Effect of Morphology of Calcium Carbonate on Toughness Behavior and Thermal Stability of Epoxy-Based Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nano-CaCO3 Nanorods
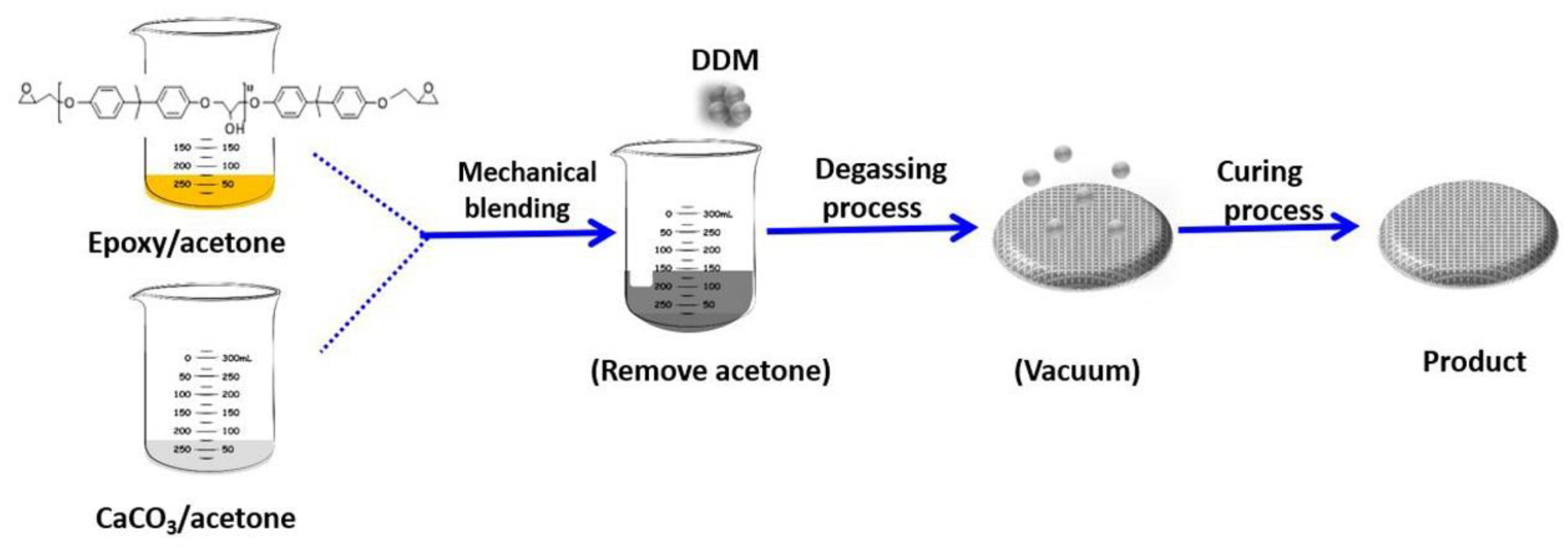
2.3. Preparation of Nano-CaCO3/Epoxy Composites
2.4. Characterization of Synthetic Compounds
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- May, C. Epoxy Resins: Chemistry and Technology; CRC Press: Boca Raton, FL, USA, 1987. [Google Scholar]
- Hsieh, T.; Kinloch, A.; Masania, K.; Taylor, A.; Sprenger, S. The mechanisms and mechanics of the toughening of epoxy polymers modified with silica nanoparticles. Polymer 2010, 51, 6284–6294. [Google Scholar] [CrossRef] [Green Version]
- Kar, S.; Banthia, A.K. Synthesis and evaluation of liquid amine-terminated polybutadiene rubber and its role in epoxy toughening. J. Appl. Polym. Sci. 2005, 96, 2446–2453. [Google Scholar] [CrossRef]
- Wang, M.; Chen, H.; Lin, W.; Li, Z.; Li, Q.; Chen, M.; Meng, F.; Xing, Y.; Yao, Y.; Wong, C.-P. Crack-free and scalable transfer of carbon nanotube arrays into flexible and highly thermal conductive composite film. ACS Appl. Mater. Interfaces 2013, 6, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Park, S.-J.; Jeong, H.-J.; Nah, C. A study of oxyfluorination of multi-walled carbon nanotubes on mechanical interfacial properties of epoxy matrix nanocomposites. Mater. Sci. Eng. A 2004, 385, 13–16. [Google Scholar] [CrossRef]
- El-Tantawy, F.; Kamada, K.; Ohnabe, H. In situ network structure, electrical and thermal properties of conductive epoxy resin–carbon black composites for electrical heater applications. Mater. Lett. 2002, 56, 112–126. [Google Scholar] [CrossRef]
- Chen, Z.-K.; Yang, J.-P.; Ni, Q.-Q.; Fu, S.-Y.; Huang, Y.-G. Reinforcement of epoxy resins with multi-walled carbon nanotubes for enhancing cryogenic mechanical properties. Polymer 2009, 50, 4753–4759. [Google Scholar] [CrossRef]
- Shen, X.-J.; Liu, Y.; Xiao, H.-M.; Feng, Q.-P.; Yu, Z.-Z.; Fu, S.-Y. The reinforcing effect of graphene nanosheets on the cryogenic mechanical properties of epoxy resins. Compos. Sci. Technol. 2012, 72, 1581–1587. [Google Scholar] [CrossRef]
- Zotti, A.; Borriello, A.; Zuppolini, S.; Antonucci, V.; Giordano, M.; Pomogailo, A.D.; Lesnichaya, V.A.; Golubeva, N.D.; Bychkov, A.N.; Dzhardimalieva, G.I. Fabrication and characterization of metal-core carbon-shell nanoparticles filling an aeronautical composite matrix. Eur. Polym. J. 2015, 71, 140–151. [Google Scholar] [CrossRef]
- Chen, L.; Chai, S.; Liu, K.; Ning, N.; Gao, J.; Liu, Q.; Chen, F.; Fu, Q. Enhanced epoxy/silica composites mechanical properties by introducing graphene oxide to the interface. ACS Appl. Mater. Interfaces 2012, 4, 4398–4404. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.-S.; Song, W.-L.; Hou, Z.-L.; Wen, B.; Yuan, J. The effects of temperature and frequency on the dielectric properties, electromagnetic interference shielding and microwave-absorption of short carbon fiber/silica composites. Carbon 2010, 48, 788–796. