Characterizing a Newly Designed Steel-Wool-Based Household Filter for Safe Drinking Water Provision: Hydraulic Conductivity and Efficiency for Pathogen Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Filter Characteristics
2.2. Media and Their Preparation for Filter
2.2.1. Sand
2.2.2. Gravel
2.2.3. Metallic Iron
2.3. Experimental Procedure
2.3.1. Sample Collection
2.3.2. Efficiency Characterization
2.4. Analytical Method
2.5. Expression of Experimental Results
2.5.1. Value of E
2.5.2. Hydraulic Conductivity
3. Results and Discussion
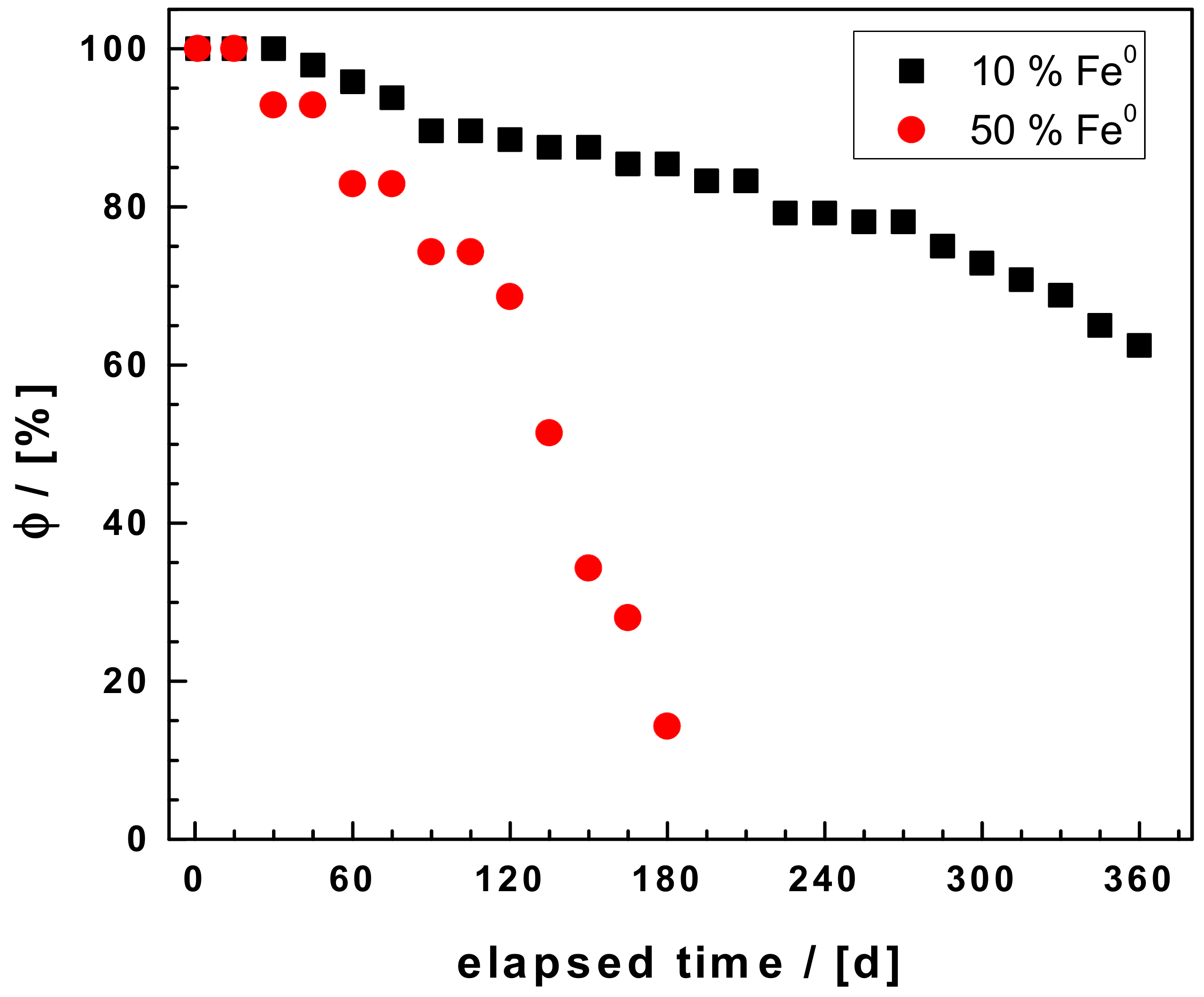
3.1. Hydraulic Conductivity
3.2. Turbidity Removal
3.3. Nitrate Removal
3.4. pH Value and Iron Breakthrough
3.5. Removal of Coliform
3.6. Discussion
3.7. Significance of the Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Progress on Drinking Water, Sanitation and Hygiene-2017 Update and SDG Baselines, WHO JMP launch version July 12 2017; WHO Library Cataloguing-in-Publication Data; WHO: Geneva, Switzerland, 2017. [CrossRef]
- Nanseu-Njiki, C.P.; Gwenzi, W.; Pengou, M.; Rahman, M.A.; Noubactep, C. Fe0/H2O filtration systems for decentralized safe drinking water: Where to from here? Water 2019, 11, 429. [Google Scholar] [CrossRef] [Green Version]
- Lauderdale, R.A.; Emmons, A.H. A method for decontaminating small volumes of radioactive water. J. Am. Water Works Assoc. 1951, 43, 327–331. [Google Scholar] [CrossRef]
- Baig, S.A.; Mahmood, Q.; Nawab, B.; Shafqat, M.N.; Pervez, A. Improvement of drinking water quality by using plant biomass through household biosand filter–A decentralized approach. Ecol. Eng. 2011, 37, 1842–1848. [Google Scholar] [CrossRef]
- Moglia, M.; Alexander, K.S.; Sharma, A. Discussion of the enabling environments for decentralised water systems. Water Sci. Technol. 2011, 63, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Hussam, A.; Munir, A.K.M. A simple and effective arsenic filter based on composite iron matrix: Development and deployment studies for groundwater of Bangladesh. J. Environ. Sci. Health A 2007, 42, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Ngai, T.K.K.; Murcott, S.; Shrestha, R.R.; Dangol, B.; Maharjan, M. Development and dissemination of Kanchan™ Arsenic Filter in rural Nepal. Water Sci. Technol. Water Supply 2006, 6, 137–146. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A.; Woafo, P. Metallic iron filters for universal access to safe drinking water. Clean Soil Air Water 2009, 37, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Naseri, E.; Ndé-Tchoupé, A.I.; Mwakabona, H.T.; Nanseu-Njiki, C.P.; Noubactep, C.; Njau, K.N.; Wydra, K.D. Making Fe0-based filters a universal solution for safe drinking water provision. Sustainability 2017, 9, 1224. [Google Scholar] [CrossRef] [Green Version]
