CoFe2O4 Nanomaterials: Effect of Annealing Temperature on Characterization, Magnetic, Photocatalytic, and Photo-Fenton Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CoFe2O4 Nanoparticles
2.3. Characterizations
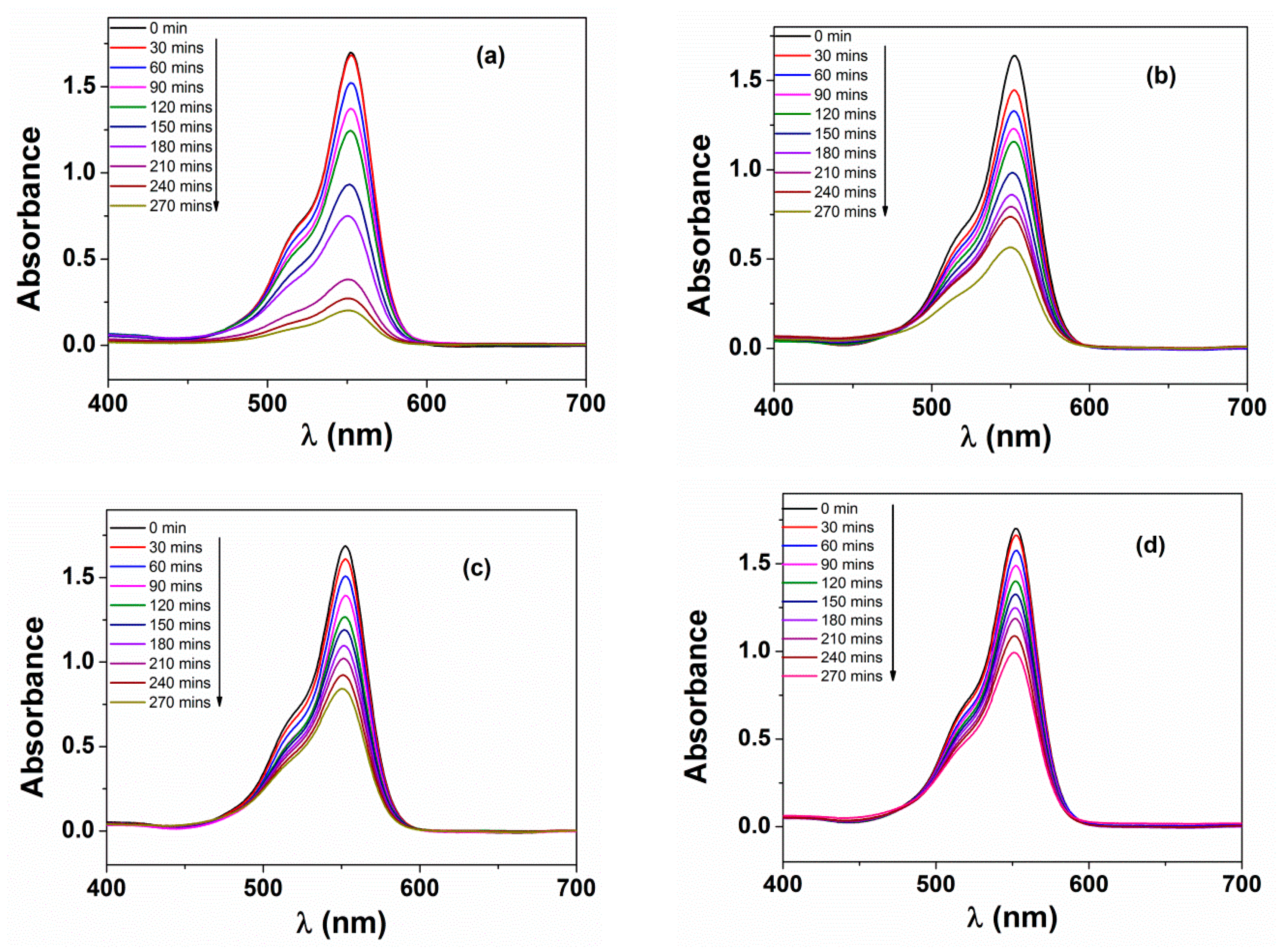
2.4. Photocatalytic Degradation of Rhodamine B
3. Results and Discussion
3.1. Structural Analysis
