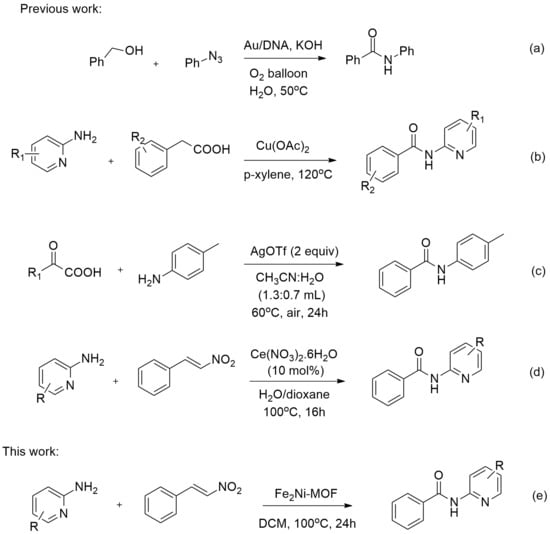
The Synthesis of N-(Pyridin-2-yl)-Benzamides from Aminopyridine and Trans-Beta-Nitrostyrene by Fe2Ni-BDC Bimetallic Metal–Organic Frameworks
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of the Catalyst
2.2. Catalyst Characterization
2.3. The Synthesis of N-Pyridinyl Benzamide
3. Results and Discussion
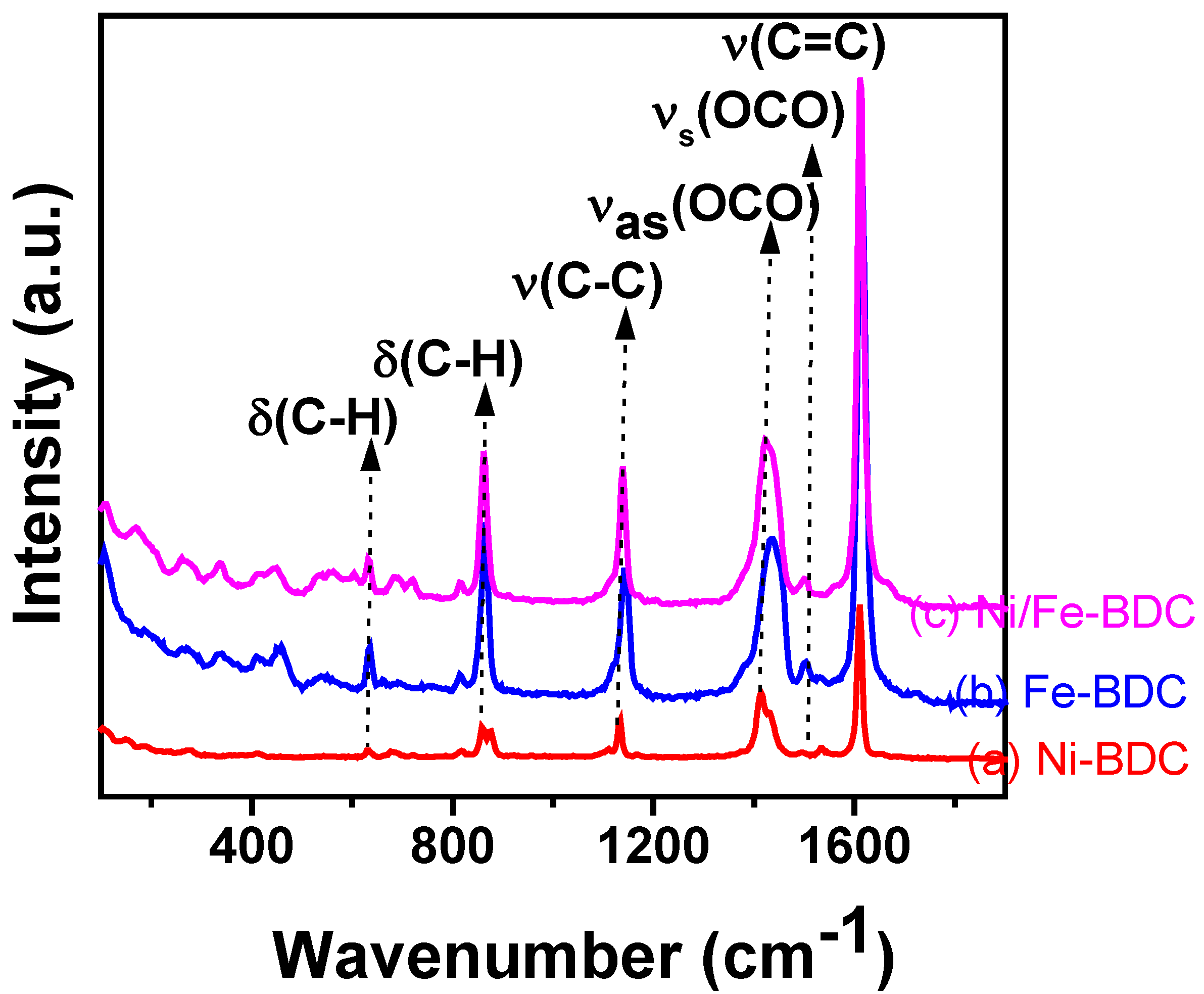
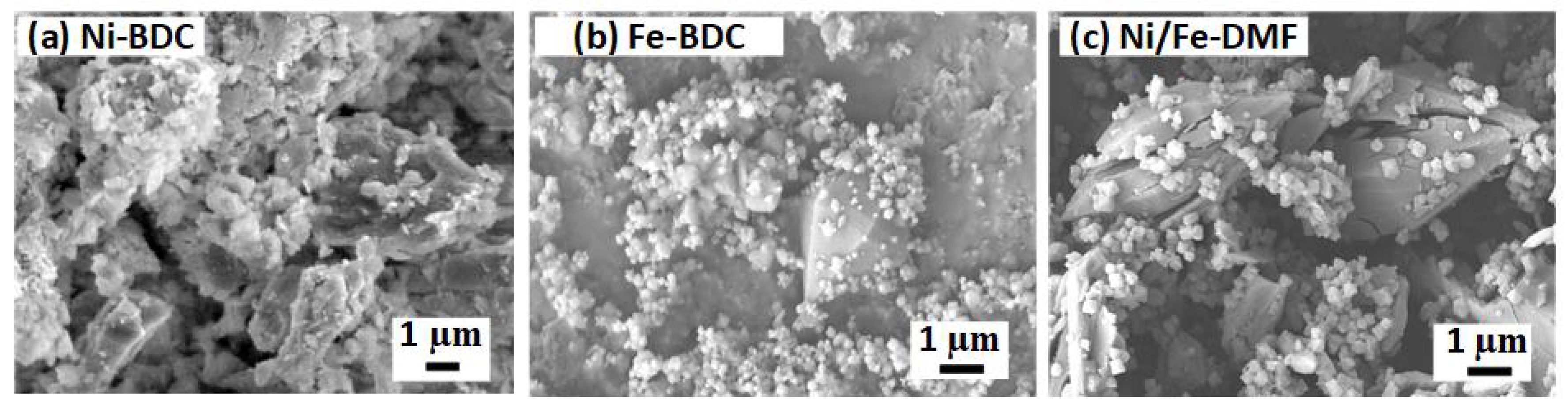
3.1. Characterization of the Ni-BDC, Fe-BDC, and Fe2Ni-BDC Catalysts
3.2. The Synthesis of N-Pyridinyl Benzamide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lundberg, H.; Tinnis, F.; Selander, N.; Adolfsson, H. Catalytic amide formation from non-activated carboxylic acids and amines. Chem. Soc. Rev. 2014, 43, 2714–2742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef] [PubMed]
- Köhn, M.; Breinbauer, R. The staudinger ligation—A gift to chemical biology. Angew. Chem. Int. Ed. 2004, 43, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Leow, D. Phenazinium salt-catalyzed aerobic oxidative amidation of aromatic aldehydes. Org. Lett. 2014, 16, 5812–5815. [Google Scholar] [CrossRef]
- Fang, X.; Li, H.; Jackstell, R.; Beller, M. Selective palladium-catalyzed aminocarbonylation of 1,3-dienes: Atom-efficient synthesis of β,γ-unsaturated amides. J. Am. Chem. Soc. 2014, 136, 16039–16043. [Google Scholar] [CrossRef]
- Cassidy, M.P.; Raushel, J.; Fokin, V.V. Practical synthesis of amides from in situ generated copper(I) acetylides and sulfonyl azides. Angew. Chem. Int. Ed. 2006, 45, 3154–3157. [Google Scholar] [CrossRef]
- Morimoto, H.; Fujiwara, R.; Shimizu, Y.; Morisaki, K.; Ohshima, T. Lanthanum(III) triflate catalyzed direct amidation of esters. Org. Lett. 2014, 16, 2018–2021. [Google Scholar] [CrossRef]
- Liu, X.; Jensen, K.F. Multistep synthesis of amides from alcohols and amines in continuous flow microreactor systems using oxygen and urea hydrogen peroxide as oxidants. Green Chem. 2013, 15, 1538–1541. [Google Scholar] [CrossRef]
- Chen, Z.W.; Jiang, H.F.; Pan, X.Y.; He, Z.J. Practical synthesis of amides from alkynyl bromides, amines, and water. Tetrahedron 2011, 67, 5920–5927. [Google Scholar] [CrossRef]