- Devonshire, E. The purification of water by means of metallic iron. J. Frankl. Inst. 1890, 129, 449–461. [Google Scholar] [CrossRef]
- Hussam, A. Contending with a Development Disaster: SONO Filters Remove Arsenic from Well Water in Bangladesh. Innovations 2009, 4, 89–102. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Impact of solids formation and gas production on the permeability of ZVI PRBs. J. Environ. Eng. 2011, 137, 689–696. [Google Scholar] [CrossRef]
- Ngai, T.K.K.; Shrestha, R.R.; Dangol, B.; Maharjan, M.; Murcott, S.E. Design for sustainable development – Household drinking water filter for arsenic and pathogen treatment in Nepal. J. Environ. Sci. Health A 2007, 42, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Banerji, T.; Chaudhari, S. A cost-effective technology for arsenic removal: Case study of zerovalent iron-based iit bombay arsenic filter in west bengal. In Water and Sanitation in the New Millennium; Nath, K., Sharma, V., Eds.; Springer: New Delhi, India, 2017. [Google Scholar]
- Tepong-Tsindé, R. Metallic Iron Filters for Safe Drinking Water in Informal Settlements of Douala (Cameroun): A Pilot Scale Study. Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2020. [Google Scholar]
- Tepong-Tsindé, R.; Crane, R.; Noubactep, C.; Nassi, A.; Ruppert, H. Testing metallic iron filtration systems for decentralized water treatment at pilot scale. Water 2015, 7, 868–897. [Google Scholar] [CrossRef] [Green Version]
- Westerhoff, P.; James, J. Nitrate removal in zero-valent iron packed columns. Water Res. 2003, 37, 1818–1830. [Google Scholar] [CrossRef]
- Ahammed, M.M.; Davra, K. Performance evaluation of biosand filter modified with iron oxide-coated sand for household treatment of drinking water. Desalination 2011, 276, 287–293. [Google Scholar] [CrossRef]
- Haig, S.J.; Collins, G.; Davies, R.L.; Dorea, C.C.; Quince, C. Biological aspects of slow sand filtration: Past, present and future. Water Sci. Technol. Water Supply 2011, 11, 468–472. [Google Scholar] [CrossRef]
- Nitzsche, K.S.; Lan, V.M.; Trang, P.T.K.; Viet, P.H.; Berg, M.; Voegelin, A.; Planer-Friedrich, B.; Zahoransky, J.; Müller, S.-K.; Byrne, J.M.; et al. Arsenic removal from drinking water by a household sand filter in Vietnam–Effect of filter usage practices on arsenic removal efficiency and microbiological water quality. Sci. Total Environ. 2015, 502, 526–536. [Google Scholar] [CrossRef]
- Jia, Y.; Aagaard, P.; Breedveld, G.D. Sorption of triazoles to soil and iron minerals. Chemosphere 2007, 67, 250–258. [Google Scholar] [CrossRef]
