Abstract
Carbon capture and sequestration (CCS) is taking the lead as a means for mitigating climate change. It is considered a crucial bridging technology, enabling carbon dioxide (CO2) emissions from fossil fuels to be reduced while the energy transition to renewable sources is taking place. CCS includes a portfolio of technologies that can possibly capture vast amounts of CO2 per year. Mineral carbonation is evolving as a possible candidate to sequester CO2 from medium-sized emissions point sources. It is the only recognized form of permanent CO2 storage with no concerns regarding CO2 leakage. It is based on the principles of natural rock weathering, where the CO2 dissolved in rainwater reacts with alkaline rocks to form carbonate minerals. The active alkaline elements (Ca/Mg) are the fundamental reactants for mineral carbonation reaction. Although the reaction is thermodynamically favored, it takes place over a large time scale. The challenge of mineral carbonation is to offset this limitation by accelerating the carbonation reaction with minimal energy and feedstock consumption. Calcium and magnesium silicates are generally selected for carbonation due to their abundance in nature. Industrial waste residues emerge as an alternative source of carbonation minerals that have higher reactivity than natural minerals; they are also inexpensive and readily available in proximity to CO2 emitters. In addition, the environmental stability of the industrial waste is often enhanced as they undergo carbonation. Recently, direct mineral carbonation has been investigated significantly due to its applicability to CO2 capture and storage. This review outlines the main research work carried out over the last few years on direct mineral carbonation process utilizing steel-making waste, with emphasis on recent research achievements and potentials for future research.
1. Introduction
Fossil fuels are used as the main source of energy globally, and now they supply over 80% of the world energy demand [1]. Fossil fuels are expected to remain the most used energy source for years to come. This is due to the ever-increasing demand for energy created by the thriving economies around the globe. International Energy Agency reported a total energy demand of 574 exajoules globally in 2014 [2]. Although there are multiple sources for atmospheric CO2, human activities, such as transportation and electricity generation, which directly burn several kinds of fossil fuels (including coal, oil, and natural gas), release more CO2 into the atmosphere. This leads to increases in the earth temperature and in turn causes global warming. Hence, mitigating CO2 emissions is a key to decrease global warming and sustain a better future for humanity [3]. Carbon capture and storage serves as the main technology for mitigating carbon emissions. Numerous conventional CO2 capture technologies based on a post-combustion approach are being used in the industry. Separation based methods, such as absorption, adsorption, and membrane separation, are the most utilized separation technologies available [4,5,6,7]. Mineral carbonation is one of few technologies that work as both capture and storage technologies [8]. It is based on the principles of natural rock weathering, where the CO2 dissolved in rainwater reacts with alkaline rocks to form carbonate minerals. The active alkaline elements (Ca/Mg) are the fundamental reactants for mineral carbonation reaction. Although the reaction is thermodynamically favored, it takes place over a large time scale. The challenge of mineral carbonation is to offset this limitation by accelerating the carbonation reaction with minimal energy and feedstock consumption. Calcium and magnesium silicates are generally selected for carbonation due to their abundance in nature. Industrial waste residues emerge as an alternative source of carbonation minerals that have higher reactivity than natural minerals; they are also inexpensive and readily available in proximity to CO2 emitters. In addition, the environmental stability of the industrial waste is often enhanced as they undergo carbonation. Recently, mineral carbonation has been investigated significantly, due to its applicability to CO2 capture and storage. Despite the growing interest in mineral carbonation research, there have not been any focused reviews that assess the status of CO2 sequestration using steel-making waste. In this review, mineral carbonation using steel-making waste is reviewed in the light of different process parameters and their effect on CO2 uptake. Potentials for future research in the area are highlighted.
1.1. CO2 Storage
Several storage techniques are used to store CO2, and the most feasible option to do so is geological sequestration [1]. Literature work investigating geological CO2 storage has seen a substantial increase in the last decade [2]. Practically, over 1 million ton CO2 is being sequestered in 14 individual different locations around the globe [3]. Estimates of CO2 storage capacity varies depending on the region at which the study has been conducted. Nonetheless, the capacity is in the range of 100–20,000 giga ton CO2 worldwide [4]. One of the mature CO2 storage techniques is to inject it into depleted gas or oil reservoirs. Carbon dioxide is used to increase reservoirs pressure to produce enough driving force to push the gas/oil out of it. In other words, it enhances oil recovery in active wells by extracting the residual oil left. Additionally, CO2 can be used to recover natural gas (methane, CH4) trapped in coal beds. The main premise behind the idea is that CH4 can be quickly displaced from coal by carbon dioxide injection, allowing CO2 to be stored in the porous structure of the coal bed [5]. Injecting CO2 into saline aquifers is also a viable option that commercially exists with an acceptable capacity [6,7]. Carbon dioxide is usually injected in its supercritical conditions [8]. At these conditions, CO2 is buoyant relative to porous rocks and saline aquifers. Thus, there is always a possibility that buoyant CO2 could leak to the surface and cause catastrophic environmental impacts. Most critically, monitoring programs for post-injection are limited and do not provide long-term detectability of the gas that can potentially escape from the storage medium [9]. Hence, these approaches cannot be taken for granted and considered as permanent and safe CO2 storage solutions. Mineral carbonation is one approach that can provide long-term storage solution in addition to being CO2 leak-free. This is due to the fact that carbonates are in a lower energy state than CO2 [10]. More importantly, it possesses extremely large sequestration capacity compared to other geological storage options, as indicated in Table 1.

Table 1.
