Optimal Design of a Two-Stage Membrane System for Hydrogen Separation in Refining Processes
Abstract
:1. Introduction
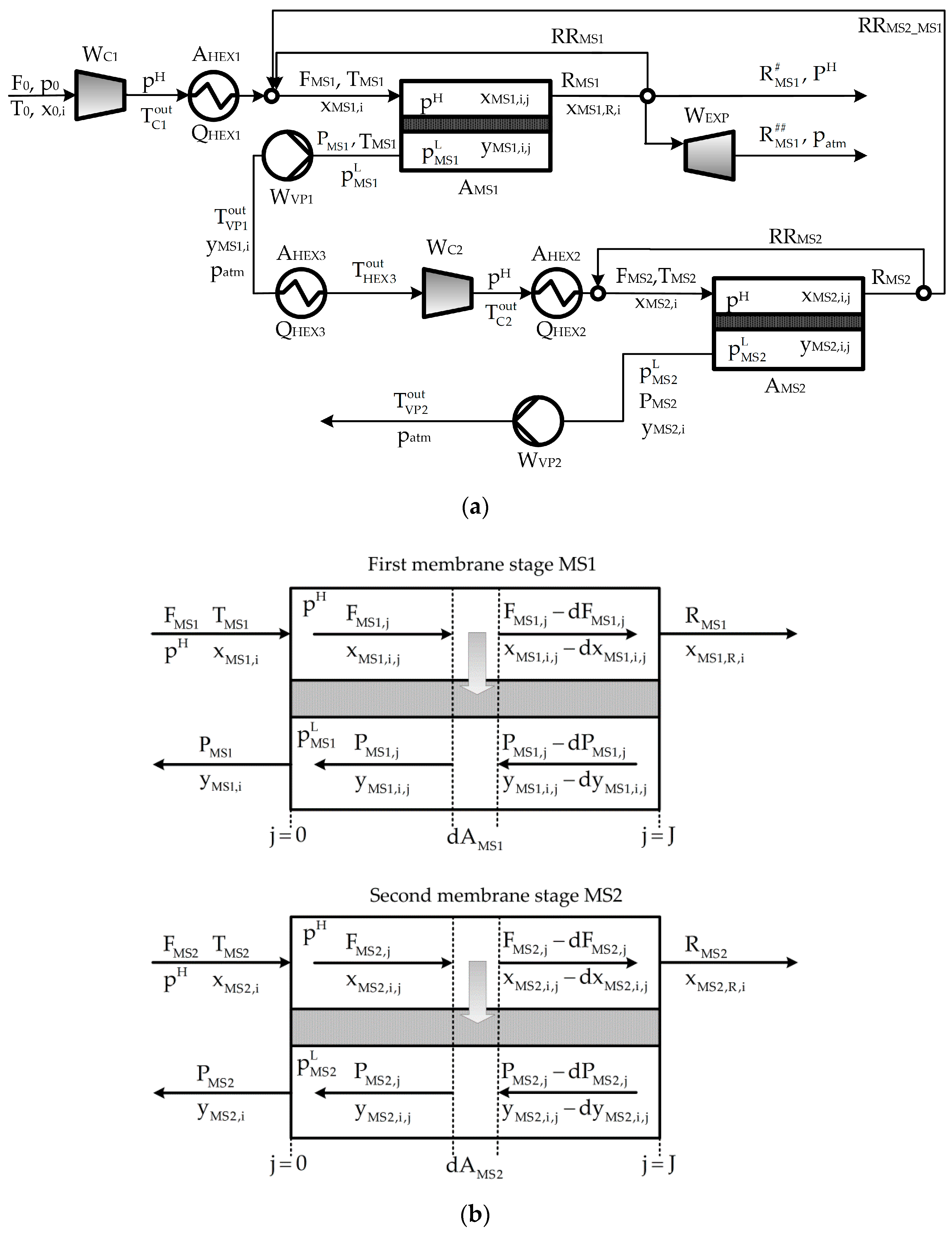
2. Process Description
- The way in which the driving force for permeation is created in each membrane stage, i.e., the solution will indicate if the driving force is created by (a) employing compression without applying vacuum, or (b) applying vacuum in the permeate streams without employing compression, or (c) combining both compression and vacuum.
- The inclusion or not of the expansion turbine for power recovery.
- The inclusion or not of (one or more) recycle streams.