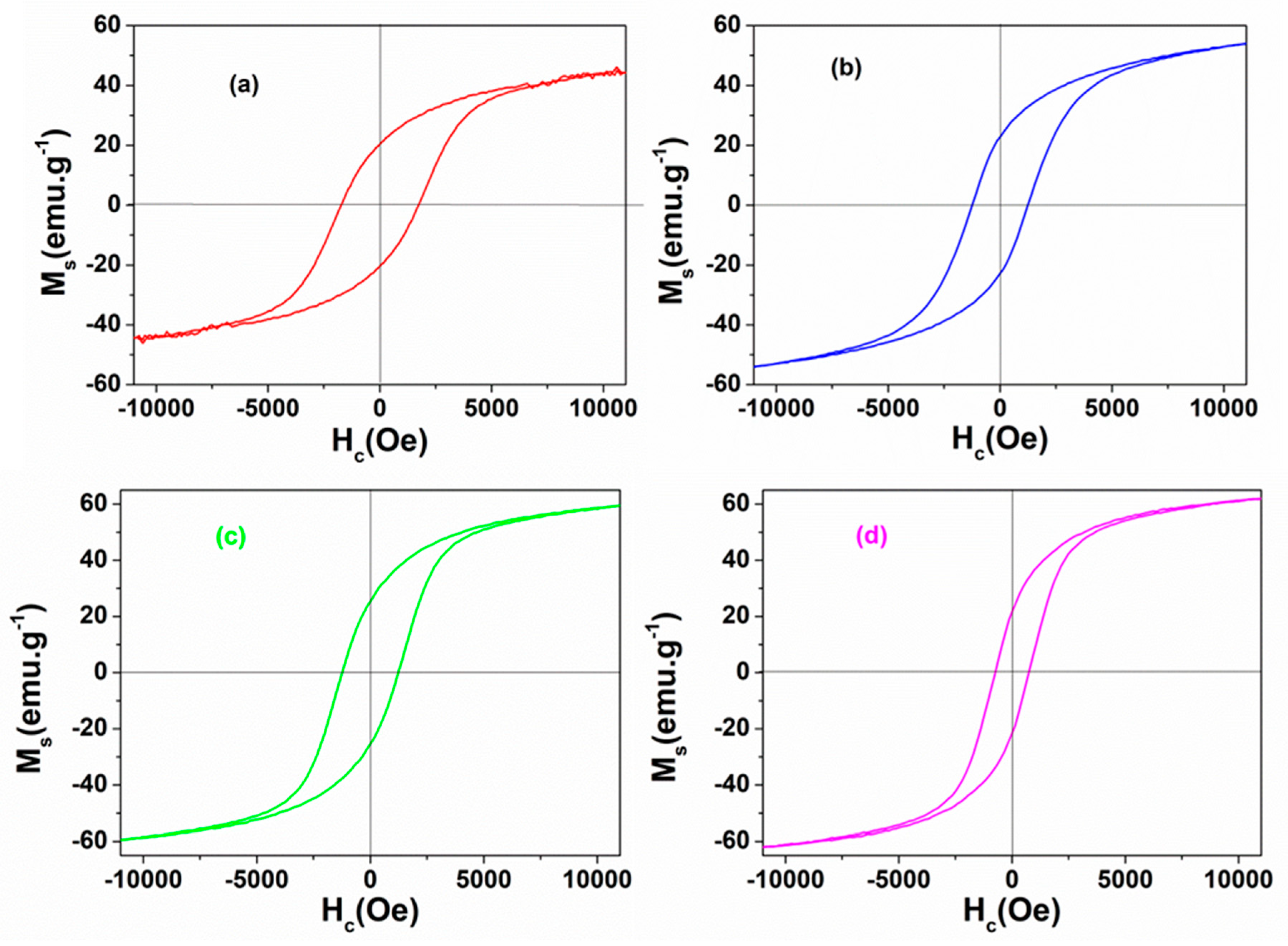
3.2. Magnetic Properties
3.3. Photochemical Activities
- (i)
- It was found that CF500 possessed the lowest pore sizes, which is indicative of a high surface area of nanoparticles. This could accelerate the adsorption of RhB molecules on the surface, leading to a higher likelihood of •OH radical formation [43].