Storage capacities for several geological storage options [11].
1.2. Mineral Carbon Sequestration
Mineral carbon sequestration is based on the principles of the natural carbonation process of natural rocks, where the CO2 dissolved in rainwater forms a weak carbonic acid. Consequently, alkali and alkaline earth metals (i.e., Ca and Mg) neutralize the acid to from insoluble carbonate minerals [12,13]. Sequestration happens in several alkaline minerals, such as calcite (CaCO3), dolomite (Ca/Mg(CO3)2), magnesite (MgCO3), siderite (FeCO3), and serpentine (Mg3Si2O5(OH)4) [14]. For mineral carbonation, having a sufficient amount of a certain natural mineral is an essential factor. Hence, magnesium-based silicates are utilized since they are available in considerable amounts globally [11]. However, increasing CO2 levels led to more CO2 absorption by the oceans, hence increasing its acidity by 30% since the industrial era started [15]. Hence, this limits the natural carbonation process. The formed carbonates are in solid form resulting from exothermic reaction (Equation (1)) and a certain amount of heat is released, depending on the type of metal oxide reacting.
Carbonates require a high amount of energy to decompose back into CO2. Hence, carbonates can be considered as thermodynamically stable CO2 sink [16]. CO2 will be fixed permanently without further monitoring to check its stability [17]. Table 2 shows the composition of different minerals rocks and the mass of CO2 that can be sequestered by a unit mineral mass (mass CO2/mass mineral). This ratio is based on the theoretical basis and considered as the maximum potential carbonation capacity for the specific mineral. Different minerals have different ratios according to their alkali metal content. Whether or not the maximum capacity can be reached, it is subject to the carbonation process and different operating parameters.

Table 2.
Carbonation potential for different naturally occurring minerals [18].
Mineral sequestration technique was first proposed by Seiftriz [19]. The proposed idea suggested introducing high purity CO2 to accelerate the carbonation process. This ensured that carbonation time can be shortened from geological time scale to hours or minutes. Since then, the literature work has expanded greatly. However, it is clear that the research progress is facing challenges in enhancing the carbonation process to be viable to deploy on a large scale, as shall be demonstrated in the following sections. Nonetheless, the technique possesses several advantages over other sequestration techniques such as ocean and geologic sequestration, due to concerns over long term carbon leakage, as described previously [20]. Mineral carbonation produces more stable products that have the potential to be profitable and usually produced in fewer steps than other techniques. Additionally, the heat of the reaction can be further utilized as a source of energy.
Mineral sequestration techniques are often divided into in situ or ex situ manner. In situ sequestration requires injecting CO2 into underground reservoirs to start a reaction with the existing underground minerals to form carbonates. Ex situ sequestration is related to carbonation process above the ground, where the raw natural mineral needs to be mined and treated before it undergoes the carbonation process. The scope of this work focuses on ex situ mineral carbonation and its related mechanisms and applications.
Although naturally occurring minerals have the potential to sequester huge amounts of CO2 due to their abundance, it is not practically feasible due to the cost of extracting and pretreatment of the minerals and the impacts associated with it [21]. In addition to numerous process challenges in terms of carbonation efficiency and energy intensity (temperature and pressure). Alkaline industrial waste rich with Mg2+ and Ca2+ is an attractive alternative for CO2 sequestration. It can be used to imitate mineral carbonation without the additional mining cost associated with natural rocks. Even so, alkaline waste is available in less amounts than natural minerals. It is available at lower cost, higher reactivity, and uptake capacity, and less pretreatment is required. Table 3 summarizes the most studied industrial alkaline waste and their alkali earth metal composition (Mg and Ca), in addition to their production rate per year and CO2 emissions associated with their production. Examples of the industrial wastes include fly ash, such as coal and shale oil ashes, cement industry waste dust, and steel slag.

Table 3.
Alkaline solids studied in the literature, their composition and global production per year and the CO2 emission.
Globally, cement industries account for 5% of the total CO2 emissions [39,40]. Furthermore, the steel industry accounts for 7% of CO2 emissions globally [41]. There are four main types of steelmaking slags, including blast furnace (BF), basic oxygen furnace (BOF), electric arc furnace (EAF), and ladle furnace (LF) slags. The slags consist of several oxides, primarily calcium, iron, and magnesium oxides that are present in different phases. On average, manufacturing 1 ton of steel produces approximately 420 kg of BOF and 180 kg of EAF [42]. There are two main approaches for ex situ alkaline waste carbonation: direct and indirect; each one has several sub-classifications based on the carbonation technique.
Direct carbonation technique implies that the carbonation process happens in one single step. On the other hand, indirect carbonation has more than one step (two or more) that usually involves pre-treatment of used minerals. Typically, mineral ores undergo a pre-treatment process where the reactive chemical components (i.e., alkali earth metals) in the rocks are separated for the mineral core. Pre-treatment usually involves mining, grinding, and activation of the rock minerals (Table 4). The end product of the pre-treatment process is almost pure carbonate form of the mineral. Then, the mineral carbonate is reacted with carbon dioxide in a separate step [12]. Table 5 shows the CO2 uptake capacity using natural rocks. It lists numerous studies that investigated the carbonation efficiency of minerals using natural minerals (i.e., olivine and serpentine) by dry and aqueous carbonation routes. The table includes details about the process parameters and carbonation conversion.

Table 4.
Description of pretreatment process.

Table 5.
Mineral carbonation studies for natural minerals.