- The optimal values of operation pressure, temperature, composition, and flow rate of each process stream.
- The optimal sizes of the process units (membrane areas, heat transfer areas, power capacity of compressors and vacuum pumps).
3. Process Modeling
3.1. Main Model Assumptions
- All the mixture components can permeate through the membrane.
- The operating pressure does not affect the component permeability.
- No pressure drop is considered in the retentate and permeate sides.
- The feed and retentate streams are at the same pressure.
- Plug flow pattern is considered at both sides of the membrane unit.
- Isothermal condition is assumed within the membrane module.
- Fick’s first law is used.
3.2. Mathematical Model
3.2.1. Mass Balances
3.2.2. Power Requirement
3.2.3. Energy Balances and Transfer Areas of Heat Exchangers
3.2.4. Connecting Constraints
3.2.5. Performance Variables
3.2.6. Cost Model
4. Results and Discussion
4.1. Comparison of Optimal and Sub-Optimal Solutions
4.2. Sensitivity Analysis
4.2.1. Sensitivity of the Optimal Solution to the H2 Product Purity Level
4.2.2. Sensitivity of the Optimal Solution to the H2 Recovery Level
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| AMS# | membrane area required in the membrane stage MS#, m2. |
| annCAPEX | annualized capital expenditures, M$·year−1. |
| CAPEX | capital expenditures, M$. |
| CRF | capital recovery factor, year−1. |
| CRM | raw material and utility cost, M$·year−1 |
| cruCW | specific cost of the cooling water, M$·kg−1. |
| cruEP | specific cost of the electricity, M$·kW−1. |
| cruMR | specific cost of the membrane replacement, M$·m−2. |
| F0 | feed flow rate, kmol·s−1. |
| FMS# | feed flow rate in the membrane stage MS#, kmol·s−1. |
| IMS# | investment for membrane area of the stage MS#, M$. |
| IHEX# | investment for the heat exchanger HEX#, M$. |
| IVP# | investment for the vacuum pump VP#, M$. |
| IC# | investment for the compressor C#, M$. |
| OPEX | operating expenditures, M$·year−1. |
| pH | high operating pressure (retentate side), MPa. |
| pLMS# | operating pressure in the permeate side of the membrane stage MS#, MPa. |
| PMS# | permeate flow rate obtained in the membrane stage MS#, kmol·s−1. |
| RMS# | retentate flow rate obtained in the membrane stage MS#, kmol·s−1. |
| TAC | total annual cost, M$·year−1. |
| T0 | feed temperature, K. |
| Tout C# | outlet temperature from the compressor C# associated with the membrane stage MS#, K. |
| TMS# | operating temperature in the membrane stage MS#, K. |
| Tout HEX# | outlet temperature from the heat exchanger HEX#, K. |
| WC# | power required by the compressor C# associated with the membrane stage MS#, MW. |
| WVP# | power required by the vacuum pump VP# in the membrane stage MS#, MW. |
| WEXP | power recovered in the expander EXP, MW. |
| xi,0 | mole fraction of component i in the feed stream, dimensionless. |
| xMS#,i | inlet composition of the component i in the membrane stage MS#, dimensionless. |
| xMS#,i,j | mole fraction of the component i in the retentate stream of the membrane stage MS# at the discretization point j, dimensionless. |
| xMS#,R,i | mole fraction of the component i in the retentate stream leaving the membrane stage MS#, dimensionless. |
| yMS#,i | mole fraction of the component i in the permeate stream leaving the membrane stage MS#, dimensionless. |
