Separation of Ag(I) by Ion Exchange and Cementation from a Raffinate Containing Ag(I), Ni(II) and Zn(II) and Traces of Cu(II) and Sn(II)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Experimental Procedure
2.2.1. Ion Exchange
2.2.2. Cementation of Ag(I) by Copper Sheet
3. Results and Discussion
3.1. Speciation of the Metals in the Raffinate at 5 M HCl
3.2. Purification of Silver(I) by Ion Exchange
3.3. Purification of Silver(I) by Cementation with Copper Sheet
3.3.1. Cementation of Silver(I)
3.3.2. Synthesis of Silver Particles
3.4. Integrated Proposed Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sulka, G.D.; Jaskuła, M. Effect of sulphuric acid and copper sulphate concentrations on the morphology of silver deposit in the cementation process. Electrochim. Acta 2006, 51, 6111–6119. [Google Scholar] [CrossRef]
- Bodsworth, C. The Extraction and Refining of Metals; CRC Press, Inc.: Boca Raton, FL, USA, 1994. [Google Scholar]
- Kononova, O.; Kuznetsova, M.; Mel’nikov, A.; Karplyakova, N.; Kononov, Y. Sorption recovery of copper (ii) and zinc (ii) from chloride aqueous solutions. J. Serbian Chem. Soc. 2014, 79, 1037–1049. [Google Scholar] [CrossRef]
- Ahmed, E.S.; Fouad, O.A.; El-Midany, A.A.; El-Sabbagh, E.A.; El-Rahman, A.A.; Ibrahim, I.A. Silver nanostructures via cementation on copper: A comparison between experimental data and statistical design model. Surf. Interface Anal. 2010, 42, 730–734. [Google Scholar] [CrossRef]
- Xing, W.; Lee, M. Leaching of gold and silver from anode slime with a mixture of hydrochloric acid and oxidizing agents. Geosyst. Eng. 2017, 20, 216–223. [Google Scholar] [CrossRef]
- Xing, W.; Lee, M.; Senanayake, G. Recovery of metals from chloride leach solutions of anode slime by solvent extraction. Part I: Recovery of gold with cyanex 272. Hydrometallurgy 2018, 180, 58–64. [Google Scholar] [CrossRef]