1.3. Indirect Carbonation
The term indirect mineral carbonation refers to the carbonation process that happens in two or more stages. Extraction of the reactive elements (Mg and Ca) form the mineral solid matrix is an essential step in indirect carbonation. Typically, strong acids are used as extracting agents. The extracted mineral then undergoes the carbonation process by reacting with CO2. One of the main advantages of using extraction is that it allows the production of almost pure mineral, as other impurities available in the natural mineral core can be removed after the reactive metal is extracted [50]. Numerous methods exist that can achieve mineral extraction, such as using acids, molten salts, caustic soda, and bioleaching. Table 6 lists all extraction methods studied in the literature with their associated extraction reactions. Every extraction technique possesses its intrinsic advantage and disadvantages. For example, using HCl produces pure alkali earth metal; however, it is significantly energy intensive if the recovery of HCl is required. Inversely, molten salts are less energy intensive in terms of regeneration. Nonetheless, molten salts are more corrosive compared to HCl [12]. Another approach of indirect carbonation is pH swing. pH swing refers to the extraction of mineral carbonate from the solid matrix at low pH condition in the first stage. In a second stage, the pH of the extraction solution is raised to improve carbonate formation [51]. Although acids are able to extract significant amounts of calcium and magnesium ions from the feedstock, as explained previously, pH plays a great role in the precipitation of calcium and magnesium carbonates. Therefore, increasing the solution pH to approximately 10 in the second stage of mineral extracting helps to increase the rate of carbonate precipitation [10]. Hence, due to the added cost of implementing a second process step and the extensive use of extraction agents, direct carbonation is a more practical mineral carbonation option. In this review, direct aqueous carbonation using steel-making waste is being reviewed.

Table 6.
Description of different indirect carbonation methods.
2. Direct Carbonation
Direct carbonation includes the reaction of CO2 with a suitable feedstock or Calcium/Magnesium rich solid residue in a single step. It is relatively easier to implement compared to indirect carbonation. Hence, it has the potential to be used in industrial scale. The following sections explain the working principles of direct carbonation, discussing the operational parameters that have the most impact on the carbonation capacity.
2.1. Gas-Solid Carbonation
The reaction of gaseous CO2 with solid minerals is the most basic and straightforward approach of direct carbonation, first studied by Lackner et al [52]. In the case of olivine carbonation:
The reaction suffers from very slow reaction rates. Hence, it is usually carried over an elevated temperature and pressure. However, due to thermodynamics limitations, the temperature is restricted between 170–400 °C for most natural minerals, as equilibrium shifts to the reactant side with increasing temperature [52]. Hence, the maximum carbonation temperature is a function of CO2 partial pressure and the specific mineral used (Table 7). The process high temperature requirement can be further utilized to generate steam that will be used to produce electricity [12]. Nevertheless, the process slow kinetics is still the main obstacle hindering further progress even at high temperature and pressure.

Table 7.
Maximum carbonation temperature for several minerals at CO2 partial pressure of 1 bar [59].
Thus, the experimentally obtained carbonation rate of direct dry rock minerals carbonation is insignificant, even at elevated temperature and pressure [12]. Kwon et al. [60] reported that introducing moisture to the flue gas in dry carbonation process can increase the carbonation rate significantly. However, due to low CO2 sequestering efficiency, 8 tons of olivine would be required to capture 1 ton of CO2. This renders the process practically not viable and reduces its wide scale applicability. Hence, focus shifted on dry carbonation of pure magnesium and calcium oxides [52]. Nevertheless, the limited availability of the used minerals hindered the research progress. However, alkali earth metals can be extracted from the mineral rocks and industrial wastes. This adds another process step (extraction) to the overall process scheme. Hence, the process becomes an indirect carbonation process, which is discussed previously.
2.2. Direct Aqueous Carbonation
As explained in the introduction section, natural carbonation occurs when CO2 is dissolved in rain water according the following equation:
The aqueous solution becomes more acidic due to the presence of protons () resulting from CO2 solubility in water. Hence, by imitating natural carbonation, carbonation of natural minerals could take place in aqueous media in a single stage process. When the rock mineral is placed in aqueous solution, calcium or magnesium element in the solid matrix leaches from mineral ore according to Equation (4):
Eventually, mineral carbonate is formed when the mineral reacts with dissolved CO2 (bicarbonate proton)
When studying direct aqueous carbonation, a good practice is to study the process parameters, such as temperature, pressure, and solution medium, that can maximize CO2 uptake capacity. For aqueous carbonation specifically, increasing process pressure enhances CO2 solubility in the aqueous medium. Therefore, according to Equation (5), the reaction will shift towards the products side, which is highly desirable. On the other hand, increasing the reaction rates can be enhanced by elevating temperature. However, this applies to a certain extent due to the decline in CO2 solubility in the solution with increasing temperature. In other words, CO2 solubility in a certain solution dictates the upper limit at which the process temperature can be elevated. Carbonation conversion is a way to measure the carbonation efficiency and is defined according to the following equation:
Hence, the efficiency is reported on the basis of magnesium and calcium content of the mineral, not the total quantity of the used mineral.
Studies investigating direct aqueous carbonation reaction mechanism revealed that aqueous carbonation proceeds in two distinct steps, as opposed to 1 step in dry carbonation [61]:
- (1)
- Dissolution of alkali earth element into the solution (leaching step).
- (2)
- Formation of mineral carbonate (carbonation step).
