%. Curving time = 7 days. | Cement addition improves compressive strength of solidified product due to the pozzolanic reactions. More than 6% of cement addition is not beneficial both for volume and economic. Final products can be used as lightweight filling materials. | [73] |
| Feedstock and Objectives | Devices | Treatment Conditions | DDRs/Leaching or Stabilization of Heavy Metals | Re-utilization of Slags | Reference |
|---|---|---|---|---|---|
| MSWIFA Melting treatment | Residual carbon furnace | T = 1200 °C or 1400 °C | DDRs are over than 99%. Metals can be separated by evaporation or physical gravity after cooling. | Construction materials. | [67] |
| MSWIFA Melting treatment | Electronic arc furnace | T = 1250–1300 °C time > 5 h. | DDRs are 99.90%. Stabilization of heavy metals is not mentioned. | Roadbed materials | [81] |
| Water extraction + MSWIFA melting process | Electric heated furnace | L/s = 10, MT = 1000–1350 °C. | Zn (70.60%), Cu (73.90%), and Pb (58.10%) were immobilized in the water-extracted fly ash, higher than those for raw fly ash. | Byproduct can be reutilization. | [84] |
| Production of glassy slag with MSWIFA by vitrification. | DC double plasma torch | T = 1500–1600 °C. | DDRs are 99.32% (99.95% in TEQ). Leaching concentrations of heavy metals in slags meet the regulatory standard but visibly volatilized. | Glassy slags were safe enough to reuse. | [79] |
| Cullet additive + melting + sintering. | Electronic Arc Furnace | Ash/Cullet = 3 TiO2 = 3 wt.%, 850 °C, 30 min | Leachate concentrations of Cu, Pb, Zn, Cr, and Cd for glass-ceramics toxicity identification standard of China. | Substitute nature marble, porcelain tiles and granite. | [80] |
| Water washing + melting + sintering of MSWIFA. | Electronic Arc Furnace | L/s = 20, TiO2 = 3 wt.%, 900 °C ash/cullet = 3, Melting/sintering time = 30 min/2 h. | Leachate concentrations of Cu, Pb, Zn, Cr, and Cd for glass-ceramics toxicity identification standard of China. | Glass-ceramics can substitute of nature materials. | [75] |
| Conversion MWIFA into harmless slag by Melting | DC thermal plasma torch | Melted for 15 min. working gas: argon. Flow rate = 12–14 L/min | DDRs > 99% in TEQ. Leaching concentration of heavy metals in the slag meet the Chinese regulatory standard. The volume reduction is 78% after melting. | Produced slag presents good performance in microstructure. | [33] |
| Production of porous materials with MSWIFA by melting process. | Thermal Plasma furnace | Bottom ash/fly ash = 1. L/s = 0.50, 1600 °C. frother = 3 wt.%. cement: slag = 1 | Concentrations of heavy metals in water-quenched slag meet the TCLP criteria of Taiwan. | Products are used as architectural and decorative materials. | [85] |
| Zero waste treatment of MSWIFA | Electric arc furnace | T = 1630–1730 °C. 2 s residence time | DDRs = 99.999%. Heavy metals in furnace dust and slag meet the regulatory thresholds of Taiwan EPA | Zero landfill | [86] |
| Objectives | Treatment Conditions | Findings and Results | Reference |
|---|---|---|---|
| Thermal treatment for MSWIFA in Switzerland | 670–1000 °C 750 °C, >6 h; at 840 °C 3–4 h; and at 920 °C 1.50 h. | Heavy metal oxides can transfer to metal chlorides and be completely evaporated. Metal evaporation would proceed as long as there are chlorides in fly ash. But it may be restricted by the formed metal silica/alumina constituents. | [88] |
| Roasting treatment for MSWIFA | Roasting for 3 h under 1000 °C. Fly ash = 3 g. CaCl2 2H2O = 0.62 g (that is 0.30 g of Cl). | CaCl2 was found to be an alternative chlorinating agent for heavy metals volatilizing. Volatilization efficiencies of recovered metals are proportional to standard free-energy changes values for the corresponding chlorination reactions. | [91] |