- Xing, W.D.; Lee, M.S.; Senanayake, G. Recovery of metals from chloride leach solutions of anode slimes by solvent extraction. Part II: Recovery of silver and copper with cyanex 272, lix 63 and alamine 336. Hydrometallurgy 2018, 180, 49–57. [Google Scholar] [CrossRef]
- Kuntyi, O.I.; Zozulya, G.I.; Kurilets, O.G. Silver cementation with magnesium in cyanide solutions. Russ. J. Appl. Chem. 2007, 80, 189–192. [Google Scholar] [CrossRef]
- Timur, S.; Cetinkaya, O.; Erturk, S.; Orhan, G. Investigating silver cementation from nitrate solutions by copper in forced convection systems. Miner. Metall. Process. 2005, 22, 205–210. [Google Scholar]
- Sulka, G.D.; Jaskuła, M. Influence of the sulphuric acid concentration on the kinetics and mechanism of silver ion cementation on copper. Hydrometallurgy 2005, 77, 131–137. [Google Scholar] [CrossRef]
- Grishina, E.P.; Ramenskaya, L.M. Silver cementation on copper in 1-butyl-3-methylimidazolium bromide–silver bromide ionic liquid medium. J. Mol. Liq. 2017, 248, 963–971. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, S.G.; Oh, J.K. Cementation behavior of gold and silver onto Zn, Al and Fe powders from acid thiourea solutions. Can. Metall. Q. 2013, 36, 149–155. [Google Scholar]
- Winand, R. Chloride hydrometallurgy. Hydrometallurgy 1991, 27, 285–316. [Google Scholar] [CrossRef]
- Zhao, H.; Chang, J.; Boika, A.; Bard, A.J. Electrochemistry of high concentration copper chloride complexes. Anal. Chem. 2013, 85, 7696–7703. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Nam, S.H. Chemical equilibria of nickel chloride in HCL solution at 25 °C. Bull. Korean Chem. Soc. 2009, 30, 2203–2207. [Google Scholar]
- Lee, M.S.; Ahn, J.G. Separation of Tin(ii) and In(iii) in hydrochloric acid solution by solvent extraction. Korean J. Met. Mater. 2015, 53, 488–494. [Google Scholar]