| yMS1,i,j | mole fraction of the component i in the permeate stream of the membrane stage MS# at the discretization point j, dimensionless. |
References
- Drioli, E.; Giorno, L.; Fontananova, E. Comprehensive Membrane Science and Engineering, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-444-63796-3. [Google Scholar]
- Castel, C.; Favre, E. Membrane separations and energy efficiency. J. Membr. Sci. 2018, 548, 345–357. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Sharif, A. Polymeric gas separation membranes: What makes them industrially more attractive? J. Membr. Sci. Res. 2018, 4, 2–3. [Google Scholar] [CrossRef]
- Ahmad, F.; Lau, K.K.; Lock, S.S.M.; Rafiq, S.; Khan, A.U.; Lee, M. Hollow fiber membrane model for gas separation: Process simulation, experimental validation and module characteristics study. J. Ind. Eng. Chem. 2015, 21, 1246–1257. [Google Scholar] [CrossRef]
- He, X.; Hägg, M.-B.; Kim, T.-J. Hybrid FSC membrane for CO2 removal from natural gas: Experimental, process simulation, and economic feasibility analysis. AIChE J. 2014, 60, 4174–4184. [Google Scholar] [CrossRef]
- Low, B.T.; Zhao, L.; Merkel, T.C.; Weber, M.; Stolten, D. A parametric study of the impact of membrane materials and process operating conditions on carbon capture from humidified flue gas. J. Membr. Sci. 2013, 431, 139–155. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, M.; Ly, J.; Vu, J.; Wijmans, J.G.; Merkel, T.C.; Jin, J.; Haldeman, A.; Wagener, E.H.; Rue, D. Membrane-based oxygen-enriched combustion. Ind. Eng. Chem. Res. 2013, 52, 10820–10834. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Freeman, B.; Hao, P.; Baker, R.; Kniep, J.; Chen, E.; Ding, J.; Zhang, Y.; Rochelle, G.T. Hybrid Membrane-absorption CO2 Capture Process. Energy Procedia 2014, 63, 605–613. [Google Scholar] [CrossRef]
- Lin, H.; He, Z.; Sun, Z.; Kniep, J.; Ng, A.; Baker, R.W.; Merkel, T.C. CO2-selective membranes for hydrogen production and CO2 capture—Part II: Techno-economic analysis. J. Membr. Sci. 2015, 493, 794–806. [Google Scholar] [CrossRef]
- Masebinu, S.O.; Aboyade, A.O.; Muzenda, E. Parametric study of single and double stage membrane configuration in methane enrichment process. In Proceedings of the World Congress on Engineering and Computer Science, San Francisco, CA, USA, 22–24 October 2014; Volume 2, pp. 581–589. [Google Scholar]
- Bounaceur, R.; Berger, E.; Pfister, M.; Ramirez Santos, A.A.; Favre, E. Rigorous variable permeability modelling and process simulation for the design of polymeric membrane gas separation units: MEMSIC simulation tool. J. Membr. Sci. 2017, 523, 77–91. [Google Scholar] [CrossRef]
- Makaruk, A.; Harasek, M. Numerical algorithm for modelling multicomponent multipermeator systems. J. Membr. Sci. 2009, 344, 258–265. [Google Scholar] [CrossRef]
- Kundu, P.K.; Chakma, A.; Feng, X. Simulation of binary gas separation with asymmetric hollow fibre membranes and case studies of air separation. Can. J. Chem. Eng. 2012, 90, 1253–1268. [Google Scholar] [CrossRef]
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. On the optimal design of membrane-based gas separation processes. J. Membr. Sci. 2017, 526, 118–130. [Google Scholar] [CrossRef]