| Thermal treatment of MSWIFA. | Fly ash/CaCl2 = 15:1 (w/w). Roasting temperature = 990 ± 10 °C for 1 h | This technology is found to be effective for removal of most heavy metals. But after ash was treated under 1000 °C, the leaching rate of Cr was increased 11 to 13%. | [92] |
| Thermal treatment of MSWIFA in Austria | 800–1200 °C for 20 h. Chloride addition: 0–200 g Cl per kg ash. Added as NaCl, CaCl2, or MgCl2 | At 1200 °C, above 95% of Zn, Cu, Pb, Cd with addition of CaCl2 or MgCl2, 75% Ni (CaCl2), and 30% Cr (MgCl2 or NaCl) could be vaporized. | [89] |
| Chloride removal by roasting and washing | Washing conditions: solid/liquid = 100 g/L, 1.50 h. Roasting conditions: 600, 800, 950, 1050 °C for 7, 4, 2, 1 h. | 1050 °C for 3 h was found to be the optimal condition and removal rate of chloride = 83%. A solid to liquid ratio of 1:10 in washing process can remove 97% of water-soluble chlorides. | [90] |
| Residues | Objectives | Treatment Conditions | Findings | Reference |
|---|---|---|---|---|
| MSWIFA from Taiwan and China. | SCW and SCWH treatment of heavy metals in MSWIFA. | The H2O2 was added. | This technique can stabilize metals in Fe-Mn oxides and residual fractions. Heavy metal leaching results meets the requirements of USEPA and Chinese EPA permits. | [74] |
| Oil-contained PCBs and heavy metals in MWIFA | The simultaneous detoxification of PCBs and heavy metals in MWIFA. | 7.00–34.40 MPa, 280–410 °C. Reaction was finally quenched by water spray with supercritical water. | The technique meets technical and cost requirements, because MWIFA is found to possess the potential catalysis ability. | [45] |
| Dioxins in MSWIFA (SCWO) | Dioxin destruction and dioxins adsorption by activated carbon | 500 °C, 20 MPa. | Decomposition efficiencies of dioxins and activated carbon were more than 99% and 99.99%. | [108] |
| Dioxin extraction in fly ash (SCWO) | Extraction of dioxins in fly ash | SC-CO2 was used, solvent/feed = 5, time = 1 h, 40 °C, 50 MPa. | Extraction efficiency of dioxins attained 99.98%. | [108] |
| MSWIFA in China | Dioxins degradation by SCWO technique | 400–500 °C, 23–29 MPa, fly ash = 0–6 g, 1–2 min, O2 = 150–300%, H2O2 = 0–40 mL. | Mass concentration of dioxins reduces from 28.20 to only 2.79 ng/g, a degradation efficiency > 90%. | [109] |
| Objectives | Optimal Treatment Conditions | Findings | Reference |
|---|---|---|---|
| HT decomposition of the dioxins in fly ash | 300 °C for 20 min; solvent is 1N NaOH solution containing 10 vol% methanol | Dioxins were completely decomposed. Toxicity of dioxins for the treated fly ash decreased to 0.03 ng I-TEQ/g. | [123] |
| Heavy metal removal from MSWIFA in Japan by washing + HT | Washing 30 min. HT: autoclave pressures = 1.2–2.0 MPa, 150 °C, 5 h, L/s = 10 mL/g. | 67% Na, 76% K, and 48% Ca were extracted by washing for 30 min. Final products can produce silicon–sulfur fertilizer after further Cr disposal. | [125] |
| HT of MSWIFA to produce stable minerals. | NaOH = 0.5 M, L/s = 10 mL/g 180 °C, 48 h. | Most of heavy metals were less released in acid environment. But the concentration of Zn and Cd cannot meet the standard. | [119] |
| Production of concrete with MSWIFA. | NaOH or Na2CO3 10 g fly ash 50 g, 375 °C, 5 h. | Heavy metal leaching concentration decreased by over 58.33%, especially Zn (81.91%/86.89% were leached out by NaOH/Na2CO3, respectively). | [126] |
| Removal of Cu in MWIFA by additives + HT. | 325 °C, 2 h, initial Cu2+ = 50 mg/L. vessel pressure = 22.2 MPa. L/s = 10 mL/g. ash/Na2CO3 = 10. | Temperature has little effect on Cu(II)removal. Removal efficiency increased from 94.80% to 99.90% with the increasing concentration of Cu(II) from 10 to 50 mg L−1. | [127] |
| Decomposition of dioxins contained in MSWIFA by HT. | 290 °C,1 h. A mixture of ferrous and ferric sulphates by 5% (wt/wt) with the Fe (III)/Fe (II) = 2. | 90.33% of dioxins were decomposed (in TEQ) with addition of Fe, but the associated decomposition rates were relatively lower. | [122] |