- Senanayake, G.; Muir, D.M. Competitive solvation and complexation of Cu(i), Cu(ll), Pb(ll), Zn(ll), and Ag(i) in aqueous ethanol, acetonitrile, and dimethylsulfoxide solutions containing chloride ion with applications to hydrometallurgy. Metall. Mater. Trans. B 1990, 21B, 439–448. [Google Scholar] [CrossRef]
- Senanayake, G. Chloride assisted leaching of chalcocite by oxygenated sulphuric acid via Cu(ii)–OH–Cl. Miner. Eng. 2007, 20, 1075–1088. [Google Scholar] [CrossRef]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A review of methods of separation of the platinum-group metals through their chloro-complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- Schweitzer, G.K.; Pesterfield, L.L. The Aqueous Chemistry of the Elements; Oxford University Press, Inc.: New York, NY, USA, 2010. [Google Scholar]
- Fritz, J.J. Thermodynamic properties of chloro-complexes of silver chloride in aqueous solution. J. Solut. Chem. 1985, 14, 865–879. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Adeva, P.; Alonso, M. Processing of residual gold(iii) solutions via ion exchange. Gold Bull. 2005, 38, 9–13. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. Separation of molybdenum (vi) and tungsten (vi) from sulfuric acid solution by ion exchange with teva resin. Sep. Sci. Technol. 2015, 50, 2060–2065. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, hsab, part I fundamental principles. J. Chem. Educ. 1968, 45, 581–587. [Google Scholar] [CrossRef]
- Wells, A.F. Structural Inorganic Chemistry, 4th ed.; Oxford University Press: Oxford, UK, 1975; p. 1127. [Google Scholar]
- Canham, G.R.; Overton, T. Descriptive Inorganic Chemistry, 4th ed.; W. H. Freeman and Company: New York, NY, USA, 2006. [Google Scholar]
- Sulka, G.D.; Jaskuła, M. Study of the mechanism of silver ions cementation onto copper from acidic sulphate solutions and the morphology of the silver deposit. Hydrometallurgy 2004, 72, 93–110. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, T.; Fu, G.; He, B.; Huang, Z. Selective recovery of silver from an acidic solution by cementation with copper. J. Cent. South Univ. Technol. 1998, 5, 113–116. [Google Scholar] [CrossRef]