- Bradley, I.; Straub, A.; Maraccini, P.; Markazi, S.; Nguyen, T.H. Iron oxide amended biosand filters for virus removal. Water Res. 2011, 45, 4501–4510. [Google Scholar] [CrossRef] [Green Version]
- Domga, R.; Togue-Kamga, F.; Noubactep, C.; Tchatchueng, J.B. Discussing porosity loss of Fe0 packed water filters at ground level. Chem. Eng. J. 2015, 263, 127–134. [Google Scholar] [CrossRef]
- Hu, R.; Noubactep, C. Redirecting research on Fe0 for environmental remediation: The search for synergy. Int. J. Environ. Res. Public Health 2019, 16, 4465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilling, N.B.; Bedworth, R.E. The oxidation of metals at high temperatures. J. Inst. Met. 1923, 29, 529–591. [Google Scholar]
- Landolt, D. Corrosion and Surface Chemistry of Metals, 1st ed.; EPFL Press: Lausanne, Switzerland, 2007; p. 615. [Google Scholar]
- Caré, S.; Crane, R.; Calabrò, P.S.; Ghauch, A.; Temgoua, E.; Noubactep, C. Modeling the permeability loss of metallic iron water filtration systems. CLEAN Soil Air Water 2013, 41, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Moraci, N.; Lelo, D.; Bilardi, S.; Calabrò, P.S. Modelling long-term hydraulic conductivity behaviour of zero valent iron column tests for permeable reactive barrier design. Can. Geotech. J. 2016, 53, 946–961. [Google Scholar] [CrossRef]
- Noubactep, C. Predicting the hydraulic conductivity of metallic iron filters: Modeling gone astray. Water 2016, 8, 162. [Google Scholar] [CrossRef] [Green Version]
- Hammonds, P. An Introduction to Corrosion and its Prevention (Chapter 4). Compr. Chem. Kinet. 1989, 28, 233–279. [Google Scholar]
- Lufingo, M.; Ndé-Tchoupé, A.I.; Hu, R.; Njau, K.N.; Noubactep, C. A novel and facile method to characterize the suitability of metallic iron for water treatment. Water 2019, 11, 2465. [Google Scholar] [CrossRef] [Green Version]
- Wakatsuki, T.; Esumi, H.; Omura, S. High performance and N, P removable on-site domestic wastewater treatment system by multi-soil-layering method. Water Sci. Technol. 1993, 27, 31–40. [Google Scholar] [CrossRef]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Phosphate removal from agricultural tile drainage with iron enhanced sand. Water 2017, 9, 672. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Bailey, E.H.; Mooney, S.J. Quantification of changes in zero valent iron morphology using X-ray computed tomography. J. Environ. Sci. 2013, 25, 2344–2351. [Google Scholar] [CrossRef]
- Özer, A.; Altundogan, H.S.; Erdem, M.; Tümen, F. A study on the Cr (VI) removal from aqueous solutions by steel wool. Environ. Pollut. 1997, 97, 107–112. [Google Scholar] [CrossRef]