- (ii)
- More importantly, CF500 nanoparticles with the lowest bandgap (1.57 eV) were more likely to form the electron–hole pair under visible light irradiation, dominating the charge carriers with oxidation of RhB [15].
- (iii)
- Samples that were calcined at a higher temperature had greater band gap values, which possibly deferred the electron–hole pair recombination induced by visible light irradiation [44].
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Phong, P.T.; Phuc, N.X.; Nam, P.H.; Chien, N.V.; Dung, D.D.; Linh, P.H. Size-controlled heating ability of CoFe2O4 nanoparticles foráhyperthermia applications. Phys. B Condens. Matter 2018, 531, 30–34. [Google Scholar] [CrossRef]
- Kumar, E.R.; Jayaprakash, R.; Kumar, S. Effect of annealing temperature on structural and magnetic properties of manganese substituted NiFe2O4 nanoparticles. Mater. Sci. Semicond. Process. 2014, 17, 173–177. [Google Scholar] [CrossRef]
- Tong, J.; Bo, L.; Li, Z.; Lei, Z.; Xia, C. Magnetic CoFe2O4 nanocrystal: A novel and efficient heterogeneous catalyst for aerobic oxidation of cyclohexane. J. Mol. Catal. A Chem. 2009, 307, 58–63. [Google Scholar] [CrossRef]
- Rao, K.S.; Choudary, G.; Rao, K.H.; Sujatha, C. Structural and magnetic properties of ultrafine CoFe2O4 nanoparticles. Procedia Mater. Sci. 2015, 10, 19–27. [Google Scholar] [CrossRef]
- Kumar, S.; Munjal, S.; Khare, N. Metal-semiconductor transition and Seebeck inversion in CoFe2O4 nanoparticles. J. Phys. Chem. Solids 2017, 105, 86–89. [Google Scholar] [CrossRef]
- Salazar-Kuri, U.; Estevez, J.O.; Silva-González, N.R.; Pal, U. Large magnetostriction in chemically fabricated CoFe2O4 nanoparticles and its temperature dependence. J. Magn. Magn. Mater. 2018, 460, 141–145. [Google Scholar] [CrossRef]
- Annie Vinosha, P.; Jerome Das, S. Investigation on the role of pH for the structural, optical and magnetic properties of cobalt ferrite nanoparticles and its effect on the photo-fenton activity. Mater. Today Proc. 2018, 5, 8662–8671. [Google Scholar] [CrossRef]
- Dong, N.; He, F.; Xin, J.; Wang, Q.; Lei, Z.; Su, B. Preparation of CoFe2O4 magnetic fiber nanomaterial via a template-assisted solvothermal method. Mater. Lett. 2015, 141, 238–241. [Google Scholar] [CrossRef]
