Phytochemical Content of Melissa officinalis L. Herbal Preparations Appropriate for Consumption
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Herbal Preparations
- Decoction (D): A suitable amount of deionized water was boiled in a Duran bottle. Then, a suitable portion of MOL (2% w/v) was added and the mixture was further boiled for 5, 10 or 15 min (namely D MOL 2% 5’ W, D MOL 2% 10’ W, D MOL 2% 15’ W).
- Infusion A: A suitable amount of boiled deionized water was immediately transferred to a Duran bottle that contained an appropriate portion of the plant material (2% w/v of MOL, CHM or OLF; namely I(A) MOL 2% 10’ W, I(A) CHM 2% 10’ W, I(A) OLF 2% 10’ W). The mixture was left to stand at room temperature for 10 min. 0.5% (I(A) MOL 0.5% 10’ W) and 4% w/v (I(A) MOL 4% 10’ W) MOL infusions were also prepared in the same way.
- Infusion B: A Duran bottle containing an appropriate amount of deionized water was placed in a water bath at 80 °C. Then, a suitable portion of MOL (2% w/v) was added and the mixture was left to stand for 10 min (I(B) MOL 2% 10’ W).
- Macerate: A suitable amount of MOL (2% w/v) was macerated in water (M MOL 2% 24 h W), ethanol (M MOL 2% 24 h E) or aqueous ethanol (EtOH/H2O: 25/75 v/v; M MOL 2% 24 h E/W) for 24 h at room temperature under periodical magnetic stirring.
- Extract by Ultrasounds: MOL (2% w/v) was treated in an ultrasonic bath (Elmas 30H Elmasonic, Elma, Singen, Germany; ultrasonic power effective 80 W, ultrasonic frequency 37 kHz) at room temperature for 10 min. Extraction solvent was water (S MOL 2% 10’ W), ethanol (S MOL 2% 10’ E) or aqueous ethanol (EtOH/H2O: 25/75 v/v; S MOL 2% 10’ E/W)
2.4. Content in Total Phenols (TP) Determined via the Folin-Ciocalteu Assay
2.5. Determination and Classification of Different Bioactive Phenolic Compounds
2.6. Antioxidant Activity Determined via the DPPH• Assay
2.7. Antioxidant Activity Determined via the Ferric Reducing Ability (FRAP) Assay
2.8. Na, K, Ca and Li Content Determination via Emission Flame Photometry
2.9. Pigment Content
2.10. Extraction Yield
2.11. Sensory Evaluation
2.12. Statistical Analysis
3. Results and Discussion
3.1. Content in TP, Hydroxycinnamic Acid Derivatives and Flavonols of Herbal Preparations
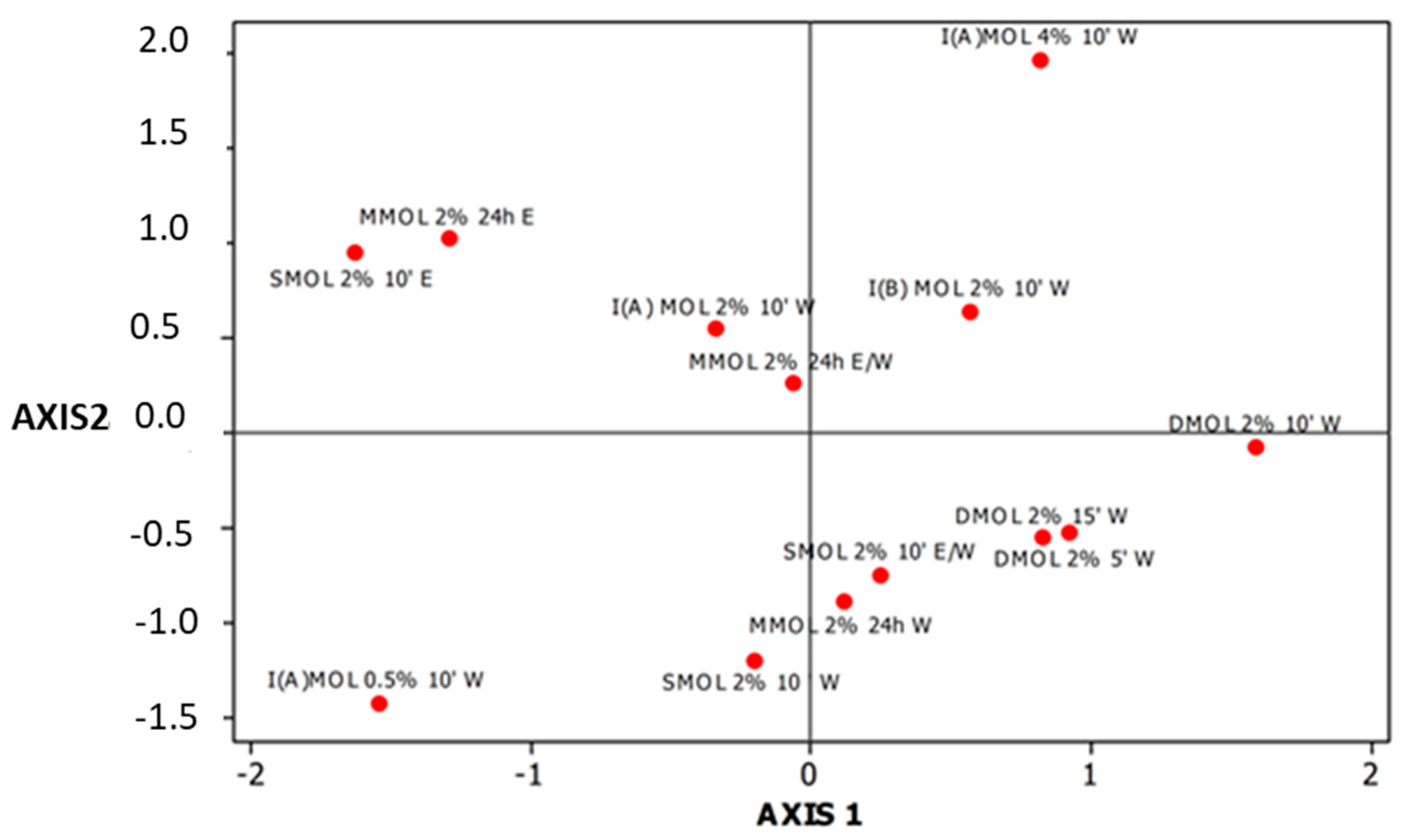
3.2. Mineral Content of Herbal Preparations
3.3. Pigment Content of Herbal Preparations
3.4. Extract Yield of Herbal Preparations
3.5. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rietjens, I.M.C.M.; Slob, W.; Galli, C.; Silano, V. Risk assessment of botanicals and botanical preparations intended for use in food and food supplements: Emerging issues. Toxicol. Lett. 2008, 180, 131–136. [Google Scholar] [CrossRef] [PubMed]
