Experimental Data of Fluid Phase Equilibria- Correlation and Prediction Models: A Review
Abstract
:1. Introduction
2. Correlation Methods
3. Predictive Methods
4. Separation Application Using Limiting Activity Coefficients
5. Separation in Ternary LLE Using COSMO-RS
6. Molecular Simulation as A Thermodynamic Tool
7. Summary and Future Perspective
Funding
Conflicts of Interest
References
- Pârvulescu, V.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef]
- Lewandowski, A.; Świderska-Mocek, A. Ionic liquids as electrolytes for Li-ion batteries—An overview of electrochemical studies. J. Power Sources 2009, 194, 601–609. [Google Scholar] [CrossRef]
- Hallet, J.P.; Welton, T. Room temperature ionic liquids: Solvents for synthesis and catalysis. Chem. Rev. 2011, 11, 3508–3676. [Google Scholar] [CrossRef] [PubMed]
- Domańska, U. Ionic Liquids in Chemical Analysis, Chapter 1, General Review of Ionic Liquids and Their Properties; CRC Press, Taylor & Francis Group: Abingdon, UK, 2008; pp. 1–72. [Google Scholar]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Hayyan, M.; Hasim, M.A.; Hayyan, A. The role of ionic liquids in desulfurization of fuels: A review. Renew. Sustain. Energy Rev. 2017, 76, 1534–1549. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Afonso, C.A.M. Deep desulfurization of diesel fuel using ionic liquids: Current status and future challenges. Green Chem. 2010, 12, 1139–1149. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Silva, F.A.E.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-liquid-mediated extraction and separation processes for bioactive compounds: Past, present, and future trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Arce, A.; Earle, M.; Katdare, S.; Rodríguez, H.; Seddon, K.S. Application of mutually immiscible ionic liquids to the separation of aromatic and aliphatic hydrocarbons by liquid extraction: A preliminary approach. Phys. Chem. Chem. Phys. 2008, 10, 2538–2542. [Google Scholar] [CrossRef]
- Meindersma, G.W.; Hansmeier, A.R.; de Haan, A.B. Ionic liquids for aromatics extraction. Present status and future outlook. Ind. Eng. Chem. Res. 2010, 49, 7530–7540. [Google Scholar] [CrossRef]
- Marciniak, A. Influence of cation and anion structure of the ionic liquid on extraction processes based on activity coefficients at infinite dilution. A review. Fluid Phase Equilib. 2010, 294, 213–233. [Google Scholar] [CrossRef]
- Domańska, U.; Pobudkowska, A.; Żołek-Tryznowska, Z. Effect of an Ionic Liquid (IL) Cation on the Ternary System (IL + p-Xylene + Hexane) at T = 298.15 K. J. Chem. Eng. Data 2007, 52, 2345–2349. [Google Scholar] [CrossRef]
- González, E.J.; González, B.; Calvar, N.; Dominguez, Á. Application of [EMpy][ESO4] ionic liquid as solvent for the liquid extraction of xylenes from hexane. Fluid Phase Equilib. 2010, 295, 249–254. [Google Scholar] [CrossRef]
- Kędra-Królik, K.; Mutelet, F.; Jaubert, J.-N. Extraction of Thiophene or Pyridine from n-Heptane Using Ionic Liquids. Gasoline and Diesel Desulfurization. Ind. Eng. Chem. Res. 2011, 50, 2296–2306. [Google Scholar] [CrossRef]
- Verdía, P.; González, E.J.; Rodríguez-Cabo, B.; Tojo, E. Synthesis and characterization of new polysubstituted pyridinium-based ionic liquids: Application as solvents on desulfurization of fuel oils. Green Chem. 2011, 13, 2768–2776. [Google Scholar] [CrossRef]
- Domańska, U.; Wlazło, M. Effect of the cation and anion of the ionic liquid on desulfurization of model fuels. Fuel 2014, 134, 114–125. [Google Scholar] [CrossRef]
- Ahmed, O.U.; Mjalli, F.S.; Hadj-Kali, M.K.; Al-Wahaibi, T.; Al-Wahaibi, T. Measurements and prediction of ternary liquid–liquid equilibria for mixtures of IL + sulfur compound + hexadecane. Fluid Phase Equilib. 2016, 421, 16–23. [Google Scholar] [CrossRef]
- Domańska, U.; Walczak, K.; Królikowski, M. Extraction desulfurization process of fuels with ionic liquids. J. Chem. Thermodyn. 2014, 77, 40–45. [Google Scholar] [CrossRef]
- Durski, M.; Naidoo, P.; Ramjugernath, D.; Domańska, U. Thermodynamics and activity coefficients at infinite dilution for organic solutes in the ionic liquid 1-butyl-1-methylpyrrolidinium dicyanamide. Fluid Phase Equilib. 2018, 473, 175–182. [Google Scholar] [CrossRef]
- Karpińska, M.; Wlazło, M.; Zawadzki, M.; Domańska, U. Liquid-liquid separation of hexane/hex-1-ene and cyclohexane/cyclohexene by dicyanamide-based ionic liquids. J. Chem. Thermodyn. 2018, 116, 299–308. [Google Scholar] [CrossRef]
- Domańska, U.; Karpińska, M.; Wlazło, M. Separation of hex-1-ene/hexane and cyclohexene/cyclohexane compounds with [EMIM]-based ionic liquids. Fluid Phase Equilib. 2016, 427, 421–428. [Google Scholar] [CrossRef]
- Karpińska, M.; Wlazło, M.; Domańska, U. Liquid-liquid separation of hex-1-ene from hexane and cyclohexene from cyclohexane with ionic liquids. J. Chem. Thermodyn. 2017, 108, 127–135. [Google Scholar] [CrossRef]
- Domańska, U.; Karpińska, M.; Wlazło, M. Bis(trifluoromethylsulfonyl)imide, or dicyanamide-based ionic liquids in the liquid-liquid extraction of hex-1-ene from hexane and cyclohexene from cyclohexane. J. Chem. Thermodyn. 2017, 105, 375–384. [Google Scholar] [CrossRef]
- Domańska, U.; Wlazło, M.; Karpińska, M. Activity coefficients at infinite dilution of organic solvents and water in the 1-butyl-3-methylimidazolium dicyanamide. A literature review of hexane/hex-1-ene separation. Fluid Phase Equilib. 2016, 417, 50–61. [Google Scholar] [CrossRef]