- Zhai, H.; Rubin, E.S. Techno-economic assessment of polymer membrane systems for postcombustion carbon capture at coal-fired power plants. Environ. Sci. Technol. 2013, 47, 3006–3014. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fu, C.; Hägg, M.-B. Membrane system design and process feasibility analysis for CO2 capture from flue gas with a fixed-site-carrier membrane. Chem. Eng. J. 2015, 268, 1–9. [Google Scholar] [CrossRef]
- Khalilpour, R.; Abbas, A.; Lai, Z.; Pinnau, I. Analysis of hollow fibre membrane systems for multicomponent gas separation. Chem. Eng. Res. Des. 2013, 91, 332–347. [Google Scholar] [CrossRef]
- Arias, A.M.; Mussati, M.C.; Mores, P.L.; Scenna, N.J.; Caballero, J.A.; Mussati, S.F. Optimization of multi-stage membrane systems for CO2 capture from flue gas. Int. J. Greenh. Gas Control 2016, 53, 371–390. [Google Scholar] [CrossRef]
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- Yuan, M.; Narakornpijit, K.; Haghpanah, R.; Wilcox, J. Consideration of a nitrogen-selective membrane for postcombustion carbon capture through process modeling and optimization. J. Membr. Sci. 2014, 465, 177–184. [Google Scholar] [CrossRef]
- Lee, S.; Binns, M.; Kim, J.-K. Automated process design and optimization of membrane-based CO2 capture for a coal-based power plant. J. Membr. Sci. 2018, 563, 820–834. [Google Scholar] [CrossRef]
- Didaskalou, C.; Kupai, J.; Cseri, L.; Barabas, J.; Vass, E.; Holtzl, T.; Szekely, G. Membrane-Grafted Asymmetric Organocatalyst for an Integrated Synthesis-Separation Platform. ACS Catal. 2018, 8, 7430–7438. [Google Scholar] [CrossRef]
- Valtcheva, I.B.; Marchetti, P.; Livingston, A.G. Crosslinked polybenzimidazole membranes for organic solvent nanofiltration (OSN): Analysis of crosslinking reaction mechanism and effects of reaction parameters. J. Membr. Sci. 2015, 493, 568–579. [Google Scholar] [CrossRef]
- Qi, R.; Henson, M.A. Membrane system design for multicomponent gas mixtures via mixed-integer nonlinear programming. Comput. Chem. Eng. 2000, 24, 2719–2737. [Google Scholar] [CrossRef] [Green Version]
- Scholz, M.; Alders, M.; Lohaus, T.; Wessling, M. Structural optimization of membrane-based biogas upgrading processes. J. Membr. Sci. 2015, 474, 1–10. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.; Kim, J. Optimization-based approach for design and integration of carbon dioxide separation processes using membrane technology. Energy Procedia 2017, 136, 336–341. [Google Scholar] [CrossRef]
- Uppaluri, R.V.S.; Linke, P.; Kokossis, A.C. Synthesis and optimization of gas permeation membrane networks. Ind. Eng. Chem. Res. 2004, 43, 4305–4322. [Google Scholar] [CrossRef]
- Zarca, G.; Urtiaga, A.; Biegler, L.T.; Ortiz, I. An optimization model for assessment of membrane-based post-combustion gas upcycling into hydrogen or syngas. J. Membr. Sci. 2018, 563, 83–92. [Google Scholar] [CrossRef]
- Brunetti, A.; Barbieri, G.; Drioli, E. New Metrics in Membrane Gas Separation. In Membrane Engineering for the Treatment of Gases; Drioli, E., Barbieri, G., Eds.; Royal Society of Chemistry: London, UK, 2011; Chapter 19; pp. 279–301. [Google Scholar]
- Abu-Zahra, M.R.M.; Niederer, J.P.M.; Feron, P.H.M.; Versteeg, G.F. CO2 capture from power plants: Part II. A parametric study of the economical performance based on mono-ethanolamine. Int. J. Greenh. Gas Control 2007, 1, 135–142. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. A Technical, Economic, and Environmental Assessment of Amine-Based CO2 Capture Technology for Power Plant Greenhouse Gas Control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef] [PubMed]