- González-Tejero, M.R.; Casares-Porcel, M.; Sánchez-Rojas, C.P.; Ramiro-Gutiérrez, J.M.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.E.; Censorii, E.; de Pasquale, C.; Della, A.; et al. Medicinal plants in the mediterranean area: Synthesis of the results of the project rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Moradkhani, H.; Sargsyan, E.; Bibak, H.; Naseri, B.; Sadat-Hosseini, M.; Fayazi-Barjin, A.; Meftahizade, H. Melissa officinalis L., a valuable medicine plant: A review. J. Med. Plants Res. 2010, 4, 2753–2759. [Google Scholar]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharm. 2016, 188, 204–228. [Google Scholar] [CrossRef] [PubMed]
- Lardos, A. The botanical materia medica of the iatrosophikon—A collection of prescriptions from a monastery in Cyprus. J. Ethnopharm. 2006, 104, 387–406. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.; Campos, M.; Órfão, J.; Ribeiro, C. Plants with neurobiological activity as potential targets for drug discovery. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 1372–1389. [Google Scholar] [CrossRef] [PubMed]
- Škrovánková, S.; Mišurcová, L.; Machů, L. Antioxidant Activity and Protecting Health Effects of Common medicinal Plants; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 75–139. [Google Scholar]
- Komes, D.; Belščak-Cvitanović, A.; Horžić, D.; Rusak, G.; Likić, S.; Berendika, M. Phenolic composition and antioxidant properties of some traditionally used medicinal plants affected by the extraction time and hydrolysis. Phytochem. Anal. 2011, 22, 172–180. [Google Scholar] [CrossRef]
- Musa Özcan, M.; Ünver, A.; Uçar, T.; Arslan, D. Mineral content of some herbs and herbal teas by infusion and decoction. Food Chem. 2008, 106, 1120–1127. [Google Scholar] [CrossRef]
- Topal, U.; Sasaki, M.; Goto, M.; Otles, S. Chemical compositions and antioxidant properties of essential oils from nine species of turkish plants obtained by supercritical carbon dioxide extraction and steam distillation. Int. J. Food Sci. Nutr. 2008, 59, 619–634. [Google Scholar] [CrossRef]
- Kim, S.; Yun, E.J.; Bak, J.S.; Lee, H.; Lee, S.J.; Kim, C.T.; Lee, J.H.; Kim, K.H. Response surface optimised extraction and chromatographic purification of rosmarinic acid from Melissa officinalis leaves. Food Chem. 2010, 121, 521–526. [Google Scholar] [CrossRef]
- Peev, G.; Penchev, P.; Peshev, D.; Angelov, G. Solvent extraction of rosmarinic acid from lemon balm and concentration of extracts by nanofiltration: Effect of plant pre-treatment by supercritical carbon dioxide. Chem. Eng. Res. Des. 2011, 89, 2236–2243. [Google Scholar] [CrossRef]
- Fecka, I.; Turek, S. Determination of water-soluble polyphenolic compounds in commercial herbal teas from Lamiaceae: Peppermint, Melissa, and Sage. J. Agric. Food Chem. 2007, 55, 10908–10917. [Google Scholar] [CrossRef] [PubMed]
- Petenatti, M.E.; Petenatti, E.M.; del Vitto, L.A.; Téves, M.R.; Caffini, N.O.; Marchevsky, E.J.; Pellerano, R.G. Evaluation of macro and microminerals in crude drugs and infusions of five herbs widely used as sedatives. Braz. J. Pharm. 2011, 21, 1144–1149. [Google Scholar] [CrossRef]