- Chapeaux, A.; Simoni, L.D.; Ronan, T.S.; Stadtherr, M.A.; Brennecke, J.F. Extraction of alcohols from water with 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. Green Chem. 2008, 10, 1301–1306. [Google Scholar] [CrossRef]
- Domańska, U.; Wlazło, M.; Paduszyński, K. Extraction butan-1-ol from aqueous solution using ionic liquids: An effect of cation revealed by experiments and thermodynamic models. Sep. Purif. Technol. 2018, 196, 71–81. [Google Scholar] [CrossRef]
- Wlazło, M.; Zawadzki, M.; Domańska, U. Separation of water/butan-1-ol based on activity coefficients at infinite dilution in 1,3-didecyl-2-methylimidazolium dicyanamide ionic liquid. J. Chem. Thermodyn. 2018, 116, 316–322. [Google Scholar] [CrossRef]
- Domańska, U.; Królikowski, M. Extraction of butan-1-ol from water with ionic liquids at T = 308.15 K. J. Chem. Thermodyn. 2012, 53, 108–113. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.; Liu, H. Liquid–Liquid Equilibria of the System 1-(2-Hydroxyethyl)-3-methylimidozolium Tetrafluoroborate or 1-(2-Hydroxyethyl)-2,3-dimethylimidozolium Tetrafluoroborate + Water + 1-Butanol at 293.15 K. J. Chem. Eng. Data 2006, 51, 691–695. [Google Scholar] [CrossRef]
- Nann, A.; Mündges, J.; Held, C.; Verevkin, S.P.; Sadowski, G. Molecular Interactions in 1-Butanol + IL Solutions by Measuring and Modeling Activity Coefficients. J. Phys. Chem. B 2013, 117, 3173–3185. [Google Scholar] [CrossRef]
- Marciniak, A.; Wlazło, M.; Gawkowska, J. Ternary (liquid + liquid) equilibria of {bis(trifluoromethylsulfonyl)-amide based ionic liquids + butan-1-ol + water}. J. Chem. Thermodyn. 2016, 94, 96–100. [Google Scholar] [CrossRef]
- Sendovski, M.; Nir, N.; Fishman, A. Bioproduction of 2-phenylethanol in a biphasic ionic liquid aqueous system. J. Agric. Food Chem. 2010, 58, 2260–2265. [Google Scholar] [CrossRef] [PubMed]
- Domańska, U.; Królikowski, M.; Zawadzki, M.; Wróblewska, A. Phase equilibrium investigation with ionic liquids and selectivity in separation of 2-phenylethanol from water. J. Chem. Thermodyn. 2016, 102, 357–366. [Google Scholar] [CrossRef]
- Domańska, U.; Paduszyński, K.; Królikowski, M.; Wróblewska, A. Separation of 2-phenylethanol from water by liquid-liquid extraction with ionic liquids—New experimental data and modelling with modern thermodynamic tools. Ind. Eng. Chem. Res. 2016, 55, 5736–5747. [Google Scholar] [CrossRef]
- Okuniewska, P.; Domańska, U.; Więckowski, M.; Mierzejewska, J. Recovery of 2-phenylethanol from aqueous solutions of biosynthesis using ionic liquids. Sep. Purif. Technol. 2017, 188, 530–538. [Google Scholar] [CrossRef]
- Domańska, U.; Marciniak, A. Phase behavior of 1-hexyloxymethyl-3-methyl- imidazolium and 1,3-dihexyloxymethyl-imidazolium based ionic liquids with alcohols, water, ketones and hydrocarbons: The effect of cation and anion on solubility. Fluid Phase Equilib. 2007, 260, 9–18. [Google Scholar] [CrossRef]
- Domańska, U.; Marciniak, A.; Królikowski, M. Phase equilibria and modelling of ammonium ionic liquid, C2NTf2, solutions. J. Phys. Chem. B 2008, 1121, 1218–1225. [Google Scholar] [CrossRef]
- Domańska, U.; Paduszyński, K. Phase equilibria study in the binary systems (tetra-n- butylphosphonium tosylate ionic liquid + 1-alcohol, or benzene, or n-alkylbenzene). J. Phys. Chem. B 2008, 112, 11054–11059. [Google Scholar] [CrossRef]
- Domańska, U.; Zawadzki, M.; Królikowski, M.; Lewandrowska, A. Phase equilibria study of binary and ternary mixtures of {N-octylisoquinolinium bis{(trifluoromethyl)sulfonyl}imide + hydrocarbon, or an alcohol, or water}. Chem. Eng. J. 2012, 181-182, 63–71. [Google Scholar] [CrossRef]
- Paduszyński, K.; Lukoskho, E.; Królikowski, M.; Domańska, U. Measurements and equation-of-state modelling of thermodynamic properties of binary mixtures of 1-butyl- 1-methylpyrrolidinium tetracyanoborate ionic liquid with molecular compounds. J. Chem. Thermodyn. 2015, 90, 317–326. [Google Scholar] [CrossRef]
- Paduszyński, K.; Okuniewski, M.; Domańska, U. Sweet-in-Green” systems based on sugars and ionic liquids: New solubility data and thermodynamic analysis. Ind. Eng. Chem. Res. 2013, 52, 18482–18491. [Google Scholar] [CrossRef]
- Domańska, U.; Królikowski, M.; Wlazło, M.; Więckowski, M. Extraction of 2-phenylethanol (PEA) from aqueous solution using ionic liquids: Synthesis, phase equilibrium investigation, selectivity in separation and thermodynamic models. J. Phys. Chem. B 2018, 122, 6188–6197. [Google Scholar] [CrossRef]
- Domańska, U.; Karpińska, M.; Wiśniewska, A.; Dąbrowski, Z. Ammonium ionic liquids in extraction of bio-butan-1-ol from water phase using activity coefficients at infinite dilution. Fluid Phase Equilib. 2019, 479, 9–16. [Google Scholar] [CrossRef]
- Domańska, U.; Wlazło, M.; Dąbrowski, Z.; Wiśniewska, A. Ammonium ionic liquids in separation of water/butan-1-ol using liquid-liquid equilibrium diagrams in ternary systems. Fluid Phase Equilib. 2019, 485, 23–31. [Google Scholar] [CrossRef]
- Domańska, U.; Wlazło, M.; Karpińska, M. Separation of water/butanol with ionic liquids in ternary liquid-liquid phase equilibrium. J. Chem. Thermodyn. 2019, 134, 76–83. [Google Scholar] [CrossRef]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many By-Products; Elsevier: Amsterdam, The Netherlands, 2000; ISBN 0-444-50351-X. [Google Scholar]
- Halayqa, M.; Zawadzki, M.; Domańska, U.; Plichta, A. Polymer-Ionic liquid-pharmaceutical conjugates as drug delivery systems. J. Mol. Struct. 2019, 1180, 573–584. [Google Scholar] [CrossRef]