- Campos, V. The effect of carbon steel-wool in removal of arsenic from drinking water. Environ. Geol. 2002, 42, 81–82. [Google Scholar] [CrossRef]
- Cornejo, L.; Lienqueo, H.; Arenas, M.; Acarapi, J.; Contreras, D.; Yáñez, J.; Mansilla, H.D. In field arsenic removal from natural water by zero-valent iron assisted by solar radiation. Environ. Pollut. 2008, 156, 827–831. [Google Scholar] [CrossRef]
- Triszcz, J.M.; Porta, A.; Einschlag, F.S.G. Effect of operating conditions on iron corrosion rates in zero-valent iron systems for arsenic removal. Chem. Eng. J. 2009, 150, 431–439. [Google Scholar] [CrossRef]
- Rao, T.S.; Murthy, D.S. Removal of arsenic (V) from water by adsorption onto low-cost and waste materials. Int. J. Res. Eng. Technol. 2013, 2, 206–212. [Google Scholar]
- George, D.; Ahammed, M.A. Effect of zero-valent iron amendment on the performance of biosand filters. Water Supply 2019, 19, 1612–1618. [Google Scholar] [CrossRef]
- Ndé-Tchoupé, A.I.; Crane, R.A.; Mwakabona, H.T.; Noubactep, C.; Njau, K.N. Technologies for decentralized fluoride removal: Testing metallic iron based filters. Water 2015, 7, 6750–6774. [Google Scholar] [CrossRef] [Green Version]
- Ndé-Tchoupé, A.I. Design and Construction of Fe0-Based Filters for Households. Ph.D. Thesis, University of Douala, Douala, Cameroon, 2019. (In French). [Google Scholar]
- Varlikli, C.; Bekiari, V.; Kus, M.; Boduroglu, N.; Oner, I.; Lianos, P.; Lyberatos, G.; Icli, S. Adsorption of dyes on Sahara desert sand. J. Hazard. Mater. 2009, 170, 27–34. [Google Scholar] [CrossRef]
- James, B.R.; Rabenhorst, M.C.; Frigon, G.A. Phosphorus sorption by peat and sand amended with iron oxides or steel wool. Water Environ. Res. 1992, 64, 699–705. [Google Scholar] [CrossRef]
- Chiew, H.; Sampson, M.L.; Huch, S.; Ken, S.; Bostick, B.C. Effect of groundwater iron and phosphate on the efficacy of arsenic removal by iron-amended biosand filters. Environ. Sci. Technol. 2009, 43, 6295–6300. [Google Scholar] [CrossRef]
- Btatkeu-K, B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Determining the optimum Fe0 ratio for sustainable granular Fe0/sand water filters. Chem. Eng. J. 2014, 247, 265–274. [Google Scholar] [CrossRef]
- Saywell, L.G.; Cunningham, B.B. Determination of iron: Colorimetric o-phenanthroline method. Ind. Eng. Chem. Anal. Ed. 1937, 9, 67–69. [Google Scholar] [CrossRef]
- Fortune, W.B.; Mellon, M.G. Determination of iron with o-phenanthroline. Ind. Eng. Chem. Anal. Ed. 1938, 10, 60–64. [Google Scholar] [CrossRef]
- Mackenzie, P.D.; Horney, D.P.; Sivavec, T.M. Mineral precipitation and porosity losses in granular iron columns. J. Hazard. Mater. 1999, 68, 1–17. [Google Scholar] [CrossRef]