- Triantaphyllou, K.; Blekas, G.; Boskou, D. Antioxidative properties of water extracts obtained from herbs of the species Lamiaceae. Int. J. Food Sci. Nutr. 2001, 52, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Papoti, V.; Pegklidou, K.; Perifantsi, E.; Nenadis, N.; Demopoulos, V.; Tsimidou, M. Antioxidant and aldose reductase inhibition activity of Ligustrum japonicum and Olea europaea L. leaf extracts. Eur. J. Lipid Sci. Technol. 2011, 113, 876–885. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R., Jr.; Prenzler, P.D.; Robards, K. Investigation of australian olive mill waste for recovery of biophenols. J. Agric. Food Chem. 2005, 53, 9911–9920. [Google Scholar] [CrossRef]
- Hatzidimitriou, E.; Nenadis, N.; Tsimidou, M.Z. Changes in the catechin and epicatechin content of grape seeds on storage under different water activity (aw) conditions. Food Chem. 2007, 105, 1504–1511. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: The frap assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls A and B of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Sentkowska, A.; Biesaga, M.; Pyrzynska, K. Polyphenolic composition and antioxidative properties of lemon balm (Melissa officinalis L.) extract affected by different brewing processes. Int. J. Food Prop. 2015, 18, 2009–2014. [Google Scholar] [CrossRef]
- Pereira, R.P.; Fachinetto, R.; de Souza Prestes, A.; Puntel, R.L.; Santos da Silva, G.N.; Heinzmann, B.M.; Boschetti, T.K.; Athayde, M.L.; Bürger, M.E.; Morel, A.F.; et al. Antioxidant effects of different extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citratus. Neurochem. Res. 2009, 34, 973–983. [Google Scholar] [CrossRef]
- Caniova, A.; Brandsteterova, E. HPLC analysis of phenolic acids in Melissa officinalis. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 2647–2659. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A knowledge base for the recovery of natural phenols with different solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef]
- Tsakona, S.; Galanakis, C.M.; Gekas, V. Hydro-ethanolic mixtures for the recovery of phenols from mediterranean plant materials. Food Bioprocess Technol. 2012, 5, 1384–1393. [Google Scholar] [CrossRef]
- Blekas, G.; Psomiadou, E.; Tsimidou, M.; Boskou, D. On the importance of total polar phenols to monitor the stability of Greek virgin olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 340–346. [Google Scholar] [CrossRef]
- Atoui, A.K.; Mansouri, A.; Boskou, G.; Kefalas, P. Tea and herbal infusions: Their antioxidant activity and phenolic profile. Food Chem. 2005, 89, 27–36. [Google Scholar] [CrossRef]