- Puvvada, G.; Tran, T. The cementation of Ag(i) ions from sodium chloride solutions onto a rotating copper disc. Hydrometallurgy 1995, 37, 193–206. [Google Scholar] [CrossRef]
- Malassis, L.; Dreyfus, R.; Murphy, R.J.; Hough, L.A.; Donnio, B.; Murray, C.B. One-step green synthesis of gold and silver nanoparticles with ascorbic acid and their versatile surface post-functionalization. RSC Adv. 2016, 6, 33092–33100. [Google Scholar] [CrossRef]
- Singha, D.; Barman, N.; Sahu, K. A facile synthesis of high optical quality silver nanoparticles by ascorbic acid reduction in reverse micelles at room temperature. J. Coll. Interface Sci. 2014, 413, 37–42. [Google Scholar] [CrossRef] [PubMed]
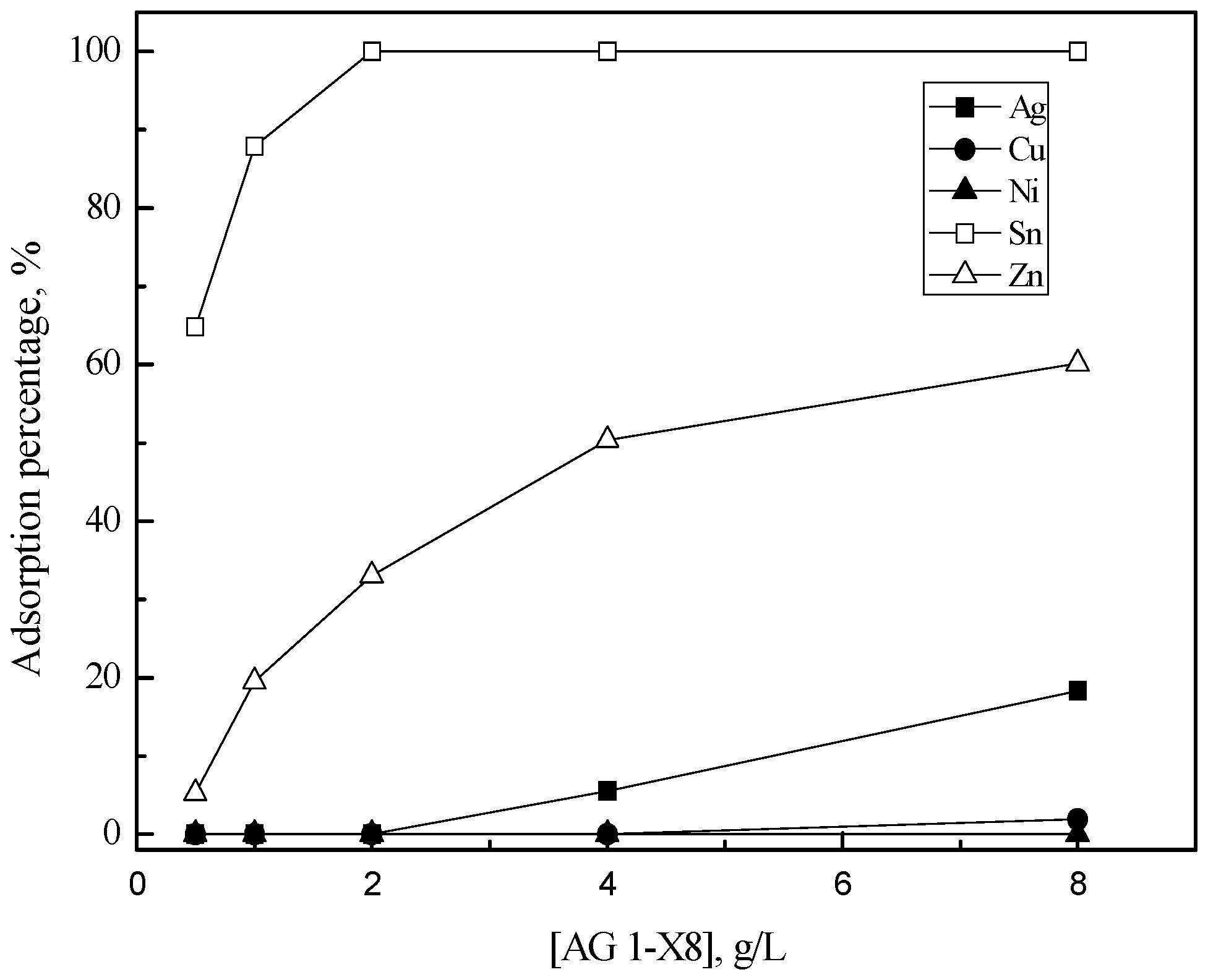
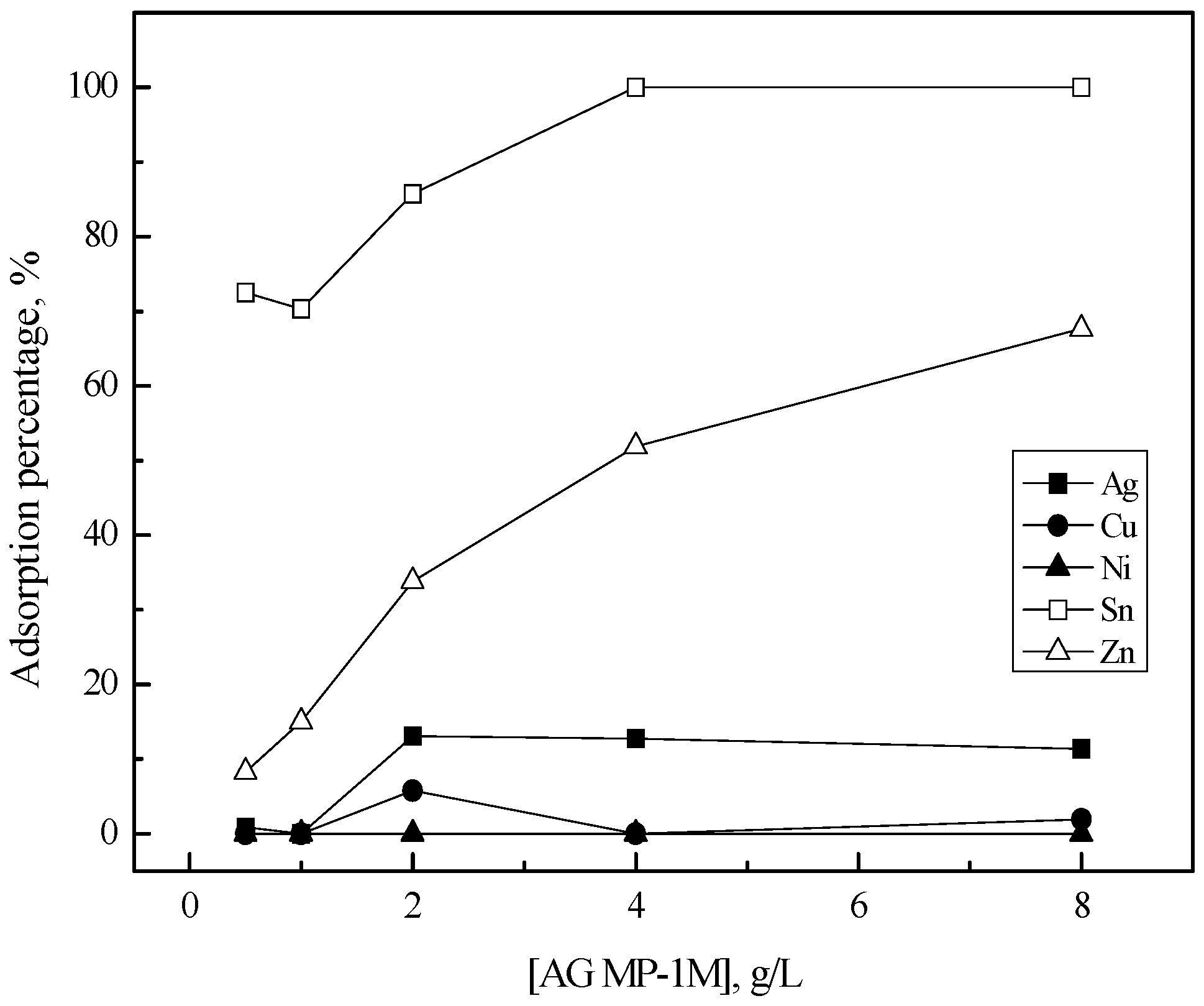










| Resin | Ionic Forms | Functional Group | Bead Size (µm) | Capacity (meq/mL) | Density (g/mL) | Matrix |
|---|---|---|---|---|---|---|
| AG 1-X8 | chloride | R-CH2N+(CH3)3 | 45–106 | 1.2 | 0.75 | / |
| AG MP-1M | chloride | R-CH2N+(CH3)3 | 75–150 | 1 | 0.70 | Macroporous |
| Diaion WA21J | free base | Polyamine | 300–1180 | 2.0 | 1.07 | Styrene-DVB, Porous |
| Lewatit MP-64 | chloride | Tertiary/quarternary amine | 300–1250 | 1.3 | 1.04 | Macroporous |
| Purolite A500 | chloride | Type 1 quaternary Ammonium | 600–850 | 1.15 | 1.08 | Macroporous polystyrene crosslinked with divinylbenzene |
| TEVA Resin | chloride | R3-N+CH3Cl−/NO3− | 100–150 | / | 0.35 | / |
| Metals | Ag | Cu | Ni | Sn | Zn |
|---|---|---|---|---|---|
| C (mg/L) | 40 | 4.5 | 10 | 10 | 14 |
| Metals | Species | Log βn (1~4) | References |
|---|---|---|---|
| Ag | AgCl, AgCl2−, AgCl32−, AgCl43− | (3.3, 5.3, 6.0, 3.6) | [20,21] |