- Ferrins, L.; Gazdik, M.; Rahmani, R.; Varghese, S.; Sykes, M.L.; Jones, A.J.; Avery, V.M.; White, K.L.; Ryan, E.; Charman, S.A.; et al. Pyridyl benzamides as a novel class of potent inhibitors for the kinetoplastid Trypanosoma brucei. J. Med. Chem. 2014, 57, 6393–6402. [Google Scholar] [CrossRef]
- Subramanian, P.; Indu, S.; Kaliappan, K.P. A one-pot copper catalyzed biomimetic route to n -heterocyclic amides from methyl ketones via oxidative c-c bond cleavage. Org. Lett. 2014, 16, 6212–6215. [Google Scholar] [CrossRef] [PubMed]
- Ragupathi, A.; Sagadevan, A.; Lin, C.C.; Hwu, J.R.; Hwang, K.C. Copper(i)-catalysed oxidative C-N coupling of 2-aminopyridine with terminal alkynes featuring a CC bond cleavage promoted by visible light. Chem. Commun. 2016, 52, 11756–11759. [Google Scholar] [CrossRef] [PubMed]
- Patel, O.P.S.; Anand, D.; Maurya, R.K.; Yadav, P.P. Copper-catalyzed highly efficient oxidative amidation of aldehydes with 2-aminopyridines in an aqueous micellar system. Green Chem. 2015, 17, 3728–3732. [Google Scholar] [CrossRef]
- Xu, X.L.; Xu, W.T.; Wu, J.W.; He, J.B.; Xu, H.J. Silver-promoted decarboxylative amidation of α-keto acids with amines. Org. Biomol. Chem. 2016, 14, 9970–9973. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Huang, B.; Liu, Y. Copper(II)-mediated, carbon degradation-based amidation of phenylacetic acids toward N-substituted benzamides. Org. Biomol. Chem. 2018, 16, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wen, X.; Qian, Y.; Liang, P.; Liu, B.; Ye, M. Ce(III)-catalyzed highly efficient synthesis of pyridyl benzamides from aminopyridines and nitroolefins without external oxidants. Org. Biomol. Chem. 2018, 16, 1247–1251. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Q. Bimetallic metal-organic frameworks for gas storage and separation. Cryst. Growth Des. 2017, 17, 1450–1455. [Google Scholar] [CrossRef]