- Varma, P.C.R.; Manna, R.S.; Banerjee, D.; Varma, M.R.; Suresh, K.G.; Nigam, A.K. Magnetic properties of CoFe2O4 synthesized by solid state, citrate precursor and polymerized complex methods: A comparative study. J. Alloys Compd. 2008, 453, 298–303. [Google Scholar] [CrossRef]
- Maleki, A.; Hosseini, N.; Taherizadeh, A. Synthesis and characterization of cobalt ferrite nanoparticles prepared by the glycine-nitrate process. Ceram. Int. 2018, 44, 8576–8581. [Google Scholar] [CrossRef]
- Gabal, M.A.; Al-Juaid, A.A.; El-Rashed, S.; Hussein, M.A. Synthesis and characterization of nano-sized CoFe2O4 via facile methods: A comparative study. Mater. Res. Bull. 2017, 89, 68–78. [Google Scholar] [CrossRef]
- Pourgolmohammad, B.; Masoudpanah, S.M.; Aboutalebi, M.R. Synthesis of CoFe2O4 powders with high surface area by solution combustion method: Effect of fuel content and cobalt precursor. Ceram. Int. 2017, 43, 3797–3803. [Google Scholar] [CrossRef]
- Rajan Babu, D.; Venkatesan, K. Synthesis of nanophasic CoFe2O4 powder by self-igniting solution combustion method using mix up fuels. J. Cryst. Growth 2017, 468, 179–184. [Google Scholar] [CrossRef]
- Yadav, N.G.; Chaudhary, L.S.; Sakhare, P.A.; Dongale, T.D.; Patil, P.S.; Sheikh, A.D. Impact of collected sunlight on ZnFe2O4 nanoparticles for photocatalytic application. J. Colloid Interface Sci. 2018, 527, 289–297. [Google Scholar] [CrossRef]
- Kalam, A.; Al-Sehemi, A.G.; Assiri, M.; Du, G.; Ahmad, T.; Ahmad, I.; Pannipara, M. Modified solvothermal synthesis of cobalt ferrite (CoFe2O4) magnetic nanoparticles photocatalysts for degradation of methylene blue with H2O2/visible light. Results Phys. 2018, 8, 1046–1053. [Google Scholar] [CrossRef]
- Vinosha, P.A.; Xavier, B.; Anceila, D.; Das, S.J. Nanocrystalline ferrite (MFe2O4, M = Ni, Cu, Mn and Sr) photocatalysts synthesized by homogeneous Co-precipitation technique. Optik 2018, 157, 441–448. [Google Scholar] [CrossRef]
- Guo, X.; Wang, K.; Li, D.; Qin, J. Heterogeneous photo-Fenton processes using graphite carbon coating hollow CuFe2O4 spheres for the degradation of methylene blue. Appl. Surf. Sci. 2017, 420, 792–801. [Google Scholar] [CrossRef]
- Kurian, M.; Nair, D.S. Heterogeneous Fenton behavior of nano nickel zinc ferrite catalysts in the degradation of 4-chlorophenol from water under neutral conditions. J. Water Process Eng. 2015, 8, e37–e49. [Google Scholar] [CrossRef]