| Acid treatment + HT and reutilization of MSWIFA | Acid treatment conditions: 30 min. pH = 6.2, L/s = 2 mL/g. HT conditions: 290 °C, 1 h, L/s = 2–2.5 mL/g. 50 g PO43−/1 kg fly ash | Heavy metal leaching concentrations meet the Chinese limits. Acid-washing reduced over 79.80% of leaching concentration of heavy metals. Higher concentrations of Cl in acid-washed fly ash were decreased than in water-washed ones. | [128] |
| HT + additives to stabilize heavy metals in MSWIFA. | CFA and diatomite/MSWIFA = 3:7, 200 °C. Seed or Tobermorite = 3%, | Leaching toxicity of all heavy metals in MSWIFA decreased to the lower than the standard values even for Pb. | [129] |
| K-zeolite syntheses from biomass incineration ash and coal fly ash via HT. | Biomass incineration ash:2–15 g, KOH: 0.5 and 2 mol/L, CFA:10 g. HT conditions: 160 °C,24 h. | The synthesized K-zeolites can be used to remove radioactive cesium. | [130] |
| Additives + HT to stabilize heavy metals in MSWIFA. | Coal fly ash/MSWIFA = 3:7, L/s = 10 mL/g. NaOH = 0.5 mol/L. HT conditions:150 °C, 48 h. | Heavy metals were detected on the surface of synthesized tobermorite crystalline. Leaching toxicity of all metals met the standard values. | [131] |
| Objectives. | Optimal Conditions and Degradation/Destruction Efficiency of Dioxins | Other Findings | References | ||||
|---|---|---|---|---|---|---|---|
| Reagent (Ratio) | Rotation Speed | Grinding Time | Grinding Type | Optimal Efficiency | |||
| Destruction of dioxins in MWIFA | CaO/fly ash = 6–60% | 400 rpm | 2 h | Planetary ball | PCDD = 76.80%; PCDF = 56.80% | Dioxin efficiency rose with increased ratio of CaO. | [3] |
| Destruction of dioxins in MWIFA | No addition | 400 rpm | 2 h | Planetary ball (XQM-0.4 L) | Destruction efficiency = 76% | - | [143] |
| Fly ash smelting with industrial secondary copper | CaO/SiO2/fly ash = 4:1:5 (wt) | 275 rpm | 12 h | Planetary ball | Dioxins = 85% | Cu served as the catalyst during dioxin reformation | [135,139] |
| Decomposition of PCBs in contaminated soil | CaO/SiO2/soil = 1:1:2 (wt) | 400 rpm | 20 h | Planetary ball | PCBs = 98% | Dioxins formed in the first 5 h were decomposed with sufficient time. | [144] |
| Destruction of dioxins and PCBs in MSWIFA | Ca/CaO/fly ash = 1/1/200 | 400 rpm | 20 h | planetary ball, PM-100 | completely detoxified | No traces of PCBs and dioxins were detected finally | [145] |
| Dechlorination/destruction of dioxins in MSWIFA | Flyash:Ca: Al-powder = 30:4:1 | 600 rpm | 10 h | planetary ball (QXQM-2) | Dioxins = 93.20% | Water washing and Fe/Al/Ca additives present better effect. | [141] |
| Heavy metal immobilization in MSWIFA with nano-Fe/Ca/CaO/PO4 reagent. | CaO:Fe:Ca = 5:2:2. ash: [PO4]:nano-Fe/Ca/CaO = 20:1:1 | 150 rpm | 2 h | planetary ball (PM-100) | Immobilization rate of heavy metals = 98–100% | Heavy metal leaching concentrations were much lower than Japan standard value. | [132] |
| Dioxin degradation by washing + Fe/Ni-SiO2 of MSWIFA | Ni/SiO2/Fe/fly ash = 1/2/4/200 | 400 rpm | 24 h | bimetallic ball | Dioxins = 93.20% | Washing conditions: L/s = 4:1, stirring speed = 400 rpm, time = 60 min. | [51] |
| Technique | Applicability to the Characteristics of MWIFA | Detoxification Effect | Cost of Treatment | Environmental Feedback | Technique Status | Merits | Demerits | Remarks | |
|---|---|---|---|---|---|---|---|---|---|
| Heavy Metals | Dioxins | ||||||||
| WWP | Removal of chlorines | No stabilization | No decomposition | Low | Environmental friendly. | established | Chlorine removal | Waste liquid may need to be treated | pretreatment method |
| ALP | Leaching out heavy metals | No stabilization | No decomposition | Low | Environmental friendly. | established | Heavy metal leaching | Waste liquid may need to be treated | pretreatment method |