- Xia, B.Y.; Yan, Y.; Li, N.; Wu, H.B.; Lou, X.W.D.; Wang, X. A metal-organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 2016, 1, 15006. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Bao, W.; Lai, Y.; Li, J.; Gan, Y.; Wang, J. Hierarchical mesoporous γ-Fe2O3/carbon nanocomposites derived from metal organic frameworks as a cathode electrocatalyst for rechargeable Li-O2 batteries. Electrochim. Acta 2014, 134, 293–301. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, Y.J.; Kim, D.H.; Lee, Y.J. Bimetallic metal-organic frameworks as efficient cathode catalysts for Li-O2 batteries. ACS Appl. Mater. Interfaces 2018, 10, 660–667. [Google Scholar] [CrossRef]
- Villajos, J.A.; Orcajo, G.; Martos, C.; Botas, J.Á.; Villacañas, J.; Calleja, G. Co/Ni mixed-metal sited MOF-74 material as hydrogen adsorbent. Int. J. Hydrog. Energy 2015, 40, 5346–5352. [Google Scholar] [CrossRef]
- Albero, J.; García, H. Metal organic frameworks as catalysts for organic reactions. In New Materials for Catalytic Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 13–40. ISBN 9780444635877. [Google Scholar]
- Gholipour-Ranjbar, H.; Soleimani, M.; Naderi, H.R. Application of Ni/Co-based metal-organic frameworks (MOFs) as an advanced electrode material for supercapacitors. New J. Chem. 2016, 40, 9187–9193. [Google Scholar] [CrossRef]
- Gao, J.; Cong, J.; Wu, Y.; Sun, L.; Yao, J.; Chen, B. Bimetallic hofmann-type metal-organic framework nanoparticles for efficient electrocatalysis of oxygen evolution reaction. ACS Appl. Energy Mater. 2018, 1, 5140–5144. [Google Scholar] [CrossRef]
- Vuong, G.T.; Pham, M.H.; Do, T.O. Direct synthesis and mechanism of the formation of mixed metal Fe 2Ni-MIL-88B. CrystEngComm 2013, 15, 9694–9703. [Google Scholar] [CrossRef]
- Vuong, G.T.; Pham, M.H.; Do, T.O. Synthesis and engineering porosity of a mixed metal Fe2Ni MIL-88B metal-organic framework. Dalton Trans. 2013, 42, 550–557. [Google Scholar] [CrossRef]
- Trinh, N.D.; Hong, S.-S. Photocatalytic decomposition of methylene blue over MIL-53(Fe) prepared using microwave-assisted process under visible light irradiation. J. Nanosci. Nanotechnol. 2014, 15, 5450–5454. [Google Scholar] [CrossRef]
- Nguyen, V.; Nguyen, T.; Bach, L.; Hoang, T.; Bui, Q.; Tran, L.; Nguyen, C.; Vo, D.-V.; Do, S. Effective photocatalytic activity of mixed Ni/Fe-base metal-organic framework under a compact fluorescent daylight lamp. Catalysts 2018, 8, 487. [Google Scholar] [CrossRef]