- Mussati, S.; Aguirre, P.; Scenna, N.J. Optimal MSF plant design. Desalination 2001, 138, 341–347. [Google Scholar] [CrossRef]
- Mussati, S.F.; Aguirre, P.A.; Scenna, N.J. Novel Configuration for a Multistage Flash-Mixer Desalination System. Ind. Eng. Chem. Res. 2003, 42, 4828–4839. [Google Scholar] [CrossRef]
- Mussati, S.F.; Aguirre, P.A.; Scenna, N.J. Improving the efficiency of the MSF once through (MSF-OT) and MSF-mixer (MSF-M) evaporators. Desalination 2004, 166, 141–151. [Google Scholar] [CrossRef]
- Mussati, S.F.; Aguirre, P.A.; Scenna, N.J. A rigorous, mixed-integer, nonlineal programming model (MINLP) for synthesis and optimal operation of cogeneration seawater desalination plants. Desalination 2004, 166, 339–345. [Google Scholar] [CrossRef]
- Mussati, S.; Aguirre, P.; Scenna, N. Dual-purpose desalination plants. Part II. Optimal configuration. Desalination 2003, 153, 185–189. [Google Scholar] [CrossRef]
- Manassaldi, J.I.; Arias, A.M.; Scenna, N.J.; Mussati, M.C.; Mussati, S.F. A discrete and continuous mathematical model for the optimal synthesis and design of dual pressure heat recovery steam generators coupled to two steam turbines. Energy 2016, 103, 807–823. [Google Scholar] [CrossRef]
- Manassaldi, J.I.; Mussati, S.F.; Scenna, N.J. Optimal synthesis and design of Heat Recovery Steam Generation (HRSG) via mathematical programming. Energy 2011, 36, 475–485. [Google Scholar] [CrossRef]
- Mores, P.; Rodríguez, N.; Scenna, N.; Mussati, S. CO2 capture in power plants: Minimization of the investment and operating cost of the post-combustion process using MEA aqueous solution. Int. J. Greenh. Gas Control 2012, 10, 148–163. [Google Scholar] [CrossRef]
- Mores, P.L.; Godoy, E.; Mussati, S.F.; Scenna, N.J. A NGCC power plant with a CO2 post-combustion capture option. Optimal economics for different generation/capture goals. Chem. Eng. Res. Des. 2014, 92, 1329–1353. [Google Scholar] [CrossRef]
- Alasino, N.; Mussati, M.C.; Scenna, N. Wastewater treatment plant synthesis and design. Ind. Eng. Chem. Res. 2007, 46, 7497–7512. [Google Scholar] [CrossRef]
- Alasino, N.; Mussati, M.C.; Scenna, N.J.; Aguirre, P. Wastewater treatment plant synthesis and design: Combined biological nitrogen and phosphorus removal. Ind. Eng. Chem. Res. 2010, 49, 8601–8612. [Google Scholar] [CrossRef]
- Serralunga, F.J.; Mussati, M.C.; Aguirre, P.A. Model Adaptation for real-time optimization in energy systems. Ind. Eng. Chem. Res. 2013, 52, 16795–16810. [Google Scholar] [CrossRef]
- Francesconi, J.A.; Mussati, M.C.; Mato, R.O.; Aguirre, P.A. Analysis of the energy efficiency of an integrated ethanol processor for PEM fuel cell systems. J. Power Sources 2007, 167, 151–161. [Google Scholar] [CrossRef]
- Oliva, D.G.; Francesconi, J.A.; Mussati, M.C.; Aguirre, P.A. Energy efficiency analysis of an integrated glycerin processor for PEM fuel cells: Comparison with an ethanol-based system. Int. J. Hydrogen Energy 2010, 35, 709–724. [Google Scholar] [CrossRef]
- Oliva, D.G.; Francesconi, J.A.; Mussati, M.C.; Aguirre, P.A. Modeling, synthesis and optimization of heat exchanger networks. Application to fuel processing systems for PEM fuel cells. Int. J. Hydrogen Energy 2011, 36, 9098–9114. [Google Scholar] [CrossRef]
