Immersion Enthalpy of Activated Carbon–Cyclohexane and Activated Carbon–Hexane. Difference in the Solid–Liquid Interaction Enthalpy Due to the Structure of the Solvent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Activated Carbons with Differences in Their Surface Chemistry
2.2. Nitrogen Adsorption Isotherms at 77 K
2.3. Determination of the Content of Total Acidic and Basic Groups
2.4. Determination of the Immersion Enthalpy in Cyclohexane and Hexane
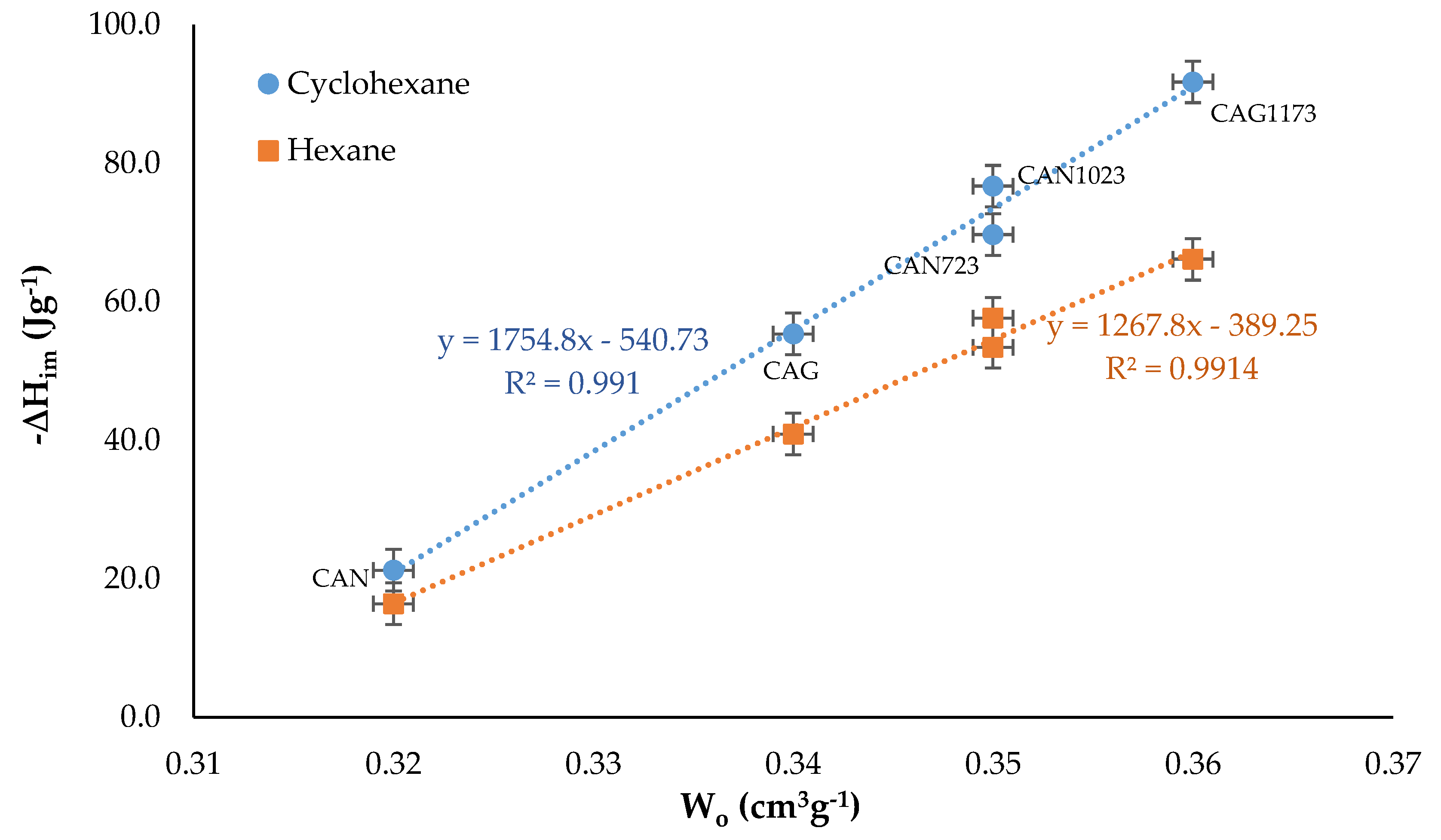
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saini, V.K.; Pires, J. Development of metal organic fromwork-199 immobilized zeolite foam for adsorption of common indoor VOCs. J. Environ. Sci. 2017, 55, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Gallego, E.; Roca, F.J.; Perales, J.F.; Guardino, X. Experimental evaluation of VOC removal efficiency of a coconut shell activated carbon filter for indoor air quality enhancement. Build Environ. 2013, 67, 14–25. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, Q.; Cui, Y.; Xie, F.; Li, W.; Li, Y.; Chen, M. Adsorption properties of activated carbon from reed with a high adsorption capacity. Ecol. Eng. 2017, 102, 443–450. [Google Scholar] [CrossRef]
- Yang, X.; Yi, H.; Tang, X.; Zhao, S.; Yang, Z.; Ma, Y.; Feng, T.; Cui, X. Behaviors and kinetics of toluene adsorption -desorption on activated carbons with varying pore structure. J. Environ. Sci. 2018, 67, 104–114. [Google Scholar] [CrossRef]
- Gil, R.R.; Ruiz, B.; Lozano, M.S.; Martín, M.J.; Fuente, E. VOCs removal by adsorption onto activated carbons from biocollagenic wastes of vegetable tanning. Chem. Eng. J. 2014, 245, 80–88. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Liu, J. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Long, C.; Li, A. Enhanced adsorption and desorption of VOCs vapor on novel micro-mesoporous polymeric adsorbents. J. Colloid Interface Sci. 2014, 428, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.H. Recent developments in the physical adsorption of toxic organic vapours by activated carbons. Adsorpt. Sci. Technol. 2011, 29, 1–28. [Google Scholar] [CrossRef]
- Wang, G.; Dou, B.; Zhang, Z.; Wang, J.; Liu, H.; Hao, Z. Adsorption of benzene, cyclohexane and hexane on ordered mesoporous carbon. J. Environ. Sci. 2015, 30, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Sarigiannis, D.A.; Karakitsios, S.P.; Gotti, A.; Liakos, I.; Katsoyiannis, L.A. Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk. Environ. Int. 2011, 37, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Delgadillo, D.P.; Giraldo, L.; Moreno-Piraján, J.C. Preparation and calorimetry characterization of nitrogen-enriched activated carbons and their application in the removal of carbon dioxide. Eur. J. Chem. 2017, 8, 130–136. [Google Scholar] [CrossRef]
- Stoeckli, H.F.; Kraehenbuehl, F.; Ballerini, L.; De Bernardini, S. Recent developments in the Dubinin equation. Carbon 1989, 27, 125–128. [Google Scholar] [CrossRef] [Green Version]
- Bansal, R.C.; Donnet, J.B.; Stoeckli, H.F. Active Carbon; Marcel Dekker: New York, NY, USA, 1988. [Google Scholar]
- Moreno, J.C.; Giraldo, L. Determination of the immersion enthalpy of activated carbon by microcalorimetry of the heat conduction. Instrum. Sci. Technol. 2000, 28, 171–178. [Google Scholar] [CrossRef]