- Jiménez-Zamora, A.; Delgado-Andrade, C.; Rufián-Henares, J.A. Antioxidant capacity, total phenols and color profile during the storage of selected plants used for infusion. Food Chem. 2016, 199, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Exarchou, V.; Nenadis, N.; Tsimidou, M.; Gerothanassis, I.P.; Troganis, A.; Boskou, D. Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J. Agric. Food Chem. 2002, 50, 5294–5299. [Google Scholar] [CrossRef] [PubMed]
- Skotti, E.; Anastasaki, E.; Kanellou, G.; Polissiou, M.; Tarantilis, P.A. Total phenolic content, antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatic plants. Ind. Crops Prod. 2014, 53, 46–54. [Google Scholar] [CrossRef]
- İnce, A.E.; Şahin, S.; Şάmnά, S.G. Extraction of phenolic compounds from Melissa using microwave and ultrasound. Turk. J. Agric. For. 2013, 37, 69–75. [Google Scholar]
- Lin, J.-T.; Chen, Y.-C.; Lee, Y.-C.; Rolis Hou, C.-W.; Chen, F.-L.; Yang, D.-J. Antioxidant, anti-proliferative and cyclooxygenase-2 inhibitory activities of ethanolic extracts from lemon balm (Melissa officinalis L.) leaves. LWT Food Sci. Technol. 2012, 49, 1–7. [Google Scholar] [CrossRef]
- Szabó, K.; Malekzadeh, M.; Radácsi, P.; Ladányi, M.; Rajhárt, P.; Inotai, K.; Tavaszi-Sárosi, S.; Németh, É. Could the variety influence the quantitative and qualitative outcome of lemon balm production? Ind. Crops Prod. 2016, 83, 710–716. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Arceusz, A.; Wesolowski, M. Quality consistency evaluation of Melissa officinalis L. Commercial herbs by HPLC fingerprint and quantitation of selected phenolic acids. J. Pharm. Biomed. Anal. 2013, 83, 215–220. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.; Chatzopoulou, P. Analysis of phenolic compounds in Greek plants of Lamiaceae family by HPLC. J. Appl. Res. Med. Aromat. Plants 2017, 6, 62–69. [Google Scholar] [CrossRef]
- Papoti, V.T.; Tsimidou, M.Z. Impact of sampling parameters on the radical scavenging potential of olive (Olea europaea L.) leaves. J. Agric. Food Chem. 2009, 57, 3470–3477. [Google Scholar] [CrossRef]
- Goulas, V.; Papoti, V.T.; Exarchou, V.; Tsimidou, M.Z.; Gerothanassis, I.P. Contribution of flavonoids to the overall radical scavenging activity of olive (Olea europaea L.) leaf polar extracts. J. Agric. Food Chem. 2010, 58, 3303–3308. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH-assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Koksal, E.; Bursal, E.; Dikici, E.; Tozoglu, F.; Gulcin, I. Antioxidant activity of Melissa officinalis leaves. J. Med. Plants Res. 2011, 5, 217–222. [Google Scholar]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Herodež, S.S.; Hadolin, M.; Škerget, M.; Knez, Ž. Solvent extraction study of antioxidants from balm (Melissa officinalis L.) leaves. Food Chem. 2003, 80, 275–282. [Google Scholar] [CrossRef]