- Hussain, A.; Farrukh, S.; Minhas, F.T. Two-Stage Membrane System for Post-combustion CO2 Capture Application. Energy Fuels 2015, 29, 6664–6669. [Google Scholar] [CrossRef]
- Fernández-Barquín, A.; Casado-Coterillo, C.; Irabien, Á. Separation of CO2-N2 gas mixtures: Membrane combination and temperature influence. Sep. Purif. Technol. 2017, 188, 197–205. [Google Scholar] [CrossRef]
- Sluijs, J.P.V.D.; Hendriks, C.A.; Blok, K. Feasibility of polymer membranes for carbon dioxide recovery from flue gases. Energy Convers. Manag. 1992, 33, 429–436. [Google Scholar] [CrossRef]
- Brinkmann, T.; Pohlmann, J.; Bram, M.; Zhao, L.; Tota, A.; Escalona, N.J.; de Graaff, M.; Stolten, D. Investigating the influence of the pressure distribution in a membrane module on the cascaded membrane system for post-combustion capture. Int. J. Greenh. Gas Control 2015, 39, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Gerber, E. Production of Electric Power from Fossil Fuel with Almost Zero Air Pollution. International Patent Application No. PCT/US2014/000213 28 May 2015. [Google Scholar]
- Li, B.; He, G.; Jiang, X.; Dai, Y.; Ruan, X. Pressure swing adsorption/membrane hybrid processes for hydrogen purification with a high recovery. Front. Chem. Sci. Eng. 2016, 10, 255–264. [Google Scholar] [CrossRef]
- Didaskalou, C.; Buyuktiryaki, S.; Kecili, R.; Fonte, C.P.; Szekely, G. Valorisation of agricultural waste with an adsorption/nanofiltration hybrid process: From materials to sustainable process design. Green Chem. 2017, 19, 3116–3125. [Google Scholar] [CrossRef]
- Akinlabi, C.O.; Gerogiorgis, D.I.; Georgiadis, M.C.; Pistikopoulos, E.N. Modelling, design and optimisation of a hybrid PSA-membrane gas separation process. In Proceedings of the 17th European Symposium on Computer Aided Process Engineering, Bucharest, Romania, 27–30 May 2007; Volume 24, pp. 363–370. [Google Scholar]









| Parameter | Value |
|---|---|
| Feed specification | |
| Flow rate, kmol·h−1 | 100 |
| Temperature, K | 313.15 |
| Pressure, kPa | 101.32 |
| Composition (mole fraction) | |
| CO2 | 0.04 |
| CO | 0.16 |
| H2 | 0.18 |
| N2 | 0.62 |
| Membrane material (Polymer) | |
| Permeance, mole·m−2·s−1·MPa−1) | |
| CO2 | 8.444 × 10−3 |
| CO | 7.457 × 10−4 |
| H2 | 2.871 × 10−2 |
| N2 | 4.078 × 10−4 |
| Optimal Sol. OPT | Sub-Optimal Sol. SUBOPT1 | Sub-Optimal Sol. SUBOPT2 | |
|---|---|---|---|
| TAC (M$·year−1) | 1.764 | 2.038 | 2.182 |
| OPEX (M$·year−1) | 1.095 | 1.262 | 1.272 |
| annCAPEX (M$·year−1) | 0.669 | 0.776 | 0.911 |
| CINV (M$) | 1.431 | 1.661 | 1.948 |
| C1 | 0.694 | 0.916 | 0.642 |
| C2 | 0.316 | 0.480 | 0.299 |
| AMS1 | 0.269 | 0.214 | 0.344 |
| VP1 | 0.077 | 0.0 | 0.080 |
| AMS2 | 0.034 | 0.015 | 0.040 |
| HEX1 | 0.020 | 0.022 | 0.020 |
| HEX2 | 0.011 | 0.013 | 0.010 |
| HEX3 | 0.010 | 0.0 | 0.010 |
| VP2 | 0.0 | 0.0 | 7.96 × 10−3 |
| EXP | 0.0 | 0.0 | 0.494 |
| CRM (M$·year−1) | 0.155 | 0.211 | 0.094 |
| EP | 0.141 | 0.198 | 0.077 |
| MR | 0.011 | 8.61 × 10−3 | 0.014 |
| CW | 2.79 × 10−3 | 3.90 × 10−3 | 2.76 × 10−3 |
| H2 Product Purity | |||||
|---|---|---|---|---|---|
| Dev. (%) | 0.89 | 0.90 | 0.91 | Dev. (%) | |
| Cost item | |||||
| TAC (M$·year−1) | −1.32 | 1.741 | 1.764 | 1.802 | +2.11 |
| OPEX (M$·year−1) | −1.30 | 1.081 | 1.095 | 1.118 | +2.04 |