- Stoeckli, H.F. Dubinin’s theory and its contribution to adsorption science. Russ. Chem. B+ 2001, 50, 2265–2272. [Google Scholar] [CrossRef]
- Hernández-Monje, D.; Giraldo, L.; Moreno-Piraján, J.C. Study of Hexane Adsorption on Activated Carbons with Differences in Their Surface Chemistry. Molecules 2018, 23, 476. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F. Adsorption at the liquid–solid interface: Thermodynamics and methodology. In Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Rouquerol, J., Rouquerol, F., Llewellyn, P., Maurinm, G., Sing, K., Eds.; Academic Press: Kidlington, UK, 2014; pp. 105–158. [Google Scholar]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Kim, Y.; Park, C. Titration Method for the Identification of Surface Functional Groups. In Materials Science and Engineering of Carbon Characterization; Inagaki, M., Kang, F., Eds.; Tsinghua University Press Limited: Hamamatsu, Japan; pp. 273–286.
- Vargas, D.P.; Giraldo, L.; Moreno-Piraján, J.C. Calorimetric study of the CO2 adsorption on carbon materials. J. Therm. Anal. Calorim. 2014, 117, 1299–1309. [Google Scholar] [CrossRef]
- Vargas, D.; Giraldo, L.; Moreno-Piraján, J.C. Accessible area and hydrophobicity of activated carbons obtained from the enthalpy characterization. Adsorption 2016, 22, 3–11. [Google Scholar] [CrossRef]
- Moreno, J.C.; Giraldo, L. Instrumentación calorimétrica aplicada a la determinación de entalpias de inmersión de sólidos porosos. In Sólidos Porosos Preparación, Caracterización y Aplicaciones; Moreno, J.C., Ed.; Uniandes: Bogotá, Colombia, 2007; pp. 281–297. [Google Scholar]
- Murillo, Y.S.; Giraldo, L.; Moreno-Piraján, J.C. Contribution enthalpic in the interaction of activated carbon with polar and apolar solvents. Arab. J. Chem. 2013, 6, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, G.; Giraldo, L.; Moreno, J. Entalpías de inmersión de telas de carbón activado como parámetro de caracterización fisicoquímica. Rev. Colomb. Quím. 2009, 38, 32–36. [Google Scholar]
- Moreno-Piraján, J.C.; Giraldo, L. Study of Carbon Foams Synthesized by the Pyrolysis of Wastes Coconut Shells of African Palm at Different Conditions and use of Immersion Calorimetry as a Tool for Characterization. Orient. J. Chem. 2013, 29, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, P.; Giraldo, L.; Moreno, J.C. Modified surface chemistry of activated carbons. Correlation with immersion enthalpy. J. Therm. Anal. Calorim. 2013, 114, 245–251. [Google Scholar] [CrossRef]
- Belhachemi, M.; Addoun, F. Effect of Heat Treatment on the Surface Properties of Activated Carbons. E- J. Chem. 2011, 8, 992–999. [Google Scholar] [CrossRef]
- Wang, R.; Lang, J.; Yan, X. Effect of surface area and heteroatom of porous carbon materials on electrochemical capacitance in aqueous and organic electrolytes. Sci. China Chem. 2014, 57, 1570–1578. [Google Scholar] [CrossRef]
- Yin, C.; Aroua, M.; Daud, W. Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep. Purif. Technol. 2007, 52, 403–411. [Google Scholar] [CrossRef]
- Mangun, C.L.; Benak, K.R.; Daley, M.A.; Economy, J. Oxidation of Activated Carbon Fibers: Effect on Pore Size, Surface Chemistry, and Adsorption Properties. Chem. Mater. 1999, 11, 3476–3483. [Google Scholar] [CrossRef]