- Annie Vinosha, P.; Xavier, B.; Ashwini, A.; Ansel Mely, L.; Jerome Das, S. Tailoring the photo-Fenton activity of nickel ferrite nanoparticles synthesized by low-temperature coprecipitation technique. Optik 2017, 137, 244–253. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Nguyen, L.T.H.; Manh, N.C.; Quoc, D.N.; Quang, H.N.; Nguyen, H.T.T.; Nguyen, D.C.; Bach, L.G. A Facile Synthesis, Characterization, and Photocatalytic Activity of Magnesium Ferrite Nanoparticles via the Solution Combustion Method. J. Chem. 2019, 2019, 3428681. [Google Scholar] [CrossRef]
- Xie, H.; Gu, S.; Zhang, J.; Hu, Q.; Yu, X.; Kong, J. Novel PEI–AuNPs–MnIIIPPIX nanocomposite with enhanced peroxidase-like catalytic activity in aqueous media. C. R. Chim. 2018, 21, 104–111. [Google Scholar] [CrossRef]
- Sahnoun, S.; Boutahala, M.; Tiar, C.; Kahoul, A. Adsorption of tartrazine from an aqueous solution by octadecyltrimethylammonium bromide-modified bentonite: Kinetics and isotherm modeling. C. R. Chim. 2018, 21, 391–398. [Google Scholar] [CrossRef]
- Khelifi, S.; Ayari, F. Modified bentonite for anionic dye removal from aqueous solutions. Adsorbent regeneration by the photo-Fenton process. C. R. Chim. 2019, 22, 154–160. [Google Scholar] [CrossRef]
- da Rosa, A.L.D.; Carissimi, E.; Dotto, G.L.; Sander, H.; Feris, L.A. Biosorption of rhodamine B dye from dyeing stones effluents using the green microalgae Chlorella pyrenoidosa. J. Clean. Prod. 2018, 198, 1302–1310. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, D.T.C.; Le, H.T.N.; Duong, C.D.; Bach, L.G.; Nguyen, H.-T.T.; Nguyen, T.D. Facile synthesis of manganese oxide-embedded mesoporous carbons and their adsorbability towards methylene blue. Chemosphere 2019, 227, 455–461. [Google Scholar] [CrossRef]
- Sanchez-Hachair, A.; Hofmann, A. Hexavalent chromium quantification in solution: Comparing direct UV–visible spectrometry with 1,5-diphenylcarbazide colorimetry. C. R. Chim. 2018, 21, 890–896. [Google Scholar] [CrossRef]
- Ignat, M.; Rotaru, R.; Samoila, P.; Sacarescu, L.; Timpu, D.; Harabagiu, V. Relationship between the component synthesis order of zinc ferrite–titania nanocomposites and their performances as visible light-driven photocatalysts for relevant organic pollutant degradation. C. R. Chim. 2018, 21, 263–269. [Google Scholar] [CrossRef]