- Santisukkasaem, U.; Das, D.B. A non-dimensional analysis of permeability loss in zero-valent iron permeable reactive barrier (PRB). Transp. Porous Media 2019, 126, 139–159. [Google Scholar] [CrossRef] [Green Version]
- Noubactep, C.; Temgoua, E.; Rahman, M.A. Designing iron-amended biosand filters for decentralized safe drinking water provision. CLEAN Soil Air Water 2012, 40, 798–807. [Google Scholar] [CrossRef] [Green Version]
- Miyajima, K. Optimizing the design of metallic iron filters for water treatment. Freib. Online Geosci. 2012, 32, 1–60. [Google Scholar]
- Lufingo, M. Investigation of Metallic Iron for Water Defluoridation. Master’s Thesis, Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania, 2019. [Google Scholar]
- Nakhla, G.; Farooq, S. Simultaneous nitrification–denitrification in slow sand filters. J. Hazard. Mater. 2003, 96, 291–303. [Google Scholar] [CrossRef]
- Murphy, H.M.; McBean, E.A.; Farahbakhsh, K. Nitrification, denitrification and ammonification in point-of-use biosand filters in rural Cambodia. J. Water Health 2010, 8, 803–817. [Google Scholar] [CrossRef] [Green Version]
- Heimann, S.; Ndé-Tchoupé, A.I.; Hu, R.; Licha, T.; Noubactep, C. Investigating the suitability of Fe0 packed-beds for water defluoridation. Chemosphere 2018, 209, 578–587. [Google Scholar] [CrossRef]
- Liu, X.; Millero, F.J. The solubility of iron hydroxide in sodium chloride solutions. Geochim. Cosmochim. Acta 1999, 63, 3487–3497. [Google Scholar] [CrossRef]
- Ghauch, A. Iron-based metallic systems: An excellent choice for sustainable water treatment. Freib. Online Geosci. 2015, 32, 1–80. [Google Scholar]
- Btatkeu-K, B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Characterizing the impact of MnO2 on the efficiency of Fe0-based filtration systems. Chem. Eng. J. 2014, 250, 416–422. [Google Scholar] [CrossRef]
- Bischof, G. Über das Reinigen des Wassers und über die Wirkung des Eisenschwammes auf unreines Wasser. Polytech. J. 1873, 210, 40–59. [Google Scholar]
- Bischof, G. On putrescent organic matter in potable water. Proc. R. Soc. Lond. 1877, 26, 258–261. [Google Scholar]
- Bischof, G. On putrescent organic matter in potable water II. Proc. R. Soc. Lond. 1878, 27, 152–156. [Google Scholar]
- Notter, J.L. The purification of water by filtration. Br. Med. J. 1878, Oct. 12, 556–557. [Google Scholar] [CrossRef] [Green Version]
- Hatton, F. On the oxidation of organic matter in water by filtration through various media; and on the reduction of nitrates by sewage, spongy iron, and other agents. J. Chem. Soc. Trans. 1881, 39, 258–276. [Google Scholar] [CrossRef] [Green Version]