- Pelczarska, A.; Ramjugernat, D.; Rarey, J.; Domańska, U. Prediction of the solubility of selected pharmaceuticals in water and alcohols with a group contribution method. J. Chem. Thermodyn. 2013, 62, 118–129. [Google Scholar] [CrossRef]
- Domańska, U.; Pobudkowska, A.; Pelczarska, A.; Żukowski, Ł. Modelling, solubility and pKa of five sparingly soluble drugs. Int. J. Pharm. 2011, 403, 115–122. [Google Scholar] [CrossRef]
- Domańska, U.; Pobudkowska, A.; Pelczarska, A. Solubility of sparingly soluble drug derivatives of anthranilic acid. J. Phys. Chem. B 2011, 115, 2547–2554. [Google Scholar] [CrossRef]
- Domańska, U.; Pelczarska, A.; Pobudkowska, A. Solubility and pKa determination of six structurally related phenothiazines. Int. J. Pharm. 2011, 421, 135–144. [Google Scholar] [CrossRef]
- Halayqa, M.; Pobudkowska, A.; Zawadzki, M.; Domańska, U. Studying od drug solubility in water and alcohols uising drug-ammonium ionic liquid-compounds. Eur. J. Pharm. Sci. 2018, 111, 270–277. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Goto, M. Ionic Liquids: Future solvents and reagents for pharmaceuticals. J. Chem. Eng. Jpn. 2011, 44, 370–381. [Google Scholar] [CrossRef]
- Bica, K.; Rijksen, C.; Nieuwenhuyzen, M.; Rogers, R.D. In search of pure liquid salt forms of aspirin: Ionic liquid approaches with acetylsalicylic acid and salicylic acid. Phys. Chem. Chem. Phys. 2010, 12, 2011–2017. [Google Scholar] [CrossRef]
- Letcher, T.M.; Ramjugernath, D.; Tumba, K.; Królikowski, M.; Domańska, U. (Solid + Liquid) and (liquid + liquid) phase equilibria and correlation of the binary systems {N-butyl-3-methylpyridinium tosylate + water, or + an alcohol, or + a hydrocarbon}. Fluid Phase Equilib. 2010, 294, 89–97. [Google Scholar] [CrossRef]
- Domańska, U.; Królikowska, M.; Królikowski, M. Thermodynamic phase behaviour and physico-chemical properties of the binary systems {(1-ethyl-3-methylimidazolium thiocyanate, or 1-ethyl-3-methylimidazolium tosylate + water, or + an alcohol}. Fluid Phase Equilib. 2010, 294, 72–93. [Google Scholar] [CrossRef]
- Wilson, G.M. Vapor-Liquid Equilibrium. XI. A New Expression for the Excess Free Energy of Mixing. J. Am. Chem. Soc. 1964, 86, 127–130. [Google Scholar] [CrossRef]
- Abrams, D.S.; Prausnitz, J.M. Statistical Thermodynamics of Liquid Mixtures: A New Expression for the Excess Gibbs Energy of Partly or Completely Miscible Systems. AIChE J. 1975, 21, 116–128. [Google Scholar] [CrossRef]
- Renon, H.; Prausnitz, J.M. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 1968, 14, 135–144. [Google Scholar] [CrossRef]
- Walas, S.W. Phase Equilibria in Chemical Engineering; Butterworth Publishers: Boston, MA, USA, 1985; pp. 279–297. [Google Scholar]
- Weidlich, U.; Gmehling, J. A modified UNIFAC model. 1. Prediction of VLE, hE, and gamma.infin. Ind. Eng. Chem. Res. 1987, 26, 1372–1381. [Google Scholar] [CrossRef]
- Gmehling, J.; Li, J.; Schiller, M. A modified UNIFAC model. 2. Present parameter matrix and results for different thermodynamic properties. Ind. Eng. Chem. Res. 1993, 32, 178–193. [Google Scholar] [CrossRef]
- Moller, B. Activity of Complex Multifunctional Organic Compounds in Common Solvents. Ph.D. Thesis, Chemical Engineering, University of KwaZulu-Natal, Durban, South Africa, 2009. [Google Scholar]
- Paduszyński, K.; Domańska, U. Solubility of aliphatic hydrocarbons in piperidinium ionic liquids: Measurements and modeling in terms of perturbed-bonding-chain statistical associating fluid theory and nonrandom hydrogen-bonding theory. J. Phys. Chem. B 2011, 115, 12537–12548. [Google Scholar] [CrossRef]
- Gross, J.; Sadowski, G. Perturbed-Chain SAFT: An Equation of State Based on a Perturbation Theory for Chain Molecules. Ind. Eng. Chem. Res. 2001, 40, 1244–1260. [Google Scholar] [CrossRef]
- Gross, J.; Sadowski, G. Application of the Perturbed-Chain SAFT Equation of State to Associating Systems. Ind. Eng. Chem. Res. 2002, 41, 5510–5515. [Google Scholar] [CrossRef]
- Klamt, A. COSMO-RS: From Quantum Chemistry to Fluid Phase Thermodynamics and Drug Design; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Domańska, U.; Zawadzki, M.; Królikowski, M. Heat capacity, excess molar volumes and excess dynamic viscosity of binary systems of N-octylisoquinolinium bis{(trifluoromethyl)sulfonyl}imide ionic liquid. Z. Phys. Chem. 2013, 227, 217–238. [Google Scholar] [CrossRef]
- Vega, J.F.; Vilaseca, O.; Llovell, F.; Andreu, J.S. Modeling ionic liquids and the solubility of gases in them: Recent advances and perspectives. Fluid Phase Equilib. 2010, 294, 15–30. [Google Scholar] [CrossRef]
- Paduszyński, K.; Chiyen, J.; Ramjugernath, D.; Letcher, T.M.; Domańska, U. Liquid-liquid phase equilibria of (piperidinium-based ionic liquid + an alcohol) binary systems in terms of NRHB model and PCP-SAFT. Fluid Phase Equilib. 2011, 305, 43–52. [Google Scholar] [CrossRef]
- Paduszyński, K.; Domańska, U. Thermodynamic modeling of ionic liquid systems. Development and detailed overviev of novel methodology based on the PC-SAFT. J. Phys. Chem. B 2012, 116, 5002–5018. [Google Scholar] [CrossRef]
- Domańska, U.; Zawadzki, M.; Tshibangu, M.; Ramjugernath, D.; Letcher, T.M. Phase equilibria study of {N-hexylisoquinolinium bis{(trifluoromethyl)sulfony}imide + aromatuic hydrocarbons or an alcohol} binary systems. J. Phys. Chem. B 2011, 115, 4003–4010. [Google Scholar] [CrossRef]
- Llovell, L.; Valente, E.; Vilaseca, O.; Vega, L.F. Modeling complex associating mixtures with [Cn-mim][Tf2N] ionic liquids: Predictions from the Soft-SAFT Equation. J. Phys. Chem. B 2011, 115, 4387–4398. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Tsivintzelis, I.; Panayiotou, C. Equation-of-state modeling of mixtures with ionic liquids. Phys. Chem. Chem. Phys. 2010, 12, 4843–4851. [Google Scholar] [CrossRef]