| annCAPEX (M$·year−1) | −1.36 | 0.660 | 0.669 | 0.684 | +2.24 |
| CINV (M$) | −1.36 | 1.411 | 1.431 | 1.463 | +2.24 |
| IC1 | −1.24 | 0.685 | 0.694 | 0.705 | +1.63 |
| IC2 | −4.72 | 0.302 | 0.316 | 0.337 | +6.46 |
| IMA_MS1 | +1.48 | 0.273 | 0.269 | 0.266 | −1.00 |
| IVP1 | −5.80 | 7.22 × 10−2 | 7.67 × 10−2 | 8.28 × 10−2 | +8.04 |
| IMA_MS2 | +15.83 | 3.94 × 10−2 | 3.40 × 10−2 | 2.96 × 10−2 | −12.88 |
| IHEX1 | −0.49 | 2.02 × 10−2 | 2.03 × 10−2 | 2.04 × 10−2 | +0.54 |
| IHEX2 | −4.11 | 1.02 × 10−2 | 1.07 × 10−2 | 1.13 × 10−2 | +5.79 |
| IHEX3 | −3.74 | 1.00 × 10−2 | 1.04 × 10−2 | 1.09 × 10−2 | +4.99 |
| CRM (M$·year−1) | −3.18 | 0.150 | 0.155 | 0.162 | +4.52 |
| CE | −3.67 | 0.136 | 0.141 | 0.148 | +5.06 |
| CMR | +3.07 | 1.175 × 10−2 | 1.140 × 10−2 | 1.113 × 10−2 | −2.36 |
| CCW | −4.30 | 2.67 × 10−3 | 2.79 × 10−3 | 2.95 × 10−3 | +5.73 |
| Design item | |||||
| pH (MPa) | −2.84 | 0.581 | 0.598 | 0.621 | +3.84 |
| TMA (m2) | +3.10 | 5878.9 | 5701.7 | 5567.3 | −2.35 |
| TW (MW) | −3.69 | 0.287 | 0.298 | 0.313 | +5.03 |
| H2 Recovery | |||||
|---|---|---|---|---|---|
| Dev. (%) | 89% | 90% | 91% | Dev. (%) | |
| Cost Item | |||||
| TAC (M$·year−1) | −1.49 | 1.738 | 1.764 | 1.793 | +1.64 |
| OPEX (M$·year−1) | −1.40 | 1.080 | 1.095 | 1.112 | +1.54 |
| annCAPEX (M$·year−1) | −1.64 | 0.658 | 0.669 | 0.681 | +1.80 |
| CINV (M$) | −1.64 | 1.407 | 1.431 | 1.457 | +1.80 |
| IC1 | −1.34 | 0.684 | 0.694 | 0.703 | +1.42 |
| IC2 | −3.22 | 0.306 | 0.316 | 0.328 | +3.58 |
| IMA_MS1 | −0.88 | 0.266 | 0.268 | 0.271 | +0.95 |
| IVP1 | −3.17 | 7.48 × 10−2 | 7.67 × 10−2 | 7.94 × 10−2 | +3.57 |
| IMA_MS2 | +3.59 | 3.52 × 10−2 | 3.40 × 10−2 | 3.27 × 10−2 | −3.76 |
| IHEX1 | −0.54 | 2.02 × 10−2 | 2.03 × 10−2 | 2.04 × 10−2 | +0.44 |
| IHEX2 | −2.24 | 10.45 × 10−3 | 10.70 × 10−3 | 10.95 × 10−3 | +2.43 |
| IHEX3 | −1.72 | 1.02 × 10−2 | 1.04 × 10−2 | 1.06 × 10−2 | +1.82 |
| CRM (M$·year−1) | −2.74 | 0.151 | 0.155 | 0.160 | +3.02 |
| CE | −2.92 | 0.137 | 0.141 | 0.145 | +3.23 |
| CMR | −0.35 | 1.136 × 10−2 | 1.140 × 10−2 | 1.145 × 10−2 | +0.43 |
| CCW | −2.86 | 2.71 × 10−3 | 2.79 × 10−3 | 2.88 × 10−3 | +3.22 |
| Design item | |||||
| pH (MPa) | −3.01 | 0.580 | 0.598 | 0.618 | +3.34 |
| TMA (m2) | −0.36 | 5680.9 | 5701.7 | 5725.2 | +0.41 |
| TW (MW) | −3.02 | 0.289 | 0.298 | 0.307 | +3.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias, A.M.; Mores, P.L.; Scenna, N.J.; Caballero, J.A.; Mussati, S.F.; Mussati, M.C. Optimal Design of a Two-Stage Membrane System for Hydrogen Separation in Refining Processes. Processes 2018, 6, 208. https://doi.org/10.3390/pr6110208
Arias AM, Mores PL, Scenna NJ, Caballero JA, Mussati SF, Mussati MC. Optimal Design of a Two-Stage Membrane System for Hydrogen Separation in Refining Processes. Processes. 2018; 6(11):208. https://doi.org/10.3390/pr6110208
Chicago/Turabian StyleArias, Ana M., Patricia L. Mores, Nicolás J. Scenna, José A. Caballero, Sergio F. Mussati, and Miguel C. Mussati. 2018. "Optimal Design of a Two-Stage Membrane System for Hydrogen Separation in Refining Processes" Processes 6, no. 11: 208. https://doi.org/10.3390/pr6110208
APA StyleArias, A. M., Mores, P. L., Scenna, N. J., Caballero, J. A., Mussati, S. F., & Mussati, M. C. (2018). Optimal Design of a Two-Stage Membrane System for Hydrogen Separation in Refining Processes. Processes, 6(11), 208. https://doi.org/10.3390/pr6110208