| Cu | CuCl+, CuCl2, CuCl3−, CuCl42− | (0.02, −0.71, −2.3) | [17] |
| Ni | Ni2+, NiCl+ | 2.1 | [15] |
| Sn | SnCl+, SnCl2, SnCl3−, SnCl42− | (1.5, 2.3, 2.0, 1.5) | [20] |
| Zn | ZnCl+, ZnCl2, ZnCl3−, ZnCl42− | (0.43, 0.61, 0.53, 0.2) | [17] |
| Ion | Ionic Radii (pm) | Charge Density (C/mm3) |
|---|---|---|
| Cu2+ | 73 | 98.2 |
| Ni2+ | 70 | 111.4 |
| Ag+ | 115 | 25.1 |
| Sn2+ | 118 | 46.5 |
| Zn2+ | 74 | 56.1 |
| Cl− | 167 | / |
| Hard | Borderline | Soft | |
|---|---|---|---|
| Acid | N3+, N+ | Ni2+, Cu2+, Zn2+, Sn2+ | Ag+ |
| Base | RNH2, N2H4 | Cl−, N3− |
| Electrode | Reaction | E0 (V vs. SHE) |
|---|---|---|
| Ag+/Ag | Ag+ + e ↔ Ag0 | 0.80 |
| Cu2+/Cu | Cu2+ + 2e ↔ Cu0 | 0.34 |
| Cu+/Cu | Cu+ + e ↔ Cu0 | 0.52 |
| Ni2+/Ni | Ni2+ + 2e ↔ Ni0 | −0.26 |
| Sn2+/Sn | Sn2+ + 2e ↔ Sn0 | −0.14 |
| Zn2+/Zn | Zn2+ + 2e ↔ Zn0 | −0.76 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, W.D.; Lee, M.S.; Choi, S.H. Separation of Ag(I) by Ion Exchange and Cementation from a Raffinate Containing Ag(I), Ni(II) and Zn(II) and Traces of Cu(II) and Sn(II). Processes 2018, 6, 112. https://doi.org/10.3390/pr6080112
Xing WD, Lee MS, Choi SH. Separation of Ag(I) by Ion Exchange and Cementation from a Raffinate Containing Ag(I), Ni(II) and Zn(II) and Traces of Cu(II) and Sn(II). Processes. 2018; 6(8):112. https://doi.org/10.3390/pr6080112
Chicago/Turabian StyleXing, Wei Dong, Man Seung Lee, and Seung Hoon Choi. 2018. "Separation of Ag(I) by Ion Exchange and Cementation from a Raffinate Containing Ag(I), Ni(II) and Zn(II) and Traces of Cu(II) and Sn(II)" Processes 6, no. 8: 112. https://doi.org/10.3390/pr6080112
APA StyleXing, W. D., Lee, M. S., & Choi, S. H. (2018). Separation of Ag(I) by Ion Exchange and Cementation from a Raffinate Containing Ag(I), Ni(II) and Zn(II) and Traces of Cu(II) and Sn(II). Processes, 6(8), 112. https://doi.org/10.3390/pr6080112