- Blas, F.J.; Vega, L.F. Thermodynamic behaviour of homonuclear and heteronuclear Lennard-Jones chains with association sites from simulation and theory. Mol. Phys. 1997, 92, 135–150. [Google Scholar] [CrossRef]
- Karakatsani, E.K.; Economu, I.G. Perturbed chain-statistical associating fluid theory extended to dipolar and quadrupolar molecular fluids. J. Phys. Chem. B 2006, 110, 9252–9261. [Google Scholar] [CrossRef]
- Karakatsani, E.K.; Kantageorgis, G.M.; Economu, I.G. Evaluation of the truncated perturbed chain-polar statistical associating fluid theory for complex mixture fluid phase equilibria. Ind. Eng. Chem. Res. 2006, 45, 6063–6074. [Google Scholar] [CrossRef]
- Tan, S.P.; Adirama, H.; Radosz, M. Recent advances and applications of statistical associating fluid theory. Ind. Eng. Chem. Res. 2008, 47, 8063–8082. [Google Scholar] [CrossRef]
- Shimizu, K.; Tariq, M.; Costa Gomes, M.; Rebelo, L.P.N.; Canongia Lopes, J.N.A. Assessing the dispersive and electrostatic components of the cohesive energy of ionic liquids using molecular dynamics simulations and molar refraction data. J. Phys. Chem. B 2010, 114, 5831–5834. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.M.; Rocha, M.A.A.; Freire, M.G.; Marrucho, I.M.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Evaluation of cation–anion interaction strength in ionic liquids. J. Phys. Chem. B 2011, 115, 4033–4041. [Google Scholar] [CrossRef] [PubMed]
- Andreu, J.S.; Vega, L.F. Modeling the solubility behavior of CO2, H2, and Xe in [Cn-mim][Tf2N] ionic liquids. J. Phys. Chem. B 2008, 112, 15398–15406. [Google Scholar] [CrossRef] [PubMed]
- Klamt, A. Conductor-like screening model for real solvents: A new approach to the quantitative calculation of solvation phenomena. J. Phys. Chem. 1995, 99, 2224–2235. [Google Scholar] [CrossRef]
- Klamt, A.; Eckert, F. COSMO-RS: A novel and efficient method for the a priori prediction of thermophysical data of liquids. Fluid Phase Equilib. 2000, 172, 43–72. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- Eckert, F.; Klamt, A. COSMOtherm (v. C3.0, Release 17.01); COSMOlogic GmbH& Co. KG: Leverkusen, Germany, 2017. [Google Scholar]
- Domańska, U.; Okuniewska, P.; Paduszyński, K.; Królikowska, M.; Zawadzki, M.; Więckowski, M. Extraction of 2-phenylethanol (PEA) from aqueous solution using ionic liquids: synthesis, phase equilibrium investigation, selectivity in separation, and thermodynamic models. J. Phys. Chem. B 2017, 121, 7689–7698. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Freire, M.G.; Ribeiro, J.C.; Lopes, F.M.; Crespo, J.G.; Coutinho, J.A.P. An Overview of the Liquid–Liquid Equilibria of (Ionic Liquid + Hydrocarbon) Binary Systems and Their Modeling by the Conductor-like Screening Model for Real Solvents. Ind. Eng. Chem. Res. 2011, 50, 5279–5294. [Google Scholar] [CrossRef]
- Domańska, U.; Zawadzki, M.; Paduszyński, K.; Królikowski, M. Perturbed-chain SAFT as a versatile tool for thermodynamic modeling of binary mixtures containing isoquinolinium ionic liqids. J. Phys. Chem. B 2012, 116, 8191–8200. [Google Scholar] [CrossRef]
- Paduszyński, K.; Domańska, U. New group contribution method for prediction of density of pure ionic liquids over a wide range of temperature and pressure. Ind. Eng. Chem. Res. 2012, 51, 591–604. [Google Scholar] [CrossRef]
- Abraham, M.H. Scales of solute hydrogen-bonding: Their construction and application to physicochemical and biochemical processes. Chem. Soc. Rev. 1993, 22, 73–85. [Google Scholar] [CrossRef]
- Hector, T.; Gmehling, J. Present status of the modified UNIFAC model for the prediction of phase equilibria and excess enthalpies for systems with ionic liquids. Fluid Phase Equilib. 2014, 371, 82–92. [Google Scholar] [CrossRef]
- Vale, W.R.; Rathke, B.; Will, S.; Schröer, W. Liquid−liquid phase behavior of solutions of 1-octyl- and 1-decyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (C8,10mimNTf2) in n-alkyl alcohols. J. Chem. Eng. Data 2010, 55, 2030–2038. [Google Scholar] [CrossRef]
- Heintz, A.; Kulikov, D.V.; Verevkin, S.P. Thermodynamic properties of mixtures containing ionic liquids. 2. Activity coefficients at infinite dilution of hydrocarbons and polar solutes in 1-methyl-3-ethyl-imidazolium bis(trifluoromethyl-sulfonyl) amide and in 1,2-dimethyl-3-ethyl-imidazolium bis(trifluoromethyl-sulfonyl) amide using gas−liquid chromatography. J. Chem. Eng. Data 2002, 47, 894–899. [Google Scholar]
- Verevkin, S.P.; Safarov, J.; Bich, E.; Hassel, E.; Heintz, A. Thermodynamic properties of mixtures containing ionic liquids: Vapor pressures and activity coefficients of n-alcohols and benzene in binary mixtures with 1-methyl-3-butyl-imidazolium bis(trifluoromethyl-sulfonyl) imide. Fluid Phase Equilib. 2005, 236, 222–228. [Google Scholar] [CrossRef]
- Kato, R.; Gmehling, J. Activity coefficients at infinite dilution of various solutes in the ionic liquids [MMIM]+[CH3SO4]−, [MMIM]+[CH3OC2H4SO4]−, [MMIM]+[(CH3)2PO4]−, [C5H5NC2H5]+[(CF3SO2)2N]− and [C5H5NH]+[C2H5OC2H4OSO3]−. Fluid Phase Equilib. 2004, 226, 37–44. [Google Scholar] [CrossRef]
- Kato, R.; Gmehling, J. Systems with ionic liquids: Measurement of VLE and γ∞ data and prediction of their thermodynamic behavior using original UNIFAC, mod. UNIFAC(Do) and COSMO-RS(Ol). J. Chem. Thermodyn. 2005, 37, 603–619. [Google Scholar] [CrossRef]