- Bache, R.M. Possible sterilization of city water. Proc. Am. Philos. Soc. 1891, 29, 26–39. [Google Scholar]
- Leffmann, H. The purification of water by metallic iron. In Proceedings of the 11th Annual Meeting of the American Water Works Association, Philadelphia, PA, USA, 14–16 April 1991; pp. 163–171. [Google Scholar]
- Tweeddale, W. Water purification. Trans. Ann. Meet. Kans. Acad. Sci. 1898, 16, 48–52. [Google Scholar] [CrossRef]
- Baker, M.N. Sketch of the history of water treatment. J. Am. Water Works Assoc. 1934, 26, 902–938. [Google Scholar] [CrossRef]
- Bojic, A.; Purenovic, M.; Kocic, B.; Perovic, J.; Ursic-Jankovic, J.; Bojic, D. The inactivation of escherichia coli by microalloyed aluminium based composite. Facta Univ. Phys. Chem. Technol. 2001, 2, 115–124. [Google Scholar]
- Lee, C.; Kim, J.H.; Lee, W.I.; Nelson, K.L.; Yoon, J.; Sedlak, D.L. Bactericidal effect of zero-valent iron nanoparticles on escherichia coli. Environ. Sci. Technol. 2008, 42, 4927–4933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, M.; Yao, M. Use of zero-valent iron nanoparticles in inactivating microbes. Water Res. 2009, 43, 5243–5251. [Google Scholar] [CrossRef]
- Crampon, M.; Joulian, C.; Ollivier, P.; Charron, M.; Hellal, J. Shift in natural groundwater bacterial community structure due to zero-valent iron nanoparticles (nZVI). Front. Microbiol. 2019, 10, 533. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Wang, J.; Sun, H.; Wang, S.; Liao, X.; Wang, J.; An, T. Roles of extracellular polymeric substances in the bactericidal effect of nanoscale zero-valent iron: Trade-offs between physical disruption and oxidative damage. Environ. Sci. Nano 2019, 6, 2061–2073. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.; Jiang, Y.; Shen, W.; Jia, F.; Wang, S.; Liao, X.; Zhang, L. Rapid aerobic inactivation and facile removal of escherichia coli with amorphous zero-valent iron microspheres: Indispensable roles of reactive oxygen species and iron corrosion products. Environ. Sci. Technol. 2019, 53, 3707–3717. [Google Scholar] [CrossRef]
- You, Y.; Han, J.; Chiu, P.C.; Jin, Y. Removal and inactivation of waterborne viruses using zerovalent iron. Environ. Sci. Technol. 2005, 39, 9263–9269. [Google Scholar] [CrossRef]
- Ingram, D.T.; Callahan, M.T.; Ferguson, S.; Hoover, D.G.; Shelton, D.R.; Millner, P.D.; Camp, M.J.; Patel, J.R.; Kniel, K.E.; Sharma, M. Use of zero-valent iron biosand filters to reduce E. coli O157:H12 in irrigation water applied to spinach plants in a field setting. J. Appl. Microbiol. 2012, 112, 551–560. [Google Scholar] [CrossRef]