- Dimić, D. The importance of specific solvent–solute interactions for studying UV–vis spectra of light-responsive molecular switches. C. R. Chim. 2018, 21, 1001–1010. [Google Scholar] [CrossRef]
- Sharma, R.; Singhal, S. Structural, magnetic and electrical properties of zinc doped nickel ferrite and their application in photo catalytic degradation of methylene blue. Phys. B Condens. Matter 2013, 414, 83–90. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, D.T.C.; Le, H.T.N.; Bach, L.G.; Vo, D.-V.N.; Lim, K.T.; Nong, L.X.; Nguyen, T.D. Combined Minimum-Run Resolution IV and Central Composite Design for Optimized Removal of Tetracycline Drug Over Metal–Organic Framework-Templated Porous Carbon. Molecules 2019, 24, 1887. [Google Scholar] [CrossRef]
- Jinisha, R.; Gandhimathi, R.; Ramesh, S.T.; Nidheesh, P.V.; Velmathi, S. Removal of rhodamine B dye from aqueous solution by electro-Fenton process using iron-doped mesoporous silica as a heterogeneous catalyst. Chemosphere 2018, 200, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Hu, X.; Wang, Y.; Duan, M.; Fang, S.; Chen, W. A PEG-tannic acid decorated microfiltration membrane for the fast removal of Rhodamine B from water. Sep. Purif. Technol. 2018, 207, 443–450. [Google Scholar] [CrossRef]
- Saleh, T.A.; Ali, I. Synthesis of polyamide grafted carbon microspheres for removal of rhodamine B dye and heavy metals. J. Environ. Chem. Eng. 2018, 6, 5361–5368. [Google Scholar] [CrossRef]
- Tuzen, M.; Sarı, A.; Saleh, T.A. Response surface optimization, kinetic and thermodynamic studies for effective removal of rhodamine B by magnetic AC/CeO2 nanocomposite. J. Environ. Manag. 2018, 206, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Guégan, R. Organoclay applications and limits in the environment. C. R. Chim. 2019, 22, 132–141. [Google Scholar] [CrossRef]
- Nabbou, N.; Belhachemi, M.; Boumelik, M.; Merzougui, T.; Lahcene, D.; Harek, Y.; Zorpas, A.A.; Jeguirim, M. Removal of fluoride from groundwater using natural clay (kaolinite): Optimization of adsorption conditions. C. R. Chim. 2019, 22, 105–112. [Google Scholar] [CrossRef]