- Nugraheni, M.; Santoso, U.; Suparmo, H.; Wuryastuti, H. In vitro antioxidant, antiproliferative and apoptosis effect of coleus Tuberosus L. Afr. J. Food Sci. 2011, 5, 232–241. [Google Scholar]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH.) tests. J. Am. Oil Chem. Soc. 2002, 79, 1191–1195. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Tsimidou, M.Z.; Vafiadis, A.P.; Bakalbassis, E.G. Structure-DPPH• scavenging activity relationships: Parallel study of catechol and guaiacol acid derivatives. J. Agric. Food Chem. 2006, 54, 5763–5768. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.M.; Pompeu, D.R.; Larondelle, Y.; Rogez, H. Optimisation of the adsorption of polyphenols from Inga edulis leaves on macroporous resins using an experimental design methodology. Sep. Purif. Technol. 2007, 53, 274–280. [Google Scholar] [CrossRef]
- Spiridon, I.; Colceru, S.; Anghel, N.; Teaca, C.A.; Bodirlau, R.; Armatu, A. Antioxidant capacity and total phenolic contents of oregano (Origanum vulgare), lavender (Lavandula angustifolia) and lemon balm (Melissa officinalis) from romania. Nat. Prod. Res. 2011, 25, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.I.; Barros, L.; Sousa, M.J.; Ferreira, I.C.F.R. Systematic comparison of nutraceuticals and antioxidant potential of cultivated, in vitro cultured and commercial Melissa officinalis samples. Food Chem. Toxicol. 2012, 50, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Kamdem, J.P.; Adeniran, A.; Boligon, A.A.; Klimaczewski, C.V.; Elekofehinti, O.O.; Hassan, W.; Ibrahim, M.; Waczuk, E.P.; Meinerz, D.F.; Athayde, M.L. Antioxidant activity, genotoxicity and cytotoxicity evaluation of lemon balm (Melissa officinalis L.) ethanolic extract: Its potential role in neuroprotection. Ind. Crops Prod. 2013, 51, 26–34. [Google Scholar] [CrossRef]
- Nicolai, M.; Pereira, P.; Vitor, R.F.; Reis, C.P.; Roberto, A.; Rijo, P. Antioxidant activity and rosmarinic acid content of ultrasound-assisted ethanolic extracts of medicinal plants. Measurement 2016, 89, 328–332. [Google Scholar] [CrossRef]
- Pytlakowska, K.; Kita, A.; Janoska, P.; Połowniak, M.; Kozik, V. Multi-element analysis of mineral and trace elements in medicinal herbs and their infusions. Food Chem. 2012, 135, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B.; Kimura, M. Harvestplus handbook for carotenoid analysis. In Harvestplus Technical Monographs; International Food Policy Research Institute (IFPRI) International Center for Tropical Agriculture (CIAT): Washington, WA, USA, 2004; pp. 1–58. [Google Scholar]
- Bernal, J.; Mendiola, J.A.; Ibáñez, E.; Cifuentes, A. Advanced analysis of nutraceuticals. J. Pharm. Biomed. Anal. 2011, 55, 758–774. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Joubert, E. Chemical and sensory analyses of spray- and freezedried extracts of rooibos tea (Aspalathus linearis). Int. J. Food Sci. Technol. 1990, 25, 344–349. [Google Scholar] [CrossRef]