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Salami-Kalajahi, M.; Mousavi, A.; Razavi, B.; Shahi, S. Preparation of organic-inorganic hybrid nanocomposites from chemically modified epoxy and novolac resins and silica-attached carbon nanotubes by sol-gel process: Investigation of thermal degradation and stability. Prog. Org. Coat. 2018, 117, 154–165. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Rostami, M.; Niroumandrad, S. Enhancement of the physical/mechanical properties of an epoxy composite by addition of aluminum nanoparticles through modification with cerium oxides and functionalization by SiO2-NH2 thin films. Prog. Org. Coat. 2017, 112, 244–253. [Google Scholar] [CrossRef]
- Ng, C.; Schadler, L.; Siegel, R. Synthesis and mechanical properties of TiO2-epoxy nanocomposites. Nanostruct. Mater. 1999, 12, 507–510. [Google Scholar] [CrossRef]
- Wetzel, B.; Rosso, P.; Haupert, F.; Friedrich, K. Epoxy nanocomposites—Fracture and toughening mechanisms. Eng. Fract. Mech. 2006, 73, 2375–2398. [Google Scholar] [CrossRef]
- Goyat, M.; Rana, S.; Halder, S.; Ghosh, P. Facile fabrication of epoxy-TiO2 nanocomposites: A critical analysis of TiO2 impact on mechanical properties and toughening mechanisms. Ultrason Sonochem. 2018, 40, 861–873. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, S.; Kumar, A.; Jain, A. Thermo-mechanical behavior of TiO2 dispersed epoxy composites. Eng. Fract. Mech. 2017, 184, 241–248. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, X.; Bai, L.; Du, R.; Liu, Y.; Zhang, Y.; Zhao, G. Effect of micro-Al2O3 contents on mechanical property of carbon fiber reinforced epoxy matrix composites. Compos. Part B-Eng. 2016, 91, 392–398. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, J.; Xue, Y.; He, C.; Zhou, X.; Xie, X.; Ye, Y.; Mai, Y.-W. Simultaneous improvement in the flame resistance and thermal conductivity of epoxy/Al2O3 composites by incorporating polymeric flame retardant-functionalized graphene. J. Mater. Chem. A 2017, 5, 13544–13556. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Akil, H.M.; Kudus, M.H.A.; Othman, M.B.H. Compressive properties and thermal stability of hybrid carbon nanotube-alumina filled epoxy nanocomposites. Compos. Part B-Eng. 2016, 91, 235–242. [Google Scholar] [CrossRef]
- Vaisakh, S.S.; Peer Mohammed, A.A.; Hassanzadeh, M.; Tortorici, J.F.; Metz, R.; Ananthakumar, S. Effect of nano-modified SiO2/Al2O3 mixed-matrix micro-composite fillers on thermal, mechanical, and tribological properties of epoxy polymers. Polym. Adv. Technol. 2016, 27, 905–914. [Google Scholar] [CrossRef]
- Li, J.; Peng, C.; Li, Z.; Wu, Z.; Li, S. The improvement in cryogenic mechanical properties of nano-ZrO2/epoxy composites via surface modification of nano-ZrO2. RSC Adv. 2016, 6, 61393–61401. [Google Scholar] [CrossRef]
- Khan, R.; Azhar, M.R.; Anis, A.; Alam, M.A.; Boumaza, M.; Al-Zahrani, S.M. Facile synthesis of epoxy nanocomposite coatings using inorganic nanoparticles for enhanced thermo-mechanical properties: A comparative study. J. Coat. Technol. Res. 2016, 13, 159–169. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, Y.; Li, J.; Liang, S.; Liu, Z. Mechanical enhancement of zirconia reinforced polyimine nanocomposites. J. Appl. Polym. Sci. 2017, 134, 45183. [Google Scholar] [CrossRef]