- Panayiotou, C.; Pantoula, M.; Stefanis, E.; Tsivintzelis, I.; Economou, I.G. Nonrandom hydrogen-bonding model of fluids and their mixtures. 1. Pure Ffuids. Ind. Eng. Chem. Res. 2004, 43, 6592–6606. [Google Scholar] [CrossRef]
- Panayiotou, C.; Tsivintzelis, I.; Economou, I.G. Nonrandom hydrogen-bonding model of fluids and their mixtures. 2. Multicomponent mixtures. Ind. Eng. Chem. Res. 2007, 46, 2628–2636. [Google Scholar] [CrossRef]
- Paduszyński, K.; Domańska, U. Limiting activity coefficients and gas-liquid partition coefficients of various solutes in piperidinium ionic liquids-measurements and LSER calculations. J. Phys. Chem. B 2011, 115, 8207–8215. [Google Scholar] [CrossRef]
- Wlazło, M.; Gawkowska, J.; Domańska, U. Separation based on limiting activity coefficients of various solutes in 1-allyl-3-methylimidazolium dicyanamide ionic liquid. Ind. Eng. Chem. Res. 2016, 55, 5054–5062. [Google Scholar] [CrossRef]
- Wlazło, M.; Karpińska, M.; Domańska, U. Thermodynamics and selectivity of separation based on activity coefficients at infinite dilution of various solutes in 1-allyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ionic liquid. J. Chem. Thermodyn. 2016, 102, 39–47. [Google Scholar] [CrossRef]
- Karpińska, M.; Wlazło, M.; Ramjugernath, D.; Naidoo, P.; Domańska, U. Assessment of certain ionic liquids for separation of binary mixtures based on gamma infinity data measurements. RSC Adv. 2017, 7, 7092–7107. [Google Scholar] [CrossRef]
- Domańska, U.; Karpińska, M.; Wlazło, M. Thermodynamic study of melecular interaction-selectivity in separation processes based on limiting activity coefficients. J. Chem. Thermodyn. 2018, 121, 112–120. [Google Scholar] [CrossRef]
- Wlazło, M.; Karpińska, M.; Domańska, U. A 1-alkylcyanopyridinium-based ionic liquid in the hexane/hex-1-ene separation. J. Chem. Thermodyn. 2016, 97, 253–260. [Google Scholar] [CrossRef]
- Karpińska, M.; Wlazło, M.; Zawadzki, M.; Domańska, U. Separation of binary mixtures hexane/hex-1-ene, cyclohexane/cyclohexene and ethylbenzene/styrene based on gamma infinity data measurements. J. Chem. Thermodyn. 2018, 118, 244–254. [Google Scholar] [CrossRef]
- Domańska, U.; Wlazło, M.; Karpińska, M.; Zawadzki, M. Separation of binary mixtures hexane/hex-1-ene. cyclohexane/cyclohexene and ethylbenzene/styrene based on limiting activity coefficients. J. Chem. Thermodyn. 2017, 110, 227–236. [Google Scholar] [CrossRef]
- Domańska, U.; Karpińska, M.; Zawadzki, M. Activity coefficients at infinite dilution for organic solutes and water in 1-ethyl-1-methylpyrrolidinium lactate. J. Chem. Thermodyn. 2015, 89, 127–133. [Google Scholar] [CrossRef]
- Domańska, U.; Karpińska, M. The use of ionic liquids for separation of binary hydrocarbons mixtures based on gamma infinity data measurements. J. Chem. Thermodyn. 2018, 127, 95–105. [Google Scholar] [CrossRef]
- Karpińska, M.; Wlazło, M.; Domańska, U. Separation of binary mixtures based on gamma infinity data using [EMIM][TCM] ionic liquid and modelling of thermodynamic functions. J. Mol. Liq. 2017, 225, 382–390. [Google Scholar] [CrossRef]
- Domańska, U.; Wlazło, M. Separation of ethylbenzene/styrene systems using ionic liquids in ternary LLE. J. Chem. Thermodyn. 2016, 103, 76–85. [Google Scholar] [CrossRef]
- Wlazło, M.; Domańska, U. Gamma infinity data for the separation of butan-1-ol-water mixtures using ionic liquid. Sep. Purif. Technol. 2016, 162, 162–170. [Google Scholar] [CrossRef]
- Domańska, U.; Wlazło, M.; Karpińska, M.; Zawadzki, M. Highly efficient water/butan-1-ol separation on investigation of limiting activity coefficients with [P8,8,8,8][NTf2] ionic liquid. Fluid Phase Equilib. 2017, 449, 1–9. [Google Scholar] [CrossRef]
- Wlazło, M.; Karpińska, M.; Domańska, U. Separation of water/butan-1-ol mixtures based on limiting activity coefficients with phosphonium-based ionic liquid. J. Chem. Thermodyn. 2017, 113, 183–191. [Google Scholar] [CrossRef]
- Marciniak, A.; Wlazło, M. Activity coefficients at infinite dilution and physicochemical properties for organic solutes and water in the ionic liquid trihexyl-tetradecyl-phosphonium tricyanomethanide. J. Chem. Thermodyn. 2018, 120, 72–78. [Google Scholar] [CrossRef]
- Wlazło, M.; Marciniak, A.; Zawadzki, M.; Dudkiewicz, B. Activity coefficients at infinite dilution and physicochemical properties for organic solutes and water in the ionic liquid 4-(3-hydroxypropyl)-4-methylmorpholinium bis(trifluoromethylsulfonyl)-amide. J. Chem. Thermodyn. 2015, 86, 154–161. [Google Scholar] [CrossRef]
- Everett, D.H. Effect of gas imperfection in GLC measurements: E refined method for determining activity coefficients and second virial coefficients. Trans. Faraday Soc. 1965, 61, 1637–1645. [Google Scholar] [CrossRef]
- Cruickshank, A.J.B.; Gainey, B.W.; Hicks, C.P.; Letcher, T.M.; Moody, R.W.; Young, C.L. Gas-liquid chromatographic determination of cross-term second virial coefficients using glycerol. Benzene + nitrogen and benzene + carbon dioxide at 50 °C. Trans. Faraday Soc. 1969, 65, 1014–1031. [Google Scholar] [CrossRef]
- Design Institute for Physical Properties, Sponsored by AICHE (2005; 2008; 2009; 2010). Dippr Project 801—Full Version. Design Institute for Physical Property Research/Aiche. Available online: http://knovel.com/web/portal/browse/display?_ext_knovel_display_bookid=1187&verticalid=0 (accessed on 12 April 2019).
- Poling, B.E.; Prausnitz, J.M. Properties of Gases and Liquids; McGraw-Hill Publishing: New York, NY, USA, 2001; Available online: http://lib.myilibrary.com?ID=91317 (accessed on 12 April 2019).