- Von Gadow, A.; Joubert, E.; Hansmann, C.F. Effect of extraction time and additional heating on the antioxidant activity of rooibos tea (Aspalathus linearis) extracts. J. Agric. Food Chem. 1997, 45, 1370–1374. [Google Scholar] [CrossRef]



| Herbal Beverage | (mg per 250 mL cup) | ||||
|---|---|---|---|---|---|
| Naa | Kb | Cac | Lid | Dry Extract Weighte | |
| I(A)MOL 0.5% 10’ W | 1.1 | 30.5 | 50.1 | 0.1 | 455 |
| I(A) MOL 2% 10’ W | 2.0 | 100.0 | 98.3 | 0.3 | 1707 |
| I(A)MOL 4% 10’ W | 2.9 | 175.7 | 137.7 | 0.4 | 3377 |
| I(B) MOL 2% 10’ W | 2.1 | 115.5 | 176.1 | 0.3 | 1742 |
| D MOL 2% 5’ W | 2.0 | 288.4 | 154.1 | 0.2 | 2856 |
| D MOL 2% 10’ W | 2.8 | 263.0 | 153.4 | 0.3 | 3381 |
| D MOL 2% 15’ W | 2.1 | 256.1 | 150.7 | 0.2 | 3185 |
| S MOL 2% 10’ W | 1.9 | 117.4 | 93.7 | 0.2 | 1564 |
| S MOL 2% 10’ E | 1.2 | 5.1 | 56.0 | 0.4 | 144 |
| S MOL 2% 10’ E/W | 2.7 | 125.0 | 101.1 | 0.2 | 1383 |
| M MOL 2% 24 h W | 1.6 | 125.1 | 97.7 | 0.2 | 1609 |
| M MOL 2% 24 h E | 0.8 | 4.1 | 65.0 | 0.4 | 283 |
| M MOL 2% 24 h E/W | 2.0 | 130.3 | 118.5 | 0.3 | 1730 |
| I CHM 2% 10’ W | 15.0 | 103.6 | 108.2 | 0.3 | 1199 |
| I OLF 2% 10’ W | 1.9 | 62.7 | 70.8 | 0.3 | 1524 |
| Variable | AXIS 1 | AXIS 2 |
|---|---|---|
| mg Ca per 250 mL | 0.914 | 0.141 |
| mg Na per 250 mL | 0.819 | 0.07 |
| mg K per 250 mL | 0.809 | −0.142 |
| mg Li per 250 mL | −0.071 | 0.989 |
| Variance | 2.164 | 1.023 |
| Var% | 54.1 | 25.6 |
| Herbal Preparations | ×80 = μg/mL | ||
|---|---|---|---|
| Chlorophyll aa | Chlorophyll bb | Carotenoidsc | |
| I(A) MOL 0.5% 10’ W | 0.1 | 0.3 | 0.5 |
| I(A) MOL 2% 10’ W | 1.0 | 2.0 | 1.8 |
| I(A) MOL 4% 10’ W | 2.1 | 4.2 | 6.6 |
| I(B) MOL 2% 10’ W | 1.2 | 2.0 | 2.5 |
| D MOL 2% 10’ W | 2.0 | 3.9 | 2.8 |
| I CHM 2% 10’ W | 0.2 | 0.4 | 0.5 |
| I OLF 2% 10’ W | 0.4 | 0.8 | 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papoti, V.T.; Totomis, N.; Atmatzidou, A.; Zinoviadou, K.; Androulaki, A.; Petridis, D.; Ritzoulis, C. Phytochemical Content of Melissa officinalis L. Herbal Preparations Appropriate for Consumption. Processes 2019, 7, 88. https://doi.org/10.3390/pr7020088
Papoti VT, Totomis N, Atmatzidou A, Zinoviadou K, Androulaki A, Petridis D, Ritzoulis C. Phytochemical Content of Melissa officinalis L. Herbal Preparations Appropriate for Consumption. Processes. 2019; 7(2):88. https://doi.org/10.3390/pr7020088
Chicago/Turabian StylePapoti, Vassiliki T., Nikolaos Totomis, Aikaterini Atmatzidou, Kyriaki Zinoviadou, Anna Androulaki, Dimitris Petridis, and Christos Ritzoulis. 2019. "Phytochemical Content of Melissa officinalis L. Herbal Preparations Appropriate for Consumption" Processes 7, no. 2: 88. https://doi.org/10.3390/pr7020088
APA StylePapoti, V. T., Totomis, N., Atmatzidou, A., Zinoviadou, K., Androulaki, A., Petridis, D., & Ritzoulis, C. (2019). Phytochemical Content of Melissa officinalis L. Herbal Preparations Appropriate for Consumption. Processes, 7(2), 88. https://doi.org/10.3390/pr7020088