- Halder, S.; Goyat, M.; Ghosh, P. Morphological, structural, and thermophysical properties of zirconium dioxide–epoxy nanocomposites. High Perform. Polym. 2016, 28, 697–708. [Google Scholar] [CrossRef]
- Eskizeybek, V.; Ulus, H.; Kaybal, H.B.; Şahin, Ö.S.; Avcı, A. Static and dynamic mechanical responses of CaCO3 nanoparticle modified epoxy/carbon fiber nanocomposites. Compos. Part B-Eng. 2018, 140, 223–231. [Google Scholar] [CrossRef]
- Jin, F.-L.; Park, S.-J. Interfacial toughness properties of trifunctional epoxy resins/calcium carbonate nanocomposites. Mater. Sci. Eng. A 2008, 475, 190–193. [Google Scholar] [CrossRef]
- Chen, J.-L.; Jin, F.-L.; Park, S.-J. Thermal stability and impact and flexural properties of epoxy resins/epoxidized castor oil/nano-CaCO3 ternary systems. Macromol. Res. 2010, 18, 862–867. [Google Scholar] [CrossRef]
- Yu, H.; Wang, L.; Shi, Q.; Jiang, G.; Zhao, Z.; Dong, X. Study on nano-CaCO3 modified epoxy powder coatings. Prog. Org. Coat. 2006, 55, 296–300. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Nguyen, T.V.; Thai, H.; Shi, X. Effect of nanoparticles on the thermal and mechanical properties of epoxy coatings. J. Nanosci. Nanotechnol. 2016, 16, 9874–9881. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Xu, Q.; Zhang, J. Fabrication of antibacterial casein-based ZnO nanocomposite for flexible coatings. Mater. Des. 2017, 113, 240–245. [Google Scholar] [CrossRef]
- Koshlaf, E.; Shahsavari, E.; Aburto-Medina, A.; Taha, M.; Haleyur, N.; Makadia, T.H.; Morrison, P.D.; Ball, A.S. Bioremediation potential of diesel-contaminated Libyan soil. Ecotoxicol. Environ. Safe 2016, 133, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Prasad, T.; Khan, N.I.; Goyat, M.; Chauhan, S.R. Superior mechanical properties of poly vinyl alcohol-assisted ZnO nanoparticle reinforced epoxy composites. Mater. Chem. Phys. 2017, 192, 198–209. [Google Scholar] [CrossRef]
- Li, L.; Zou, H.; Shao, L.; Wang, G.; Chen, J. Study on mechanical property of epoxy composite filled with nano-sized calcium carbonate particles. J. Mater. Sci. 2005, 40, 1297–1299. [Google Scholar] [CrossRef]
- He, H.; Li, K.; Wang, J.; Sun, G.; Li, Y.; Wang, J. Study on thermal and mechanical properties of nano-calcium carbonate/epoxy composites. Mater. Des. 2011, 32, 4521–4527. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Yuan, J.; Wu, M. Toughness improvement of epoxy resin mortar by incorporation of ground calcium carbonate. Constr. Build. Mater. 2015, 100, 122–128. [Google Scholar] [CrossRef]
- Zotti, A.; Borriello, A.; Martone, A.; Antonucci, V.; Giordano, M.; Zarrelli, M. Effect of sepiolite filler on mechanical behaviour of a bisphenol A-based epoxy system. Compos. Part B-Eng. 2014, 67, 400–409. [Google Scholar] [CrossRef]
- Ulus, H.; Şahin, Ö.S.; Avcı, A. Enhancement of flexural and shear properties of carbon fiber/epoxy hybrid nanocomposites by boron nitride nano particles and carbon nano tube modification. Fibers Polym. 2015, 16, 2627–2635. [Google Scholar] [CrossRef]