- Domańska, U.; Królikowska, M.; Acree, W.E., Jr.; Baker, G.A. Activity coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 1-ethyl-3-methylimidazolium tetracyanoborate. J. Chem. Thermodyn. 2011, 43, 1050–1057. [Google Scholar] [CrossRef]
- Grant, D.W. Gas-Liquid Chromatography; van Nostrand Reihold: London, UK, 1971. [Google Scholar]
- Domańska, U.; Laskowska, M. Measurements of activity coefficients at infinite dilution of aliphatic and aromatic hydrocarbons, alcohols, thiophene, tetrahydrofuran, MTBE, and water in ionic liquid [BMIM][SCN] using GLC. J. Chem. Thermodyn. 2009, 41, 645–650. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Coutinho, J.A.P.; Pinho, S.P.; Domańska, U. Measurements of activity coefficients at infinite dilution of organic solutes and water on polar imidazolium-based ionic liquids. J. Chem. Thermodyn. 2015, 91, 194–203. [Google Scholar] [CrossRef]
- Ge, M.-L.; Deng, X.-M.; Zhang, L.-H.; Chen, J.-Y.; Xiong, J.-M.; Li, W.-H. Activity coefficients at infinite dilution of organic solutes in the ionic liquid 1-butyl-3-methylimidazolium methyl sulfate. J. Chem. Thermodyn. 2014, 77, 7–13. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, L.-S. Activity Coefficients at Infinite Dilution of Alkanes, Alkenes, and Alkyl Benzenes in 1-Butyl-3-methylimidazolium Tetrafluoroborate Using Gas-Liquid Chromatography. J. Chem. Eng. Data 2006, 51, 1698–1701. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, Y.; Chen, J.; Fei, W. Measurement of activity coefficients at infinite dilution for hydrocarbons in imidazolium-based ionic liquids and QSPR model. Front. Chem. Eng. China 2007, 1, 190–194. [Google Scholar] [CrossRef]
- Królikowski, M.; Królikowska, M. The study of activity coefficients at infinite dilution for organic solutes and water in 1-butyl-4-methylpyridinium dicyanamide, [B4MPy][DCA] using GLC. J. Chem. Thermodyn. 2014, 68, 138–144. [Google Scholar] [CrossRef]
- Paduszyński, K. An overview of the performance of the COSMO-RS approach in predicting the activity coefficients of molecular solutes in ionic liquids and derived properties at infinite dilution. Phys. Chem. Chem. Phys. 2017, 19, 11835–11850. [Google Scholar] [CrossRef] [PubMed]
- Paduszyński, K. Extensive Evaluation of the Conductor-like Screening Model for Real Solvents Method in Predicting Liquid–Liquid Equilibria in Ternary Systems of Ionic Liquids with Molecular Compounds. J. Phys. Chem. B 2018, 122, 4016–4028. [Google Scholar] [CrossRef]
- Paduszyński, K.; Domańska, U. COSMO-RS screening for ionic liquid to be applied in extraction of 2-phenylethanol from aqueous solutions. J. Mol. Liq. 2018, 271, 305–312. [Google Scholar] [CrossRef]