Typically, leaching of alkali metal into aqueous solution is the rate limiting step. Nonetheless, altering the process parameters, such as temperature and pressure, can make the carbonation step the rate limiting step [62]. This is explained by the formation of the carbonation products on the surface of the minerals that will increase the mass transfer resistance between the dissolved CO2 and mineral core. Hence, controlling the dissolution rate and finding the best process parameters is a must to ensure sufficient carbonation efficiency. Different minerals have different dissolution rates [63]. The rate depends on the morphology of the mineral (surface area and structure) [64]. Thus, pre-treatment techniques stated in Table 4 can be used to enhance the dissolution rate, hence, the carbonation efficiency.
3. Steelmaking Waste Mineral Carbonation
Solid industrial wastes are generally alkaline and rich in Ca/Mg and can therefore be applied as an additional feedstock for mineral CO2 sequestration. The main advantages of industrial waste are that they are available at low to no costs in proximity to industrial emitters, almost no pre-treatment is needed, and they are more reactive in less energy intensive conditions. In addition, the end product of the sequestration can be used in several applications, i.e., as a construction material and in fertilizers. The fundamental working principles for mineral CO2 sequestration apply for industrial waste in the same way. In fact, the major elements of e.g., steel slag (Mg, Ca, Si, and Fe) are present in a comparable concentration as in natural rocks. However, trace metals and soluble salt concentrations are available in more quantities compared to the average composition of natural rocks. Thus, steel industry waste can undergo the same direct and indirect carbonation techniques previously explained. Presently, the research is going towards optimizing the uptake capacity of CO2 by modifying the operating parameters including pressure, temperature, liquid-to-solid ratio, CO2 gas flowrate, solid particle size, and pretreatment. Table 8 presents a summary of steel making waste which have been tested as mineral carbonation in terms of feedstocks; feed composition, the experimental CO2 capture capacity, and the different process conditions were investigated. The mineral carbonation uptake is a function of process temperature, CO2 partial pressure, and steel waste surface area, which affect the carbon dioxide dissolution rate, the diffusion rate of ions through the reaction with steel slag. The pH value is an additional essential parameter in mineral carbonation process. Optimum pH for aqueous carbonation is achieved at pH of 10 [65]. pH of the process influences the carbonation reaction, as the reaction is more favorable in alkaline mediums. In addition, the pH decreases continuously as carbonation, due to CO2 being dissociated into the solution. Eventually, the pH value remains unchanged at around 7 after the carbonation process ends. This signifies that the mineral carbonation process will not proceed in acidic mediums. Figure 1 summarizes the different aspects that affect the ex-situ mineral carbonation process, such as different reactor types and process parameters.

Table 8.
Summary of the tested steel slag for mineral carbonation in terms of CO2 capture capacity, feedstock composition and process parameters.
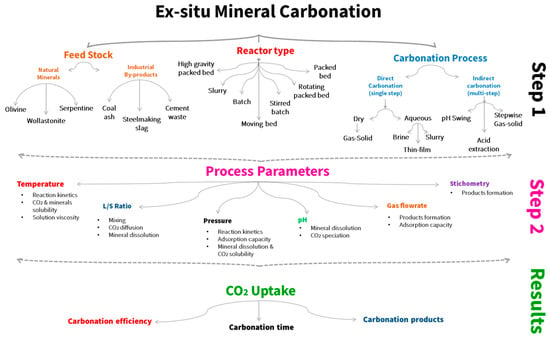
Figure 1.
Ex-situ mineral carbonation literature summary.
3.1. Temperature and Particle Size
Huijgen et al. [21] were among the first to utilized steel slag as feed stock for mineral carbonation. The authors studied parameters that could affect the carbonation rate, which include reaction temperature and steel slag particle size. An autoclave reactor was used to carry out the reactor, and a 450 mL of the slurry was used with a liquid to solid ratio of 20 kg/kg. A maximum conversion of 70% of the calcium in the feed stock was carbonated at a pressure of 19 bar and temperature of 100 °C was achieved. The authors reported that at higher temperatures leaching of calcium from steel slag components will proceed faster, hence increasing the reaction rate, but the solubility of CO2 in the solution decreases. This was also observed by Han et al. [65]. To achieve this carbonation percentage, the particle size was reduced from <2 mm to <38 μm. Reducing the particle size will produce more surface area for the carbonation reaction to occur, hence increasing the conversion. Particle size and specific surface area are among the most important factors affecting the dissolution kinetics of any kind of material. Mineral particle size determines its reactive surface area in addition to its leaching mechanisms. Typically, grinding is used to achieve a specific particle size. However, it is an energy intensive process. Hence, determining the optimal particles size will help in reducing the process cost in addition to increasing its efficiency. Baciocchi et al. [43] reported that the parameter that most affected the CO2 uptake of the slag was particle size, especially the specific surface of the particles. An increase in temperature also had a positive effect, achieving a maximum uptake of 130 g CO2/kg slag. The authors reported that an average particle size of less than 150 micrometers is considered as optimum.
3.2. Liquid to Solid Ratio
Liquid to solid ratio is defined by the amount of steel slag that is being utilized in a certain amount of aqueous medium (mass/mass). Revathy et al. reported that the carbonation efficiency increased when the S/L ratio decreased. The results indicate that when L/S is increased from 5 to 10 g/g, the carbonation degree of steel slag also increases. A further L/S increase causes a decrease in the carbonation degree of steel slag. This is caused due to the presence of extra liquid that leads to dilution of calcium ion concentration in the aqueous medium [66]. In a similar manner, the sequestration capacity of slag water slurry increased with the L/S ratio from 2 to 10, after which it decreased. This is due to the fact that a high amount of water inside the reactor causes blocking in the diffusion of gas molecules in the slurry [67].