- Abidi, N.; Duplay, J.; Jada, A.; Errais, E.; Ghazi, M.; Semhi, K.; Trabelsi-Ayadi, M. Removal of anionic dye from textile industries’ effluents by using Tunisian clays as adsorbents. Ζeta potential and streaming-induced potential measurements. C. R. Chim. 2019, 22, 113–125. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, D.T.C.; Nguyen, H.-T.T.; Nanda, S.; Vo, D.-V.N.; Do, S.T.; Van Nguyen, T.; Thi, T.A.D.; Bach, L.G.; Nguyen, T.D. Application of Fe-based metal-organic framework and its pyrolysis products for sulfonamide treatment. Environ. Sci. Pollut. Res. 2019, 26, 28106–28126. [Google Scholar] [CrossRef]
- Sundararajan, M.; John Kennedy, L.; Nithya, P.; Judith Vijaya, J.; Bououdina, M. Visible light driven photocatalytic degradation of rhodamine B using Mg doped cobalt ferrite spinel nanoparticles synthesized by microwave combustion method. J. Phys. Chem. Solids 2017, 108, 61–75. [Google Scholar] [CrossRef]
- Nadumane, A.; Shetty, K.; Anantharaju, K.S.; Nagaswarupa, H.P.; Rangappa, D.; Vidya, Y.S.; Nagabhushana, H.; Prashantha, S.C. Sunlight photocatalytic performance of Mg-doped nickel ferrite synthesized by a green sol-gel route. J. Sci. Adv. Mater. Devices 2019, 4, 89–100. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Güner, S.; Nawaz, M.; Baykal, A.; Aldakheel, F.; Akhtar, S.; Ercan, I.; Belenli, İ.; Ozçelik, B. Magnetic and structural characterization of Nb3+-substituted CoFe2O4 nanoparticles. Ceram. Int. 2019, 45, 8222–8232. [Google Scholar] [CrossRef]
- Sharma, R.; Bansal, S.; Singhal, S. Augmenting the catalytic activity of CoFe2O4 by substituting rare earth cations into the spinel structure. RSC Adv. 2016, 6, 71676–71691. [Google Scholar] [CrossRef]
- Han, L.; Zhou, X.; Wan, L.; Deng, Y.; Zhan, S. Synthesis of ZnFe2O4 nanoplates by succinic acid-assisted hydrothermal route and their photocatalytic degradation of rhodamine B under visible light. J. Environ. Chem. Eng. 2014, 2, 123–130. [Google Scholar] [CrossRef]
- Wu, X.; Wang, W.; Li, F.; Khaimanov, S.; Tsidaeva, N.; Lahoubi, M. PEG-assisted hydrothermal synthesis of CoFe2O4 nanoparticles with enhanced selective adsorption properties for different dyes. Appl. Surf. Sci. 2016, 389, 1003–1011. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, D.T.C.; Le, H.T.N.; Nguyen, O.T.K.; Nguyen, V.H.; Nguyen, T.T.; Bach, L.G.; Nguyen, T.D. A hollow mesoporous carbon from metal-organic framework for robust adsorbability of ibuprofen drug in water. R. Soc. Open Sci. 2019, 6, 190058. [Google Scholar] [CrossRef] [Green Version]