- Ahlrichs, R.; Bar, M.; Haser, M.; Horn, H.; Kolmel, C. Electronic structure calculations on workstation computers: The program system turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- MarvinSketch, Version 16.3.28, Release 2016; ChemAxon Ltd.: Budapest, Hungary, 2016.
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behaviour. Phys. Rev. A 1988, 38, 3098–3107. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the in homogeneous electron gas. Phys. Rev. B 1986, 33, 8822. [Google Scholar] [CrossRef]
- Schafer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Domańska, U.; Lukoshko, E.V.; Królikowski, M. Separation of thiophene from heptane with ionic liquids. J. Chem. Thermodyn. 2013, 61, 126–131. [Google Scholar] [CrossRef]
- Paduszyński, K.; Królikowski, M.; Zawadzki, M.; Orzeł, P. Computer-aided molecular design of new task-specific ionic liquids for extractive desulfurization of gasoline. ACS Sustain. Chem. Eng. 2017, 5, 9032–9042. [Google Scholar] [CrossRef]
- Maginn, E.J. Molecular simulation of ionic liquids: Current status and future opportunities. J. Phys. Condens. Matter 2009, 21, 373101–373113. [Google Scholar] [CrossRef]
- Smiatek, J. Aqueous ionic liquids and their effects on protein structures: An overview on recent theoretical and experimental results. J. Phys. Condens. Matter 2017, 29, 233001–233009. [Google Scholar] [CrossRef]
- Shah, J.K.; Maginn, E.J. Molecular Simulation of Ionic Liquids: Where We Are and the Path Forward. Ionic Liquids Further, Un COILed: Critical Experts Overview; Plechkova, N.V., Seddon, K.R., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2014; Chapter 6. [Google Scholar]
- Gong, Z.; Sun, H. Extension of TEAM Force-Field Database to Ionic Liquids. J. Chem. Eng. Data 2019. [Google Scholar] [CrossRef]




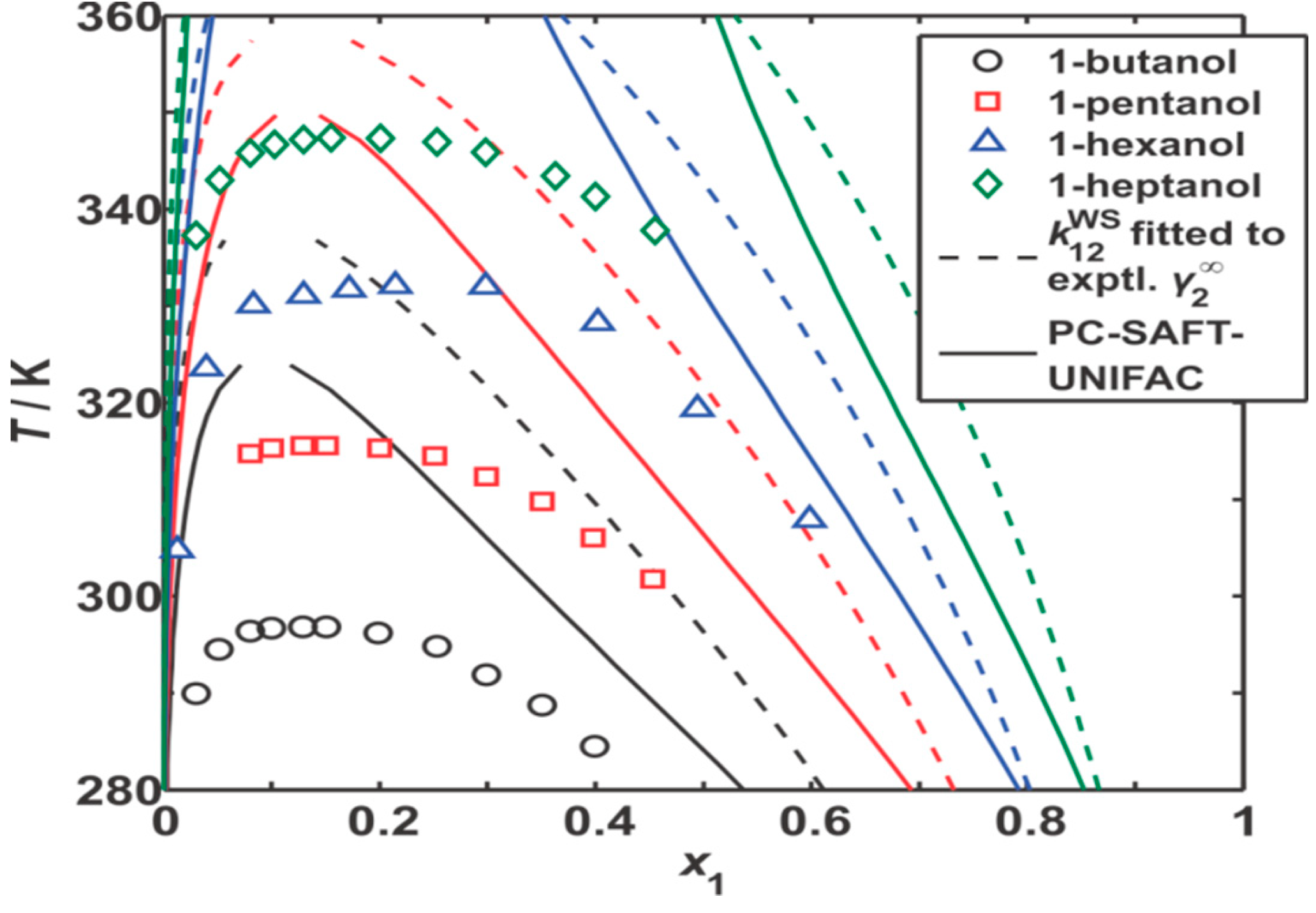
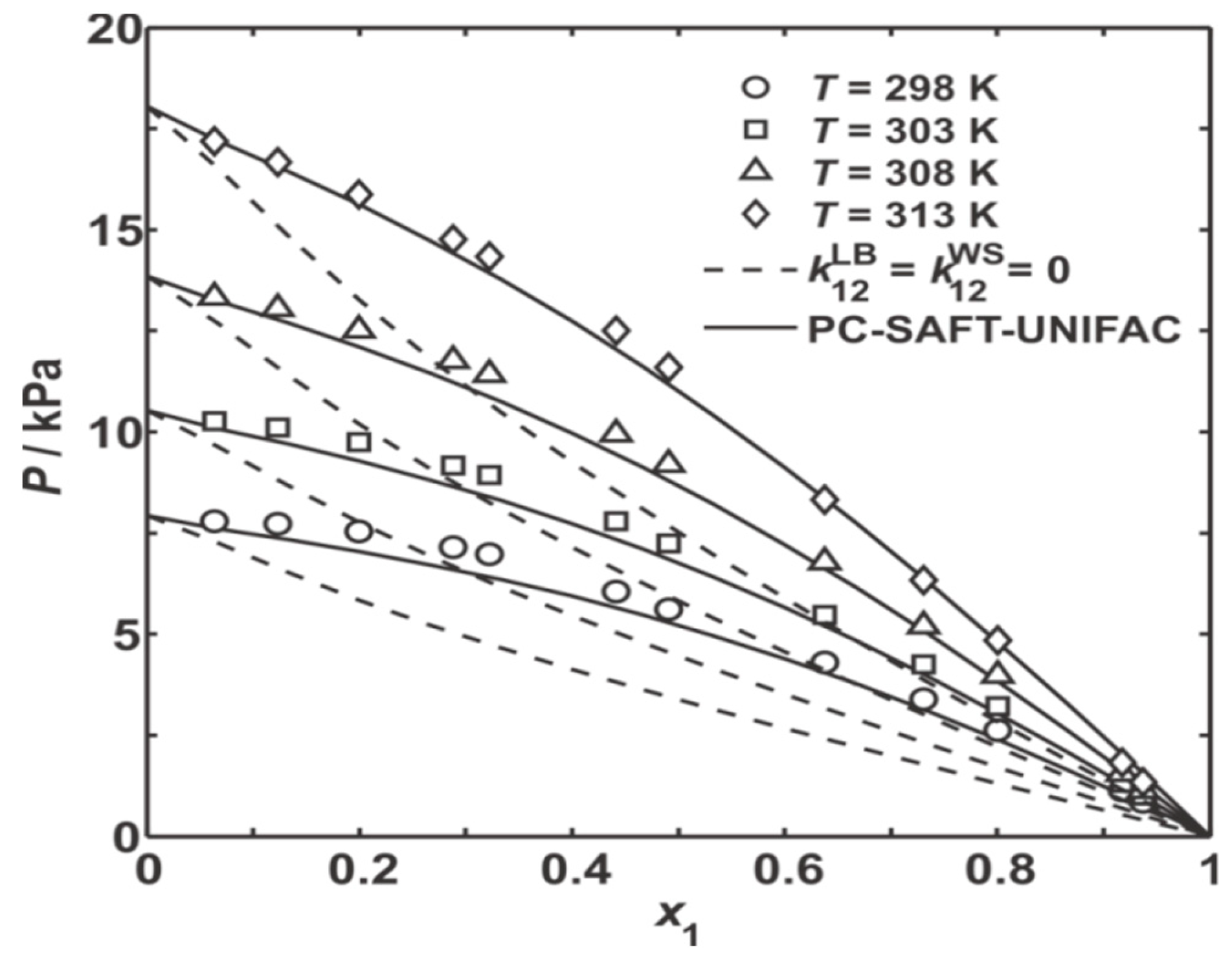


 ) [BzMIM][DCA]; (○) [EMIM][TCM]; (□) [EMPyr][Lac]; (Δ) [BCN4PY][NTf2]; (◊) [AMIM][NTf2]; (
) [BzMIM][DCA]; (○) [EMIM][TCM]; (□) [EMPyr][Lac]; (Δ) [BCN4PY][NTf2]; (◊) [AMIM][NTf2]; (  ) [BzMIM][NTf2].
) [BzMIM][NTf2].
 ) [BzMIM][DCA]; (○) [EMIM][TCM]; (□) [EMPyr][Lac]; (Δ) [BCN4PY][NTf2]; (◊) [AMIM][NTf2]; (
) [BzMIM][DCA]; (○) [EMIM][TCM]; (□) [EMPyr][Lac]; (Δ) [BCN4PY][NTf2]; (◊) [AMIM][NTf2]; (  ) [BzMIM][NTf2].
) [BzMIM][NTf2].
 ) [N-C3OHMMor][DCA]; (●) [EMMOR][DCA]; (■) [N-C3OHPY][DCA]; (▲) [N-C3OHMIM][DCA]; (♦) [AMIM][DCA]; (
) [N-C3OHMMor][DCA]; (●) [EMMOR][DCA]; (■) [N-C3OHPY][DCA]; (▲) [N-C3OHMIM][DCA]; (♦) [AMIM][DCA]; (  ) [BzMIM][DCA]; (○) [EMIM][TCM]; (□) [EMPyr][Lac]; (Δ) [BCN4PY][NTf2]; (◊) [AMIM][NTf2]; (
) [BzMIM][DCA]; (○) [EMIM][TCM]; (□) [EMPyr][Lac]; (Δ) [BCN4PY][NTf2]; (◊) [AMIM][NTf2]; (  ) [BzMIM][NTf2].
) [BzMIM][NTf2].