3.3. Pressure
At constant temperature, CO2 gas solubility increases along with pressure according to Henry’s law. Hence, CO2 molecules that are involved in the carbonation process will be more as the pressure is elevated. The effect of pressure on CO2 uptake was tested at 10, 50, 100, and 150 bar under the same condition (50 °C, L/S = 1) by Han et al. [65]. The carbonation conversion was found to be 21% and 50.2% for 10 and 150 bar, respectively. Similarly, Ghacham et al. [68] reported that a higher CO2 partial pressure caused more CO2 to be soluble in aqueous medium, forming carbonic acid and consequently increasing the bicarbonate ions formation. Therefore, more bicarbonates will react with calcium ions. Hence, higher CO2 uptake. Fagerlund et al. [55] reported that that the carbonation rate and degree might increase exponentially with time, as long as a high enough CO2 pressure could be maintained. Additionally, high pressure will cause the reaction time to be shorter, hence, having lower carbonation time. Similarly, Eloneva et al. [69] reported shorter reaction times as the partial pressure of CO2 is increased.
4. Summary and Future Prospective
Carbon capture and sequestration can be achieved through different techniques that have the potential to capture substantial amounts of CO2 and help reduce its emissions. Mineral carbonation is evolving as a possible candidate to sequester CO2 from medium-sized emissions point sources. The process of natural carbonation forms the basis of mineral carbonation process. Active alkaline elements (Ca and Mg) are the fundamental reactants for mineral carbonation reaction. Industrial alkaline wastes, such as steel-making waste, are rich with these alkaline compounds, especially calcium and magnesium oxides. Hence, they are studied in the literature as a possible mineral carbonation process feedstock. Several parameters govern the carbonation process, including process temperature, pressure, and liquid to solid ratio. There is still a room for improvement by targeting higher CO2 uptake value. This can be achieved by using a different aqueous medium to carry out the carbonation process, i.e., reject brine and the development of reactor systems that minimize mass transfer limitations. Optimizing the interactions between process parameters, such as the interplay between temperature, pressure, and the degree of mixing, will contribute to the carbonation process. In addition, studying the adsorption behavior of CO2 on other elements, such as iron oxide, will give more insights into increasing CO2 uptake.
Author Contributions
Conceptualization, M.H.E.-N. and M.H.I.; Formal Analysis, M.H.I. and A.B.; Investigation, A.B., M.H.E.-N. and M.H.I.; Resources, S.S.A. and Z.Z.; Writing-Original Draft Preparation, M.H.E.-N. and M.H.I.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Gale, J.; Abanades, J.C.; Bachu, S.; Jenkins, C. Special Issue commemorating the 10th year anniversary of the publication of the Intergovernmental Panel on Climate Change Special Report on CO2 Capture and Storage. Int. J. Greenh. Gas Control 2015, 40, 1–5. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Chunbao Charles, X.U.; Cang, D.Q. A brief overview of low CO2 emission technologies for iron and steel making. J. Iron Steel Res. Int. 2010, 17, 1–7. [Google Scholar]
- White, C.M.; Strazisar, B.R.; Granite, E.J.; Hoffman, J.S.; Pennline, H.W. Separation and capture of CO2 from large stationary sources and sequestration in geological formations—Coalbeds and deep saline aquifers. J. Air Waste Manag. Assoc. 2003, 53, 645–715. [Google Scholar] [CrossRef] [PubMed]
- Torp, T.A.; Gale, J. Demonstrating storage of CO2 in geological reservoirs: The Sleipner and SACS projects. Energy 2004, 29, 1361–1369. [Google Scholar] [CrossRef]
- Maldal, T.; Tappel, I.M. CO2 underground storage for Snøhvit gas field development. Energy 2004, 29, 1403–1411. [Google Scholar] [CrossRef]
- Matter, J.M.; Kelemen, P.B. Permanent storage of carbon dioxide in geological reservoirs by mineral carbonation. Nat. Geosci. 2009, 2, 837–841. [Google Scholar] [CrossRef]
- Michael, K.; Golab, A.; Shulakova, V.; Ennis-King, J.; Allinson, G.; Sharma, S.; Aiken, T. Geological storage of CO2 in saline aquifers-A review of the experience from existing storage operations. Int. J. Greenh. Gas Control 2010, 4, 659–667. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Hamidi, H.; Junin, R.; Karaei, M.A. A review on carbon dioxide mineral carbonation through pH-swing process. Chem. Eng. J. 2015, 279, 615–630. [Google Scholar] [CrossRef]