- Quaresimin, M.; Schulte, K.; Zappalorto, M.; Chandrasekaran, S. Toughening mechanisms in polymer nanocomposites: From experiments to modelling. Compos. Sci. Technol. 2016, 123, 187–204. [Google Scholar] [CrossRef]
- Al-Ajaj, I.A.; Abd, M.M.; Jaffer, H.I. Mechanical properties of micro and nano TiO2/epoxy composites. Int. J. Min. Metall. Eng. 2013, 1, 2320–4060. [Google Scholar]
- Kagitci, Y.C.; Tarakcioglu, N. The effect of weld line on tensile strength in a polymer composite part. Int. J. Adv. Manuf. Technol. 2016, 85, 1125–1135. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B-Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Ozdemir, N.G.; Zhang, T.; Aspin, I.; Scarpa, F.; Hadavinia, H.; Song, Y. Toughening of carbon fibre reinforced polymer composites with rubber nanoparticles for advanced industrial applications. Express Polym. Lett. 2016, 10, 394–407. [Google Scholar] [CrossRef]
- Doyle, C. Estimating thermal stability of experimental polymers by empirical thermogravimetric analysis. Anal. Chem. 1961, 33, 77–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Choi, J.R.; Park, S.-J. Thermal conductivity and thermo-physical properties of nanodiamond-attached exfoliated hexagonal boron nitride/epoxy nanocomposites for microelectronics. Compos. Part A Appl. Sci. Manuf. 2017, 101, 227–236. [Google Scholar] [CrossRef]
- Kim, S.H.; Heo, Y.-J.; Park, M.; Min, B.-G.; Rhee, K.Y.; Park, S.-J. Effect of hydrophilic graphite flake on thermal conductivity and fracture toughness of basalt fibers/epoxy composites. Compos. Part B-Eng. 2018, 153, 9–16. [Google Scholar] [CrossRef]
- Horowitz, H.H.; Metzger, G. A new analysis of thermogravimetric traces. Anal. Chem. 1963, 35, 1464–1468. [Google Scholar] [CrossRef]
- Landel, R.F.; Nielsen, L.E. Mechanical Properties of Polymers and Composites; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]






| Sample | IDT (°C) | T5% (°C) | Tmax (°C) | A1 | A2 | A3 | A* | K* | IPDT (°C) | Et (kJ mol−1) | Char Yield at 800 °C (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neat epoxy | 376.4 | 230.5 | 381.6 | 32349 | 5689 | 39005 | 0.49 | 1.17 | 476.8 | 156.58 | 7.3 |
| EP-cube-1.5 | 376.7 | 276.4 | 385.4 | 32796 | 6984 | 37226 | 0.51 | 1.21 | 512.5 | 172.18 | 9.0 |
| EP-rod-1.5 | 381.6 | 281.0 | 386.6 | 32670 | 7893 | 36441 | 0.52 | 1.24 | 533.6 | 175.14 | 10.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Heo, Y.-J.; Park, S.-J. Effect of Morphology of Calcium Carbonate on Toughness Behavior and Thermal Stability of Epoxy-Based Composites. Processes 2019, 7, 178. https://doi.org/10.3390/pr7040178
Yang G, Heo Y-J, Park S-J. Effect of Morphology of Calcium Carbonate on Toughness Behavior and Thermal Stability of Epoxy-Based Composites. Processes. 2019; 7(4):178. https://doi.org/10.3390/pr7040178
Chicago/Turabian StyleYang, Guijun, Young-Jung Heo, and Soo-Jin Park. 2019. "Effect of Morphology of Calcium Carbonate on Toughness Behavior and Thermal Stability of Epoxy-Based Composites" Processes 7, no. 4: 178. https://doi.org/10.3390/pr7040178