- Shi, C.; Wei, J.; Jin, Y.; Kniel, K.E.; Chiu, P.C. Removal of viruses and bacteriophages from drinking water using zero-valent iron. Sep. Purif. Technol. 2012, 84, 72–78. [Google Scholar] [CrossRef]
- Marik, C.M.; Anderson-Coughlin, B.; Gartley, S.; Craighead, S.; Kniel, K.E. The efficacy of zero valent iron-sand filtration on the reduction of Escherichia coli and Listeria monocytogenes in surface water for use in irrigation. Environ. Res. 2019, 173, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Gwenzi, G.; Sipowo, R.; Noubactep, C. Water treatment using metallic iron: A tutorial review. Processes 2019, 7, 622. [Google Scholar] [CrossRef] [Green Version]
- Tucker, W.G. The purification of water by chemical treatment. Science 1892, 20, 34–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mwakabona, H.T.; Ndé-Tchoupé, A.I.; Njau, K.N.; Noubactep, C.; Wydra, K.D. Metallic iron for safe drinking water provision: Considering a lost knowledge. Water Res. 2017, 117, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, N.; Deluchat, V.; Wazne, M.; Mallet, M.; Courtin-Nomade, A.; Kazpard, V.; Baudu, M. Phosphate removal from aqueous solution using ZVI/sand bedreactor: Behavior and mechanism. Water Res. 2016, 99, 56–65. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Noubactep, C.; Mukome, F.N.D. Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision. J. Environ. Manag. 2017, 197, 732–749. [Google Scholar] [CrossRef]
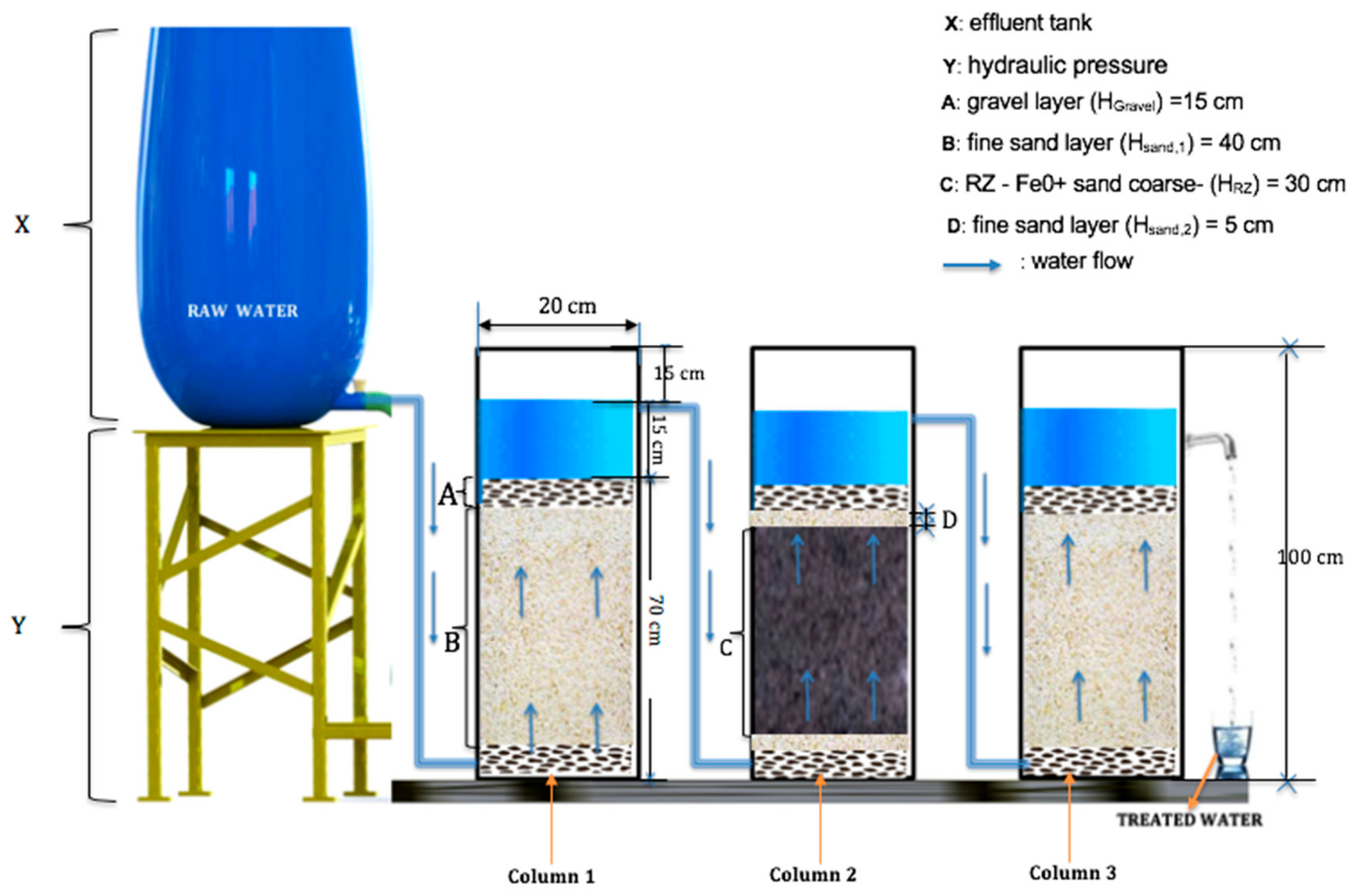

| Designation | Height (cm) | ||
|---|---|---|---|
| Column 1 | Column 2 | Column 3 | |
| Gravel (Hgravel 1) | 15.0 | 15.0 | 15.0 |
| Sand (HSand2) | - | 5.0 | - |
| Sand (HSand1) | 40.0 | - | 40.0 |
| RZ (Fe0/coarse sand) (HRZ) | - | 30.0 | - |
| Sand (HSand2) | - | 5.0 | - |
| Gravel (Hgravel 2) | 15.0 | 15.0 | 15.0 |
| Parameter | Unit | Well Water | WHO (Guideline) |
|---|---|---|---|
| Turbidity | (NTU) | 35 ± 2 | <5 |
| Conductivity | (μS cm−1) | 296 ± 7 | 250 |
| Total Iron | (mg L−1) | 1.45 ± 0.25 | <0.2 |
| Nitrate | (mg L−1) | 23.5 ± 4.5 | <50 |
| pH value (25 °C) | (-) | 4.9 ± 0.2 | 6.5–8.5 |
| Total coliform (TC) | (UFC mL−1) | 1948.6 ± 45 | 0.0 |
| Fecal coliforms (FC) | (UFC mL−1) | 1495.0 ± 97 | 0.0 |
| t | pH | ϕ | Total Coliforms | Turbidity | Nitrate |
|---|---|---|---|---|---|
| (d) | (-) | (L h−1) | (CFU/100 mL) | (NTU) | (mg L−1) |
| Raw water | 4.9 | 0.0 | 1,950 | 35.0 | 24.0 |
| 1 | 6.6 | 20.0 | 0.11 | 1.09 | 0.21 |
| 15 | 6.7 | 20.0 | 0.11 | 1.07 | 0.19 |
| 30 | 6.6 | 20.0 | 0.11 | 1.00 | 0.20 |
| 45 | 6.8 | 20.0 | 0.10 | 1.00 | 0.20 |
| 60 | 6.8 | 19.6 | 0.10 | 1.01 | 0.15 |
| 75 | 6.9 | 19.6 | 0.10 | 1.00 | 0.15 |
| 90 | 6.7 | 18.8 | 0.10 | 0.99 | 0.12 |