- Metz, B.; Davidson, O.; de Coninck, H.; Loos, M.; Meyer, L. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Kunzler, C.; Alves, N.; Pereira, E.; Nienczewski, J.; Ligabue, R.; Einloft, S.; Dullius, J. CO2 storage with indirect carbonation using industrial waste. Energy Procedia 2011, 4, 1010–1017. [Google Scholar] [CrossRef]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Harrould-kolieb, E.R. A governing framework for international ocean acidification policy. Mar. Policy 2019, 102, 10–20. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Wilson, S.A.; Kelemen, P.B.; Hitch, M.; Southam, G. Carbon Mineralization: From Natural Analogues to Engineered Systems. Rev. Mineral. Geochem. 2013, 77, 305–360. [Google Scholar] [CrossRef]
- Mun, M.; Cho, H. Mineral carbonation for carbon sequestration with industrial waste. Energy Procedia 2013, 37, 6999–7005. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed]
- Seifritz, W. CO2 Disposal by Means of Silicates; Nature Publishing Group: London, UK, 1990. [Google Scholar]
- Gunning, P.J.; Hills, C.D.; Carey, P.J. Accelerated carbonation treatment of industrial wastes. Waste Manag. 2010, 30, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Huijgen, W.J.J.; Witkamp, G.J.; Comans, R.N.J. Mineral CO2 sequestration by steel slag carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, W.; Hu, J.; Wang, L.; Gao, J.; Liang, B.; Yue, H.; Zhang, G.; Luo, D.; Li, C. Energy-efficient mineral carbonation of blast furnace slag with high value-added products. J. Clean. Prod. 2018, 197, 242–252. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.W.; Chae, S.; Bang, J.H.; Lee, S.W. CO2 sequestration technology through mineral carbonation: An extraction and carbonation of blast slag. J. CO2 Util. 2016, 16, 336–345. [Google Scholar] [CrossRef]
- Dri, M.; Sanna, A.; Maroto-Valer, M.M. Mineral carbonation from metal wastes: Effect of solid to liquid ratio on the efficiency and characterization of carbonated products. Appl. Energy 2014, 113, 515–523. [Google Scholar] [CrossRef]
- Sanna, A.; Dri, M.; Hall, M.R.; Maroto-Valer, M. Waste materials for carbon capture and storage by mineralisation (CCSM)—A UK perspective. Appl. Energy 2012, 99, 545–554. [Google Scholar] [CrossRef]
- Dananjayan, R.R.T.; Kandasamy, P.; Andimuthu, R. Direct mineral carbonation of coal fly ash for CO2 sequestration. J. Clean. Prod. 2016, 112, 4173–4182. [Google Scholar] [CrossRef]
- Nyambura, M.G.; Mugera, G.W.; Felicia, P.L.; Gathura, N.P. Carbonation of brine impacted fractionated coal fly ash: Implications for CO2 sequestration. J. Environ. Manag. 2011, 92, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, M.C.; Andrés, J.M.; Gimeno, M.P. Optimization of mineral carbonation process for CO2 sequestration by lime-rich coal ashes. Fuel 2013, 106, 448–454. [Google Scholar] [CrossRef]
- Ji, L.; Yu, H.; Wang, X.; Grigore, M.; French, D.; Gözükara, Y.M.; Yu, J.; Zeng, M. CO2 sequestration by direct mineralisation using fly ash from Chinese Shenfu coal. Fuel Process. Technol. 2017, 156, 429–437. [Google Scholar] [CrossRef]
- Mazzella, A.; Errico, M.; Spiga, D. CO2 uptake capacity of coal fly ash: Influence of pressure and temperature on direct gas-solid carbonation. J. Environ. Chem. Eng. 2016, 4, 4120–4128. [Google Scholar] [CrossRef]
- Wee, J.H. A review on carbon dioxide capture and storage technology using coal fly ash. Appl. Energy 2013, 106, 143–151. [Google Scholar] [CrossRef]
- Reddy, K.J.; John, S.; Weber, H.; Argyle, M.D.; Bhattacharyya, P.; Taylor, D.T.; Christensen, M.; Foulke, T.; Fahlsing, P. Simultaneous capture and mineralization of coal combustion flue gas carbon dioxide (CO2). Energy Procedia 2011, 4, 1574–1583. [Google Scholar] [CrossRef]
- Jo, H.Y.; Kim, J.H.; Lee, Y.J.; Lee, M.; Choh, S.J. Evaluation of factors affecting mineral carbonation of CO2 using coal fly ash in aqueous solutions under ambient conditions. Chem. Eng. J. 2012, 183, 77–87. [Google Scholar] [CrossRef]
- Uibu, M.; Velts, O.; Kuusik, R. Developments in CO2 mineral carbonation of oil shale ash. J. Hazard. Mater. 2010, 174, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Araizi, P.K.; Hills, C.D.; Maries, A.; Gunning, P.J.; Wray, D.S. Enhancement of accelerated carbonation of alkaline waste residues by ultrasound. Waste Manag. 2016, 50, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R.; Prigiobbe, V. Comparison of different reaction routes for carbonation of APC residues. Energy Procedia 2009, 1, 4851–4858. [Google Scholar] [CrossRef]
- Gadikota, G.; Fricker, K.J.; Jang, S.-H.; Park, A.-H.A. Carbonation of silicate minerals and industrial wastes and their potential use as sustainable construction materials. In Advances in CO2 Capture, Sequestration, and Conversion; American Chemical Society: Washington, DC, USA, 2015; pp. 115–137. [Google Scholar]
- Gomes, H.I.; Mayes, W.M.; Rogerson, M.; Stewart, D.I.; Burked, I.T. Alkaline residues and the environment: A review of impacts, management practices and opportunities. J. Clean. Prod. 2016, 112, 3571–3582. [Google Scholar] [CrossRef]