- Van Tran, T.; Nguyen, D.T.C.; Le, H.T.N.; Bach, L.G.; Vo, D.-V.N.; Hong, S.S.; Phan, T.-Q.T.; Nguyen, T.D. Tunable Synthesis of Mesoporous Carbons from Fe3O (BDC) 3 for Chloramphenicol Antibiotic Remediation. Nanomaterials 2019, 9, 237. [Google Scholar] [CrossRef] [Green Version]
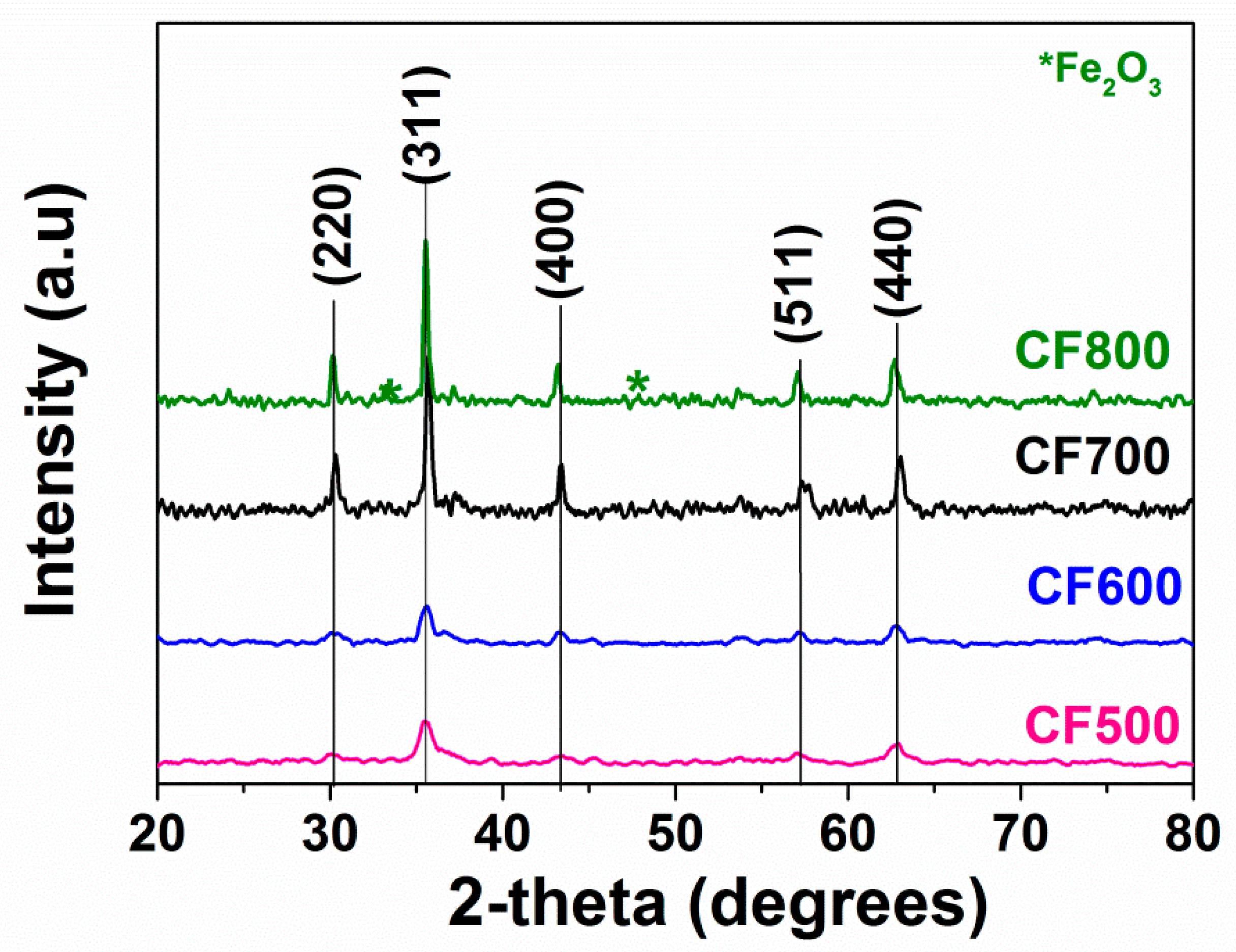




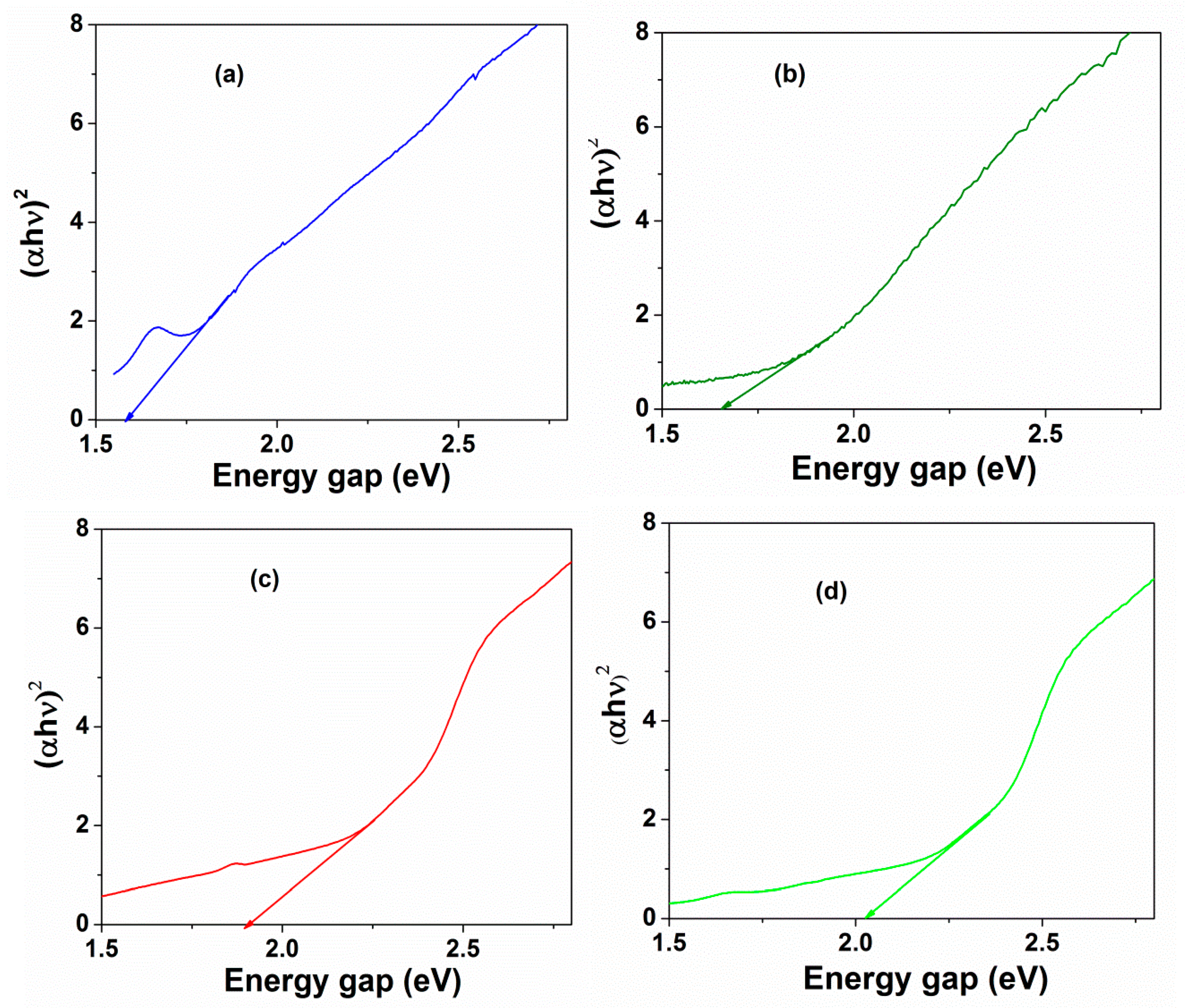


| Samples | r (nm) | a (Å) | V (Å3) | ν1 (cm−1) | ν2 (cm−1) | SBET (m2·g−1) |
|---|---|---|---|---|---|---|
| CF500 | 9 | 8.37 | 587.32 | 526 | 411 | 12.69 |
| CF600 | 11 | 8.36 | 584.53 | 530 | 409 | 7.55 |
| CF700 | 23 | 8.33 | 578.98 | 532 | 408 | 3.94 |
| CF800 | 29 | 8.36 | 585.23 | 522 | 412 | 1.58 |
| Samples | Ms (emu/g) | Hc (Oe) | Mr (emu/g) |
|---|---|---|---|
| CF500 | 44.41 | 1739.45 | 20.36 |
| CF600 | 53.86 | 1234.10 | 22.88 |
| CF700 | 59.40 | 1234.20 | 27.89 |
| CF800 | 61.80 | 762.04 | 27.83 |
| Samples | Rate Constant, (k × 10−2 min−1) | R2 |
|---|---|---|
| CF500 | 0.839 | 0.960 |
| CF600 | 0.394 | 0.967 |
| CF700 | 0.280 | 0.997 |
| CF800 | 0.216 | 0.990 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
To Loan, N.T.; Hien Lan, N.T.; Thuy Hang, N.T.; Quang Hai, N.; Tu Anh, D.T.; Thi Hau, V.; Van Tan, L.; Van Tran, T. CoFe2O4 Nanomaterials: Effect of Annealing Temperature on Characterization, Magnetic, Photocatalytic, and Photo-Fenton Properties. Processes 2019, 7, 885. https://doi.org/10.3390/pr7120885
To Loan NT, Hien Lan NT, Thuy Hang NT, Quang Hai N, Tu Anh DT, Thi Hau V, Van Tan L, Van Tran T. CoFe2O4 Nanomaterials: Effect of Annealing Temperature on Characterization, Magnetic, Photocatalytic, and Photo-Fenton Properties. Processes. 2019; 7(12):885. https://doi.org/10.3390/pr7120885
Chicago/Turabian StyleTo Loan, Nguyen Thi, Nguyen Thi Hien Lan, Nguyen Thi Thuy Hang, Nguyen Quang Hai, Duong Thi Tu Anh, Vu Thi Hau, Lam Van Tan, and Thuan Van Tran. 2019. "CoFe2O4 Nanomaterials: Effect of Annealing Temperature on Characterization, Magnetic, Photocatalytic, and Photo-Fenton Properties" Processes 7, no. 12: 885. https://doi.org/10.3390/pr7120885





