Electrolyte Effects on Poly (Acrylic Acid)-Based Aircraft De-icing Fluids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Rheology Measurements
2.3. Test of the Gelation Characteristics of PAA Mixtures
3. Results
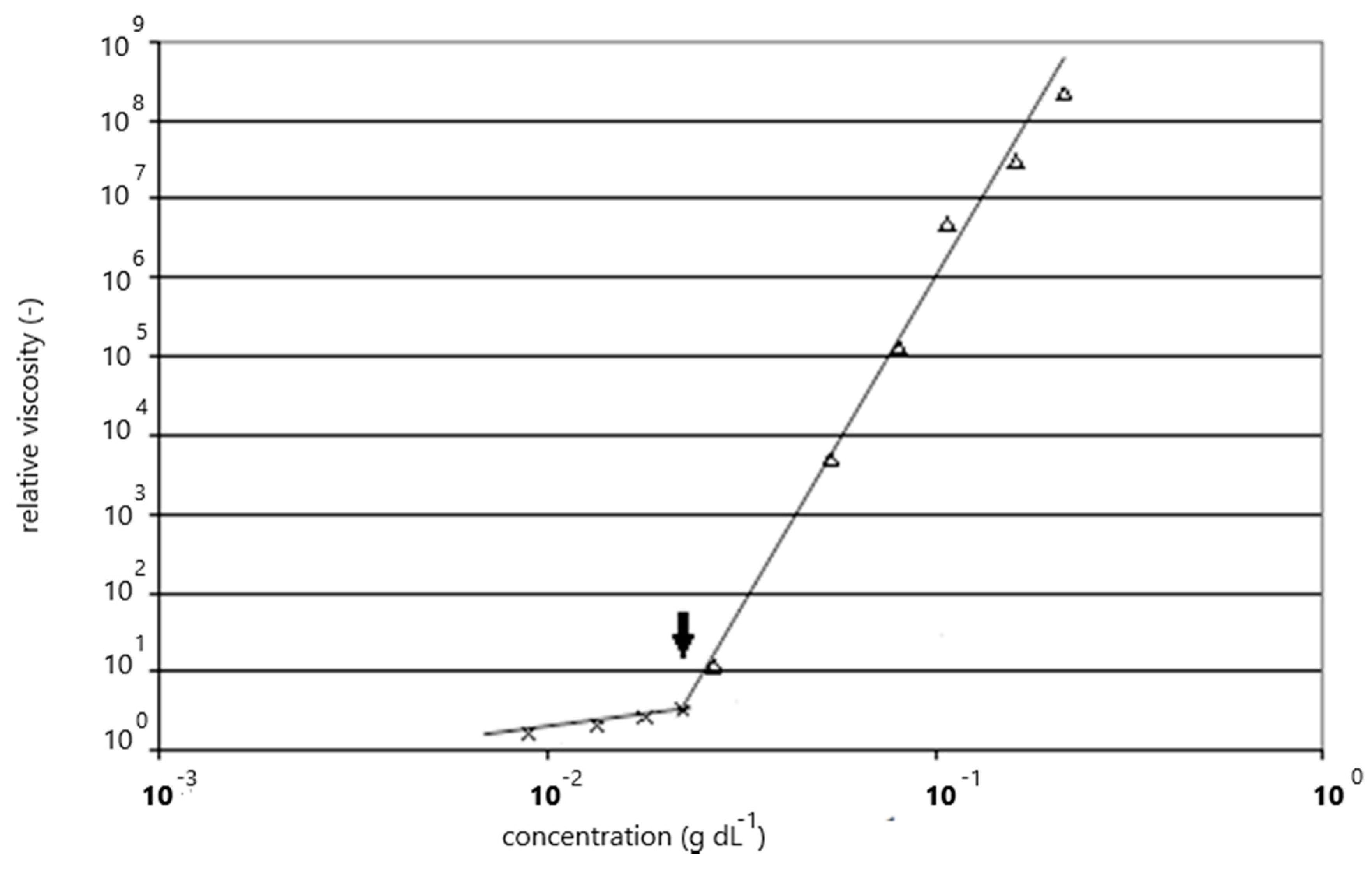
3.1. Intrinsic Viscosity Measurements