- Xi, F.; Davis, S.J.; Ciais, P.; Crawford-Brown, D.; Guan, D.; Pade, C.; Shi, T.; Syddall, M.; Lv, J.; Ji, L.; et al. Substantial global carbon uptake by cement carbonation. Nat. Geosci. 2016, 9, 880–883. [Google Scholar] [CrossRef]
- Damiani, D.; Litynski, J.T.; McIlvried, H.G.; Vikara, D.M.; Srivastava, R.D. The US Department of Energy’s R&D program to reduce greenhouse gas emissions through beneficial uses of carbon dioxide. Greenh. Gases Sci. Technol. 2012, 2, 9–19. [Google Scholar]
- Pan, S.-Y. CO2 Capture by accelerated carbonation of alkaline wastes: A review on its principles and applications. Aerosol Air Qual. Res. 2012, 12, 770–791. [Google Scholar] [CrossRef]
- Said, A.; Laukkanen, T.; Järvinen, M. Pilot-scale experimental work on carbon dioxide sequestration using steelmaking slag. Appl. Energy 2016, 177, 602–611. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R. Influence of particle size on the carbonation of stainless steel slag for CO2 storage. Energy Procedia 2009, 1, 4859–4866. [Google Scholar] [CrossRef]
- Jung, W.M.; Kang, S.H.; Kim, W.S.; Choi, C.K. Particle morphology of calcium carbonate precipitated by gas-liquid reaction in a Couette-Taylor reactor. Chem. Eng. Sci. 2000, 55, 733–747. [Google Scholar] [CrossRef]
- McKelvy, M.J.; Chizmeshya, A.V.G.; Diefenbacher, J.; Béarat, H.; Wolf, G. Exploration of the role of heat activation in enhancing serpentine carbon sequestration reactions. Environ. Sci. Technol. 2004, 38, 6897–6903. [Google Scholar] [CrossRef] [PubMed]
- Maroto-Valer, M.M.; Fauth, D.J.; Kuchta, M.E.; Zhang, Y.; Andrésen, J.M. Activation of magnesium rich minerals as carbonation feedstock materials for CO2 sequestration. Fuel Process. Technol. 2005, 86, 1627–1645. [Google Scholar] [CrossRef]
- Kleiv, R.A.; Thornhill, M. Mechanical activation of olivine. Miner. Eng. 2006, 19, 340–347. [Google Scholar] [CrossRef]
- Veetil, S.P.; Mercier, G.; Blais, J.F.; Cecchi, E.; Kentish, S. Magnetic separation of serpentinite mining residue as a precursor to mineral carbonation. Int. J. Miner. Process. 2015, 140, 19–25. [Google Scholar] [CrossRef]
- Said, A.; Mattila, O.; Eloneva, S.; Järvinen, M. Enhancement of calcium dissolution from steel slag by ultrasound. Chem. Eng. Process. Process Intensif. 2015, 89, 1–8. [Google Scholar] [CrossRef]
- Eloneva, S.; Teir, S.; Salminen, J.; Fogelholm, C.J.; Zevenhoven, R. Fixation of CO2 by carbonating calcium derived from blast furnace slag. Energy 2008, 33, 1461–1467. [Google Scholar] [CrossRef]
- Park, A.H.A.; Fan, L.S. CO2 mineral sequestration: Physically activated dissolution of serpentine and pH swing process. Chem. Eng. Sci. 2004, 59, 5241–5247. [Google Scholar] [CrossRef]
- Lackner, K.S.; Butt, D.P.; Wendt, C.H. Progress on binding CO2 in mineral substrates. Energy Convers. Manag. 1997, 38, 259–264. [Google Scholar] [CrossRef]
- O’Connor, W.; Dahlin, D.; Nilsen, D. Research status on the sequestration of carbon dioxide by direct aqueous mineral carbonation. In Proceedings of the 18th Annual International Pittsburgh Coal Conference, Newcastle, Australia, 3–7 December 2001; p. 12. [Google Scholar]
- Huijgen, W.J.J.; Ruijg, G.J.; Comans, R.N.J.; Witkamp, G.J. Energy consumption and net CO2 sequestration of aqueous mineral carbonation. Ind. Eng. Chem. Res. 2006, 45, 9184–9194. [Google Scholar] [CrossRef]
- Fagerlund, J.; Nduagu, E.; Zevenhoven, R. Recent developments in the carbonation of serpentinite derived Mg(OH)2 using a pressurized fluidized bed. Energy Procedia 2011, 4, 4993–5000. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, J.; Zhao, Y.; Liu, R.; Zheng, C. CO2 sequestration by direct aqueous mineral carbonation under low-medium pressure conditions. J. Chem. Eng. Jpn. 2015, 48, 937–946. [Google Scholar] [CrossRef]
- Bhardwaj, R.; van Ommen, J.R.; Nugteren, H.W.; Geerlings, H. Accelerating natural CO2 mineralization in a fluidized bed. Ind. Eng. Chem. Res. 2016, 55, 2946–2951. [Google Scholar] [CrossRef]
- Power, I.M.; Dipple, G.M.; Southam, G. Bioleaching of ultramafic tailings by Acidithiobacillus spp. for CO2 sequestration. Environ. Sci. Technol. 2010, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, E.L.; Sharp, D.H. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Kwon, S.; Fan, M.; Dacosta, H.F.M.; Russell, A.G. Factors affecting the direct mineralization of CO2 with olivine. J. Environ. Sci. 2011, 23, 1233–1239. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Ling, T.-C.; Park, A.-H.A.; Chiang, P.-C. An overview: Reaction mechanisms and modelling of CO2 utilization via mineralization. Aerosol Air Qual. Res. 2018, 18, 829–848. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Witkamp, G.J.; Comans, R.N.J. Mechanisms of aqueous wollastonite carbonation as a possible CO2 sequestration process. Chem. Eng. Sci. 2006, 61, 4242–4251. [Google Scholar] [CrossRef]