- Schejn, A.; Falk, V.; Mozet, K.; Schneider, R.; Aboulaich, A.; Balan, L.; Lalevée, J.; Medjahdi, G.; Aranda, L. Cu2+-doped zeolitic imidazolate frameworks (ZIF-8): Efficient and stable catalysts for cycloadditions and condensation reactions. Catal. Sci. Technol. 2015, 5, 1829–1839. [Google Scholar] [CrossRef]
- Wu, M.S.; Chen, F.Y.; Lai, Y.H.; Sie, Y.J. Electrocatalytic oxidation of urea in alkaline solution using nickel/nickel oxide nanoparticles derived from nickel-organic framework. Electrochim. Acta 2017, 258, 167–174. [Google Scholar] [CrossRef]
- Wu, M.S.; Chen, C.Y.; Chen, Y.R.; Shih, H.C. Synthesis of bimodal mesoporous carbon with embedded nickel nanoparticles through pyrolysis of nickel-organic framework as a counter-electrode catalyst for dye-sensitized solar cells. Electrochim. Acta 2016, 215, 50–56. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Bach, L.G.; Do, S.T.; Thuong, N.T.; Nguyen, T.D. Photoluminescence properties of Eu-doped MIL-53(Fe) obtained by solvothermal synthesis. J. Nanosci. Nanotechnol. 2018, 19, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Khan, N.A.; Park, H.J.; Jhung, S.H. Synthesis of a metal-organic framework material, iron terephthalate, by ultrasound, microwave, and conventional electric heating: A kinetic study. Chem. A Eur. J. 2010, 16, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, M.; Li, K.; Han, Y.; Zuo, Y.; Chai, F.; Song, C.; Zhang, G.; Guo, X. Synthesis of Fe/M (M = Mn, Co, Ni) bimetallic metal organic frameworks and their catalytic activity for phenol degradation under mild conditions. Inorg. Chem. Front. 2017, 4, 144–153. [Google Scholar] [CrossRef]
- Vu, T.A.; Le, G.H.; Dao, C.D.; Dang, L.Q.; Nguyen, K.T.; Nguyen, Q.K.; Dang, P.T.; Tran, H.T.K.; Duong, Q.T.; Nguyen, T.V.; et al. Arsenic removal from aqueous solutions by adsorption using novel MIL-53(Fe) as a highly efficient adsorbent. RSC Adv. 2015, 5, 5261–5268. [Google Scholar] [CrossRef]