- Jaramillo, J.; Álvarez, P.; Gómez-Serrano, V. Preparation and ozone-surface modification of activated carbon. Thermal stability of oxygen surface groups. Appl. Surf. Sci. 2010, 256, 5232–5236. [Google Scholar] [CrossRef]
- Silvestre-Albero, J.; Silvestre-Albero, A.; Rodríguez-Reinoso, F.; Thommes, M. Physical characterization of activated carbons with narrow microporosity by nitrogen (77.4 K), carbon dioxide (273 K) and argon (87.3 K) adsorption in combination with immersion calorimetry. Carbon 2012, 50, 3128–3133. [Google Scholar] [CrossRef]
- Gokce, Y.; Aktas, Z. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl. Surf. Sci. 2014, 313, 352–359. [Google Scholar] [CrossRef]
- Soudani, N.; Souissi-najar, S.; Ouederni, A. Influence of Nitric Acid Concentration on Characteristics of Olive Stone Based Activated Carbon. Chin. J. Chem. Eng. 2013, 21, 1425–1430. [Google Scholar] [CrossRef]
- Pereira, L.; Pereira, R.; Pereira, M.F.R.; van der Zee, F.P.; Cervantes, F.J.; Alves, M.M. Thermal modification of activated carbon surface chemistry improves its capacity as redox mediator for azo dye reduction. J. Hazard. Mater. 2010, 183, 931–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centeno, T.A.; Stoeckli, F. The assessment of surface areas in porous carbons by two model-independent techniques, the DR equation and DFT. Carbon 2010, 48, 2478–2486. [Google Scholar] [CrossRef] [Green Version]
- Montes-Morán, M.A.; Suárez, D.; Angel-Menéndez, J.; Fuente, E. The Basicity of Carbons. In Novel Carbon Adsorbents; Tascón, J.M.D., Ed.; Elsevier: London, UK, 2012; pp. 173–203. [Google Scholar]
- Kazmierczak-Razna, J.; Nowicki, P.; Wiśniewska, M.; Nosal-Wiercińska, A.; Pietrzak, R. Thermal and physicochemical properties of phosphorus-containing activated carbons obtained from biomass. J. Taiwan Inst. Chem. Eng. 2017, 80, 1006–1013. [Google Scholar] [CrossRef]








| Sample | N2 Adsorption | Surface Chemical Groups | |||
|---|---|---|---|---|---|
| BET area (m2g−1) | Wo (cm3g−1) | Vtotal (cm3g−1) | Total Acidity (mol g−1) | Total Basicity (mol g−1) | |
| CAG | 841 | 0.34 | 0.38 | 0.20 | 0.08 |
| CAN | 810 | 0.32 | 0.37 | 0.39 | 0.05 |
| CAG1173 | 996 | 0.36 | 0.42 | 0.05 | 0.31 |
| CAN1023 | 935 | 0.35 | 0.41 | 0.06 | 0.26 |
| CAN723 | 903 | 0.35 | 0.40 | 0.28 | 0.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Monje, D.; Giraldo, L.; Moreno-Piraján, J.C. Immersion Enthalpy of Activated Carbon–Cyclohexane and Activated Carbon–Hexane. Difference in the Solid–Liquid Interaction Enthalpy Due to the Structure of the Solvent. Processes 2019, 7, 180. https://doi.org/10.3390/pr7040180
Hernández-Monje D, Giraldo L, Moreno-Piraján JC. Immersion Enthalpy of Activated Carbon–Cyclohexane and Activated Carbon–Hexane. Difference in the Solid–Liquid Interaction Enthalpy Due to the Structure of the Solvent. Processes. 2019; 7(4):180. https://doi.org/10.3390/pr7040180
Chicago/Turabian StyleHernández-Monje, Diana, Liliana Giraldo, and Juan Carlos Moreno-Piraján. 2019. "Immersion Enthalpy of Activated Carbon–Cyclohexane and Activated Carbon–Hexane. Difference in the Solid–Liquid Interaction Enthalpy Due to the Structure of the Solvent" Processes 7, no. 4: 180. https://doi.org/10.3390/pr7040180