 ) [N-C3OHMMor][DCA]; (●) [EMMOR][DCA]; (■) [N-C3OHPY][DCA]; (▲) [N-C3OHMIM][DCA]; (♦) [AMIM][DCA]; (
) [N-C3OHMMor][DCA]; (●) [EMMOR][DCA]; (■) [N-C3OHPY][DCA]; (▲) [N-C3OHMIM][DCA]; (♦) [AMIM][DCA]; (  ) [BzMIM][DCA]; (○) [EMIM][TCM]; (□) [EMPyr][Lac]; (Δ) [BCN4PY][NTf2]; (◊) [AMIM][NTf2]; (
) [BzMIM][DCA]; (○) [EMIM][TCM]; (□) [EMPyr][Lac]; (Δ) [BCN4PY][NTf2]; (◊) [AMIM][NTf2]; (  ) [BzMIM][NTf2].
) [BzMIM][NTf2].
 ) [N-C3OHMMor][DCA]; (●) [EMMOR][DCA]; (■) [N-C3OHPY][DCA]; (▲) [N-C3OHMIM][DCA]; (♦) [AMIM][DCA]; (
) [N-C3OHMMor][DCA]; (●) [EMMOR][DCA]; (■) [N-C3OHPY][DCA]; (▲) [N-C3OHMIM][DCA]; (♦) [AMIM][DCA]; (  ) [BzMIM][DCA]; (○) [EMIM][TCM]; (□) [EMPyr][Lac]; (Δ) [BCN4PY][NTf2]; (◊) [AMIM][NTf2]; (
) [BzMIM][DCA]; (○) [EMIM][TCM]; (□) [EMPyr][Lac]; (Δ) [BCN4PY][NTf2]; (◊) [AMIM][NTf2]; (  ) [BzMIM][NTf2].
) [BzMIM][NTf2].
 ) [N-C3OHMMor][DCA]; (●) [EMMOR][DCA]; (■) [N-C3OHPY][DCA]; (▲) [N-C3OHMIM][DCA]; (♦) [AMIM][DCA]; (
) [N-C3OHMMor][DCA]; (●) [EMMOR][DCA]; (■) [N-C3OHPY][DCA]; (▲) [N-C3OHMIM][DCA]; (♦) [AMIM][DCA]; (  ) [BzMIM][DCA]; (○) [EMIM][TCM]; (□) [EMPyr][Lac]; (Δ) [BCN4PY][NTf2]; (◊) [AMIM][NTf2]; (
) [BzMIM][DCA]; (○) [EMIM][TCM]; (□) [EMPyr][Lac]; (Δ) [BCN4PY][NTf2]; (◊) [AMIM][NTf2]; (  ) [BzMIM][NTf2].
) [BzMIM][NTf2].


| Structure | Name, Abbreviation | Ref. |
|---|---|---|
| Hexane/hex-1-ene, or cyclohexane/cyclohexene, or ethylbenzene/styrene separation | ||
 | 1-Allyl-3-methylimidazolium dicyanamide [AMIM][DCA] | [100] |
 | [AMIM][NTf2] | [101] |
 | 1-Benzyl-3-methylimidazolium dicyanamide, [BzMIM][DCA] | [102] |
 | [BzMIM][NTf2] | [102] |
 | dicyanamide, [EMMOr][DCA] | [103] |
 | [BCN4PY][NTf2] | [104] |
 | [N-C3OHMIM][DCA] | [105] |
 | [N-C3OHPY][DCA] | [106] |
 | [EMPyr][Lac] | [107] |
 | [N-C3OHMor][DCA] | [105] |
 | [EMIM][TCM] | [109] |
| Water/butan-1-ol separation | ||
 | [DoMIM][NTf2] | [110] |
 | [N8,2,2,2][NTf2] | [111] |
 | [D2MIM][DCA] | [27] |
 | [P8,8,8,8][NTf2] | [112] |
 | [P14,4,4,4][DBS] | [113] |
 | [P14,6,6,6][TCM] | [114] |
 | [N-C3OHMMor][NTf2] | [115] |
| Ionic Liquid | ||
|---|---|---|
| [EMMor][DCA] | 2.85 | 0.007 |
| [N-C3OHMIM][DCA] | 2.52 | 0.010 |
| [BzMIM][DCA] | 2.50 | 0.026 |
| [AMIM][DCA] | 2.45 | 0.019 |
| [EMIM][TCM] | 2.35 | 0.045 |
| [EMPYR][Lac] | 2.24 | 0.020 |
| [BCN4PY][NTf2] | 2.13 | 0.074 |
| [AMIM][NTf2] | 1.94 | 0.094 |
| [BzMIM][NTf2] | 1.90 | 0.108 |
| Ionic Liquid | ||
|---|---|---|
| [N-C3OHMMor][DCA] | 3.98 | 0.251 |
| [EMMor][DCA] | 3.04 | 0.329 |
| [N-C3OHPY][DCA] | 2.79 | 0.359 |
| [N-C3OHMIM][DCA] | 2.64 | 0.379 |
| [BzMIM][DCA] | 2.46 | 0.406 |
| [BzMIM][NTf2] | 1.77 | 0.565 |
| Ionic Liquid | ||
|---|---|---|
| [N-C3OHMMor][DCA] | 2.55 | 0.097 |
| [N-C3OHMIM][DCA] | 2.38 | 0.160 |
| [EMMor][DCA] | 2.32 | 0.152 |
| [N-C3OHPY][DCA] | 2.27 | 0.138 |
| [AMIM][DCA] | 2.19 | 0.242 |
| [BzMIM][DCA] | 2.16 | 0.322 |
| [EMIM][TCM] | 2.01 | 0.474 |
| [BCN4PY][NTf2] | 1.85 | 0.694 |
| [AMIM][NTf2] | 1.75 | 0.629 |
| [BzMIM][NTf2] | 1.71 | 0.758 |
| Ionic Liquid | ||
|---|---|---|
| [P8,8,8,8][NTf2] | 5.75 | 0.781 |
| [P14,6,6,6][TCM] | 4.53 | 2.30 |
| [P14,4,4,4][DBS] | 3.77 | 5.29 |
| [DoMIM][NTf2] | 2.76 | 0.637 |
| [N8,2,2,2][NTf2] | 2.46 | 0.515 |
| [N-C3OHMMor][NTf2] | 0.44 | 0.326 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domańska, U. Experimental Data of Fluid Phase Equilibria- Correlation and Prediction Models: A Review. Processes 2019, 7, 277. https://doi.org/10.3390/pr7050277
Domańska U. Experimental Data of Fluid Phase Equilibria- Correlation and Prediction Models: A Review. Processes. 2019; 7(5):277. https://doi.org/10.3390/pr7050277
Chicago/Turabian StyleDomańska, Urszula. 2019. "Experimental Data of Fluid Phase Equilibria- Correlation and Prediction Models: A Review" Processes 7, no. 5: 277. https://doi.org/10.3390/pr7050277