- Yadav, S.; Mehra, A. Dissolution of steel slags in aqueous media. Environ. Sci. Pollut. Res. 2017, 24, 16305–16315. [Google Scholar] [CrossRef] [PubMed]
- De Windt, L.; Chaurand, P.; Rose, J. Kinetics of steel slag leaching: Batch tests and modeling. Waste Manag. 2011, 31, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Han, D.R.; Namkung, H.; Lee, H.M.; Huh, D.G.; Kim, H.T. CO2 sequestration by aqueous mineral carbonation of limestone in a supercritical reactor. J. Ind. Eng. Chem. 2015, 21, 792–796. [Google Scholar] [CrossRef]
- Tu, M.; Zhao, H.; Lei, Z.; Wang, L.; Chen, D.; Yu, H.; Qi, T. Aqueous carbonation of steel slag: A kinetics study. ISIJ Int. 2015, 55, 2509–2514. [Google Scholar] [CrossRef]
- Revathy, T.D.R.; Palanivelu, K.; Ramachandran, A. Direct mineral carbonation of steelmaking slag for CO2 sequestration at room temperature. Environ. Sci. Pollut. Res. 2016, 23, 7349–7359. [Google Scholar] [CrossRef] [PubMed]
- Ghacham, A.B.; Pasquier, L.C.; Cecchi, E.; Blais, J.F.; Mercier, G. CO2 sequestration by mineral carbonation of steel slags under ambient temperature: Parameters influence, and optimization. Environ. Sci. Pollut. Res. 2016, 23, 17635–17646. [Google Scholar] [CrossRef] [PubMed]
- Ukwattage, N.L.; Ranjith, P.G.; Li, X. Steel-making slag for mineral sequestration of carbon dioxide by accelerated carbonation. Measurement 2017, 97, 15–22. [Google Scholar] [CrossRef]
- Lekakh, S.N.; Rawlins, C.H.; Robertson, D.G.C.; Richards, V.L.; Peaslee, K.D. Kinetics of aqueous leaching and carbonization of steelmaking slag. Metall. Mater. Trans. B 2008, 39, 125–134. [Google Scholar] [CrossRef]
- Bonenfant, D.; Kharoune, L.; Sauve, S.; Hausler, R.; Niquette, P.; Mimeault, M.; Kharoune, M. CO2 sequestration potential of steel slags at ambient pressure and temperature. Ind. Eng. Chem. Res. 2008, 47, 7610–7616. [Google Scholar] [CrossRef]
- Kodama, S.; Nishimoto, T.; Yamamoto, N.; Yogo, K.; Yamada, K. Development of a new pH-swing CO2 mineralization process with a recyclable reaction solution. Energy 2008, 33, 776–784. [Google Scholar] [CrossRef]
- Doucet, F.J. Effective CO2-specific sequestration capacity of steel slags and variability in their leaching behaviour in view of industrial mineral carbonation. Miner. Eng. 2010, 23, 262–269. [Google Scholar] [CrossRef]
- Morales-Flórez, V.; Santos, A.; Lemus, A.; Esquivias, L. Artificial weathering pools of calcium-rich industrial waste for CO2 sequestration. Chem. Eng. J. 2011, 166, 132–137. [Google Scholar] [CrossRef]
- Chang, E.E.; Chen, C.H.; Chen, Y.H.; Pan, S.Y.; Chiang, P.C. Performance evaluation for carbonation of steel-making slags in a slurry reactor. J. Hazard. Mater. 2011, 186, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Pan, S.Y.; Chen, Y.H.; Tan, C.S.; Chiang, P.C. Accelerated carbonation of steelmaking slags in a high-gravity rotating packed bed. J. Hazard. Mater. 2012, 227–228, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Chiang, P.C.; Chen, Y.H.; Tan, C.S.; Chang, E.E. Ex Situ CO2 capture by carbonation of steelmaking slag coupled with metalworking wastewater in a rotating packed bed. Environ. Sci. Technol. 2013, 47, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Chiu, A.C.; Pan, S.Y.; Chen, Y.H.; Tan, C.S.; Chiang, P.C. Carbonation of basic oxygen furnace slag with metalworking wastewater in a slurry reactor. Int. J. Greenh. Gas Control 2013, 12, 382–389. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, J.; Li, K.; Yan, F.; Chen, X. Performance of steel slag in carbonation–calcination looping for CO2 capture from industrial flue gas. RSC Adv. 2014, 4, 6858–6862. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chiang, P.C.; Chen, Y.H.; Chang, E.E.; da Chen, C.; Shen, A.L. Process intensification of steel slag carbonation via a rotating packed Bed: Reaction kinetics and mass transfer. Energy Procedia 2014, 63, 2255–2260. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; di Gianfilippo, M.; Polettini, A.; Pomi, R.; Stramazzo, A. Thin-film versus slurry-phase carbonation of steel slag: CO2 uptake and effects on mineralogy. J. Hazard. Mater. 2015, 283, 302–313. [Google Scholar] [CrossRef] [PubMed]
- El-Naas, M.H.; el Gamal, M.; Hameedi, S.; Mohamed, A.M.O. CO2 sequestration using accelerated gas-solid carbonation of pre-treated EAF steel-making bag house dust. J. Environ. Manag. 2015, 156, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Liu, H.L.; Chang, E.E.; Kim, H.; Chen, Y.H.; Chiang, P.C. Multiple model approach to evaluation of accelerated carbonation for steelmaking slag in a slurry reactor. Chemosphere 2016, 154, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Polettini, A.; Pomi, R.; Stramazzo, A. CO2 sequestration through aqueous accelerated carbonation of BOF slag: A factorial study of parameters effects. J. Environ. Manag. 2016, 167, 185–195. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).