- Feng, X.; Chen, H.; Jiang, F. In-situ ethylenediamine-assisted synthesis of a magnetic iron-based metal-organic framework MIL-53(Fe) for visible light photocatalysis. J. Colloid Interface Sci. 2017, 494, 32–37. [Google Scholar] [CrossRef]






| Entry | Reactant 1 | Reactant 2 | Product | Isolated Yields (%) |
|---|---|---|---|---|
| 1 | 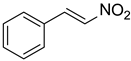 | 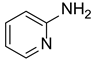 | 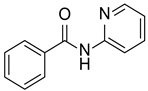 | 82 (85 [16]) |
| 2 |  |  | 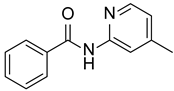 | 78 |
| 3 |  |  | 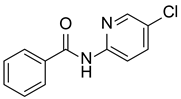 | 68 |
| 4 | 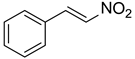 | 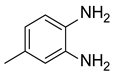 |  | 63 |
| 5 |  | 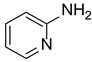 |  | 74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.D.; Nguyen, O.K.T.; Tran, T.V.; Nguyen, V.H.; Bach, L.G.; Tran, N.V.; Vo, D.-V.N.; Nguyen, T.V.; Hong, S.-S.; Do, S.T. The Synthesis of N-(Pyridin-2-yl)-Benzamides from Aminopyridine and Trans-Beta-Nitrostyrene by Fe2Ni-BDC Bimetallic Metal–Organic Frameworks. Processes 2019, 7, 789. https://doi.org/10.3390/pr7110789
Nguyen TD, Nguyen OKT, Tran TV, Nguyen VH, Bach LG, Tran NV, Vo D-VN, Nguyen TV, Hong S-S, Do ST. The Synthesis of N-(Pyridin-2-yl)-Benzamides from Aminopyridine and Trans-Beta-Nitrostyrene by Fe2Ni-BDC Bimetallic Metal–Organic Frameworks. Processes. 2019; 7(11):789. https://doi.org/10.3390/pr7110789
Chicago/Turabian StyleNguyen, Trinh Duy, Oanh Kim Thi Nguyen, Thuan Van Tran, Vinh Huu Nguyen, Long Giang Bach, Nhan Viet Tran, Dai-Viet N. Vo, Tuyen Van Nguyen, Seong-Soo Hong, and Sy Trung Do. 2019. "The Synthesis of N-(Pyridin-2-yl)-Benzamides from Aminopyridine and Trans-Beta-Nitrostyrene by Fe2Ni-BDC Bimetallic Metal–Organic Frameworks" Processes 7, no. 11: 789. https://doi.org/10.3390/pr7110789
APA StyleNguyen, T. D., Nguyen, O. K. T., Tran, T. V., Nguyen, V. H., Bach, L. G., Tran, N. V., Vo, D. -V. N., Nguyen, T. V., Hong, S. -S., & Do, S. T. (2019). The Synthesis of N-(Pyridin-2-yl)-Benzamides from Aminopyridine and Trans-Beta-Nitrostyrene by Fe2Ni-BDC Bimetallic Metal–Organic Frameworks. Processes, 7(11), 789. https://doi.org/10.3390/pr7110789