| CST | High PAC reduces the compressive strength and increases metal leachability | stabilization | No decomposition | Low/moderate | Questionable | Mature | Easy implementing and low expense | consumes mass of cement; products cannot be used; landfill sites are limited | just one of the expedients |
| MT | High carbon content is harmful to graphite electrode | Partial stabilization | Decomposition | High | Existing a secondary pollution | Developing/Developed | molten glasses or slag byproducts can be used as road/construction materials | High expense and energy consumption. Metal chlorides were easy volatile. | residual carbon melting furnace is suitable |
| Roasting | It is suitable for chloride-/metal-enriched ash | Recovery | Decomposition | Moderate | Slight pollution | Developed | No mentioned | higher facility requirements, energy consumption/costs | Theoretically applicable |
| LTTT | No mention | Partly stabilization | Decomposition | Moderate | Slight pollution | Developed | Simple operation and low energy consumption | Heavy metals were not considered. | can be a sequential dioxin removal step |
| CHD | Facilities are easily corroded by HCl. | No stabilization | Decomposition | Moderate | Potentially pollution | laboratory stage | High efficiency, strong selectivity, stable reaction | dioxin regeneration may happen | can be a sequential dioxin removal step |
| SCWO | Applicability for MWIFA is unknown | Stabilization | Decomposition | High/moderate | Potentially secondary pollution | Developing | Effective, harmless, fast, and violent, and no heat transfer resistance problem | high requirement for equipment and high energy consumption. | Dioxins may regenerated. |
| HTT | Less Si and Al were bad for the formation of zeolite and tobermorite crystalline | Partial stabilization | Decomposition | Low/moderate | Less pollution | small scale engineering | Energy saving, low facility requirement, and products can be reused. | Catalysts are liable to be poisoned by toxic methanol. Facility is easily corroded | Obstacles need to overcome |
| MCT | No mentioned | Partial removal or stabilization | Decomposition | Moderate | Environmental friendly. | small scale engineering | Nonthermal process and nontail gas disposal method | Unknown difficulties may need be overcome | has bright prospect of industry application |
| Flotation | Suitable for high carbon, chlorines and dioxins | Removal | Removal | Low | Environmental friendly | Emerging | Flexibility, less reagent usage, | No actual engineering application | pretreatment method, flotation + reburning is promising |
| MWT | Suitable for high carbon, and dioxins | Stabilization | Decomposition | Moderate | Less pollution | small scale engineering | Rapid/interior/volumetric/selective heating | Obstacles need to overcome for industry application | Flotation + MWT is a promising method |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Liu, H.-Q.; Wei, G.-X.; Zhang, R.; Zeng, T.-T.; Liu, G.-S.; Zhou, J.-H. Characteristics and Treatment Methods of Medical Waste Incinerator Fly Ash: A Review. Processes 2018, 6, 173. https://doi.org/10.3390/pr6100173
Liu F, Liu H-Q, Wei G-X, Zhang R, Zeng T-T, Liu G-S, Zhou J-H. Characteristics and Treatment Methods of Medical Waste Incinerator Fly Ash: A Review. Processes. 2018; 6(10):173. https://doi.org/10.3390/pr6100173
Chicago/Turabian StyleLiu, Fang, Han-Qiao Liu, Guo-Xia Wei, Rui Zhang, Tong-Tong Zeng, Gui-Sheng Liu, and Jian-Hua Zhou. 2018. "Characteristics and Treatment Methods of Medical Waste Incinerator Fly Ash: A Review" Processes 6, no. 10: 173. https://doi.org/10.3390/pr6100173
APA StyleLiu, F., Liu, H.-Q., Wei, G.-X., Zhang, R., Zeng, T.-T., Liu, G.-S., & Zhou, J.-H. (2018). Characteristics and Treatment Methods of Medical Waste Incinerator Fly Ash: A Review. Processes, 6(10), 173. https://doi.org/10.3390/pr6100173