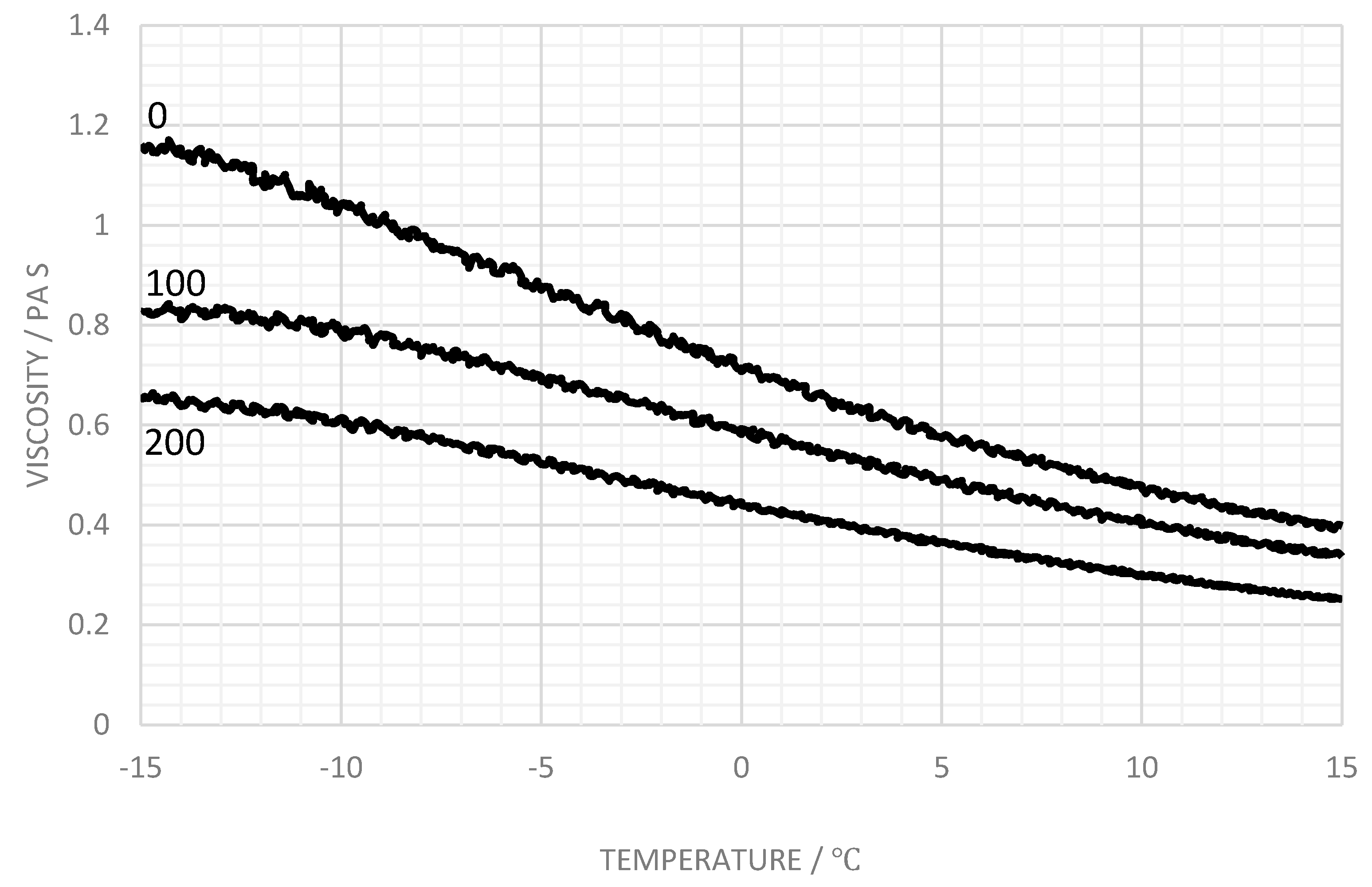
3.2. Concentration Dependence of Viscosity for Carbomer A
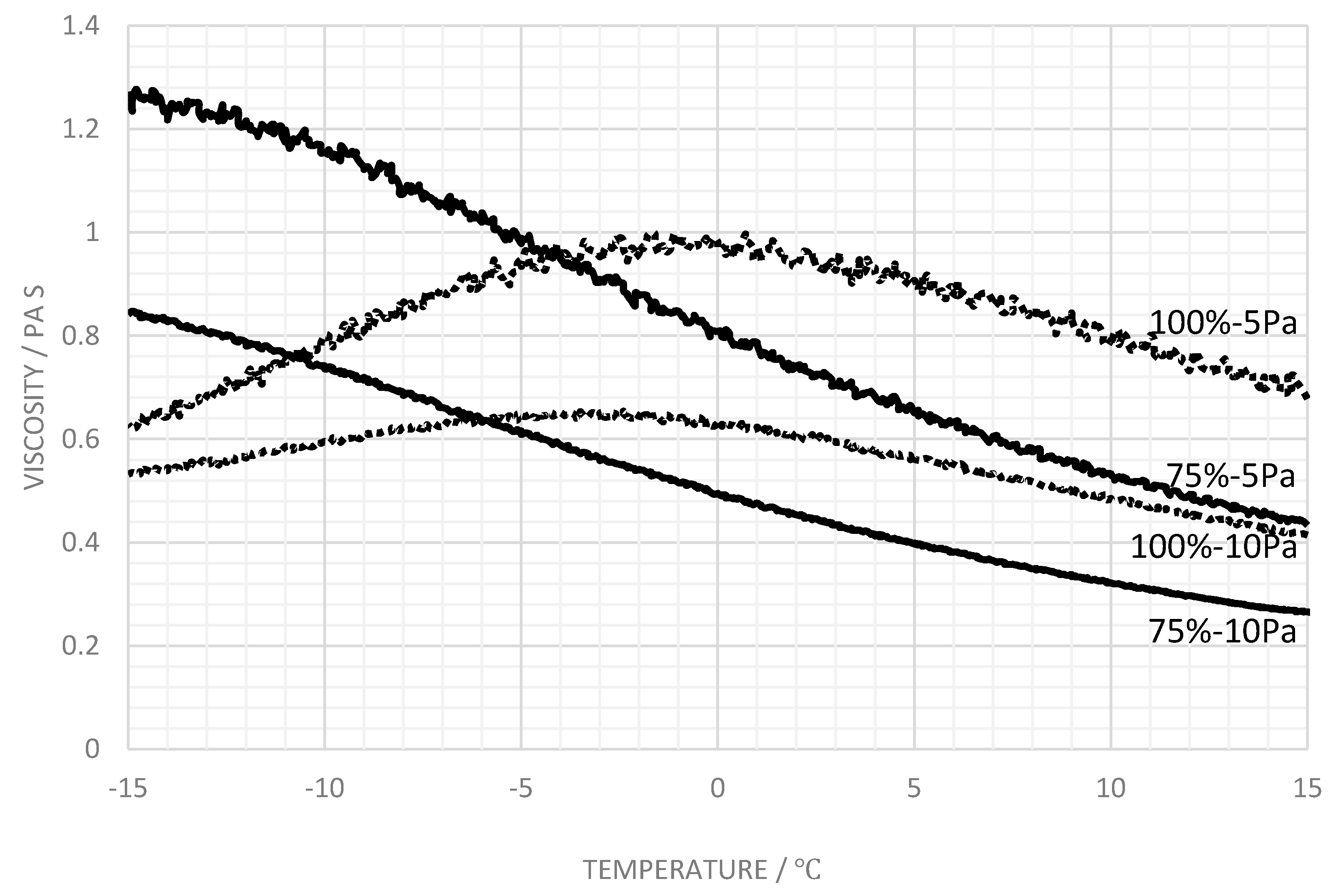