| 105 | 6.6 | 18.8 | 0.08 | 0.80 | <0.1 |
| 120 | 6.8 | 17.9 | 0.08 | 0.90 | <0.1 |
| 135 | 7.0 | 17.5 | 0.08 | 0.90 | <0.1 |
| 150 | 7.1 | 17.5 | 0.08 | 0.80 | <0.1 |
| 165 | 7.3 | 17.1 | 0.08 | 0.80 | <0.1 |
| 180 | 7.4 | 17.1 | 0.08 | 0.80 | <0.1 |
| 195 | 7.5 | 16.7 | 0.02 | 0.90 | <0.1 |
| 210 | 7.5 | 16.7 | <0.02 | 0.90 | <0.1 |
| 225 | 7.5 | 15.8 | <0.02 | 0.80 | <0.1 |
| 240 | 7.6 | 15.8 | <0.02 | 0.90 | <0.1 |
| 255 | 7.6 | 15.6 | <0.02 | 0.90 | <0.1 |
| 270 | 7.7 | 15.6 | <0.02 | 0.80 | <0.1 |
| 285 | 7.5 | 15.0 | <0.02 | 0.80 | <0.1 |
| 300 | 7.6 | 15.8 | <0.02 | 0.70 | <0.1 |
| 315 | 7.9 | 15.2 | <0.02 | 0.80 | <0.1 |
| 330 | 8.1 | 13.8 | <0.02 | 0.70 | <0.1 |
| 345 | 8.3 | 13.0 | <0.02 | 0.80 | <0.1 |
| 360 | 8.6 | 12.5 | <0.02 | 0.70 | <0.1 |
| L | D | Fe0/Sand | ϕ0 | t | Fe0 Type | miron | Reference |
|---|---|---|---|---|---|---|---|
| (cm) | (cm) | (vol/vol) | (L h−1) | (d) | (-) | (kg) | |
| 10 | 3.8 | 50:50 | 0.06 | 10 | granular | 0.15 | [75] |
| 20 | n.s. | 10:90 | 0.03 | 300 | SW | 0.26 | [22] |
| 0.77 | 0.14 | 50:50 * | n.s. | 15 | granular | 23.0 | [76] |
| 10 | 3.8 | 50:50 | 4.38 | n.s. | granular | n.s. | [77] |
| non SI | non SI | 35:65 | 222 | 154 | granular | n.s. | [78] |
| n.s. | n.s. | n.s. | n.s. | 120 | granular | 7.5 | [40] |
| 100 | 20 | 10:90 | 20.4 | 365 | SW | 0.30 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tepong-Tsindé, R.; Ndé-Tchoupé, A.I.; Noubactep, C.; Nassi, A.; Ruppert, H. Characterizing a Newly Designed Steel-Wool-Based Household Filter for Safe Drinking Water Provision: Hydraulic Conductivity and Efficiency for Pathogen Removal. Processes 2019, 7, 966. https://doi.org/10.3390/pr7120966
Tepong-Tsindé R, Ndé-Tchoupé AI, Noubactep C, Nassi A, Ruppert H. Characterizing a Newly Designed Steel-Wool-Based Household Filter for Safe Drinking Water Provision: Hydraulic Conductivity and Efficiency for Pathogen Removal. Processes. 2019; 7(12):966. https://doi.org/10.3390/pr7120966
Chicago/Turabian StyleTepong-Tsindé, Raoul, Arnaud Igor Ndé-Tchoupé, Chicgoua Noubactep, Achille Nassi, and Hans Ruppert. 2019. "Characterizing a Newly Designed Steel-Wool-Based Household Filter for Safe Drinking Water Provision: Hydraulic Conductivity and Efficiency for Pathogen Removal" Processes 7, no. 12: 966. https://doi.org/10.3390/pr7120966
APA StyleTepong-Tsindé, R., Ndé-Tchoupé, A. I., Noubactep, C., Nassi, A., & Ruppert, H. (2019). Characterizing a Newly Designed Steel-Wool-Based Household Filter for Safe Drinking Water Provision: Hydraulic Conductivity and Efficiency for Pathogen Removal. Processes, 7(12), 966. https://doi.org/10.3390/pr7120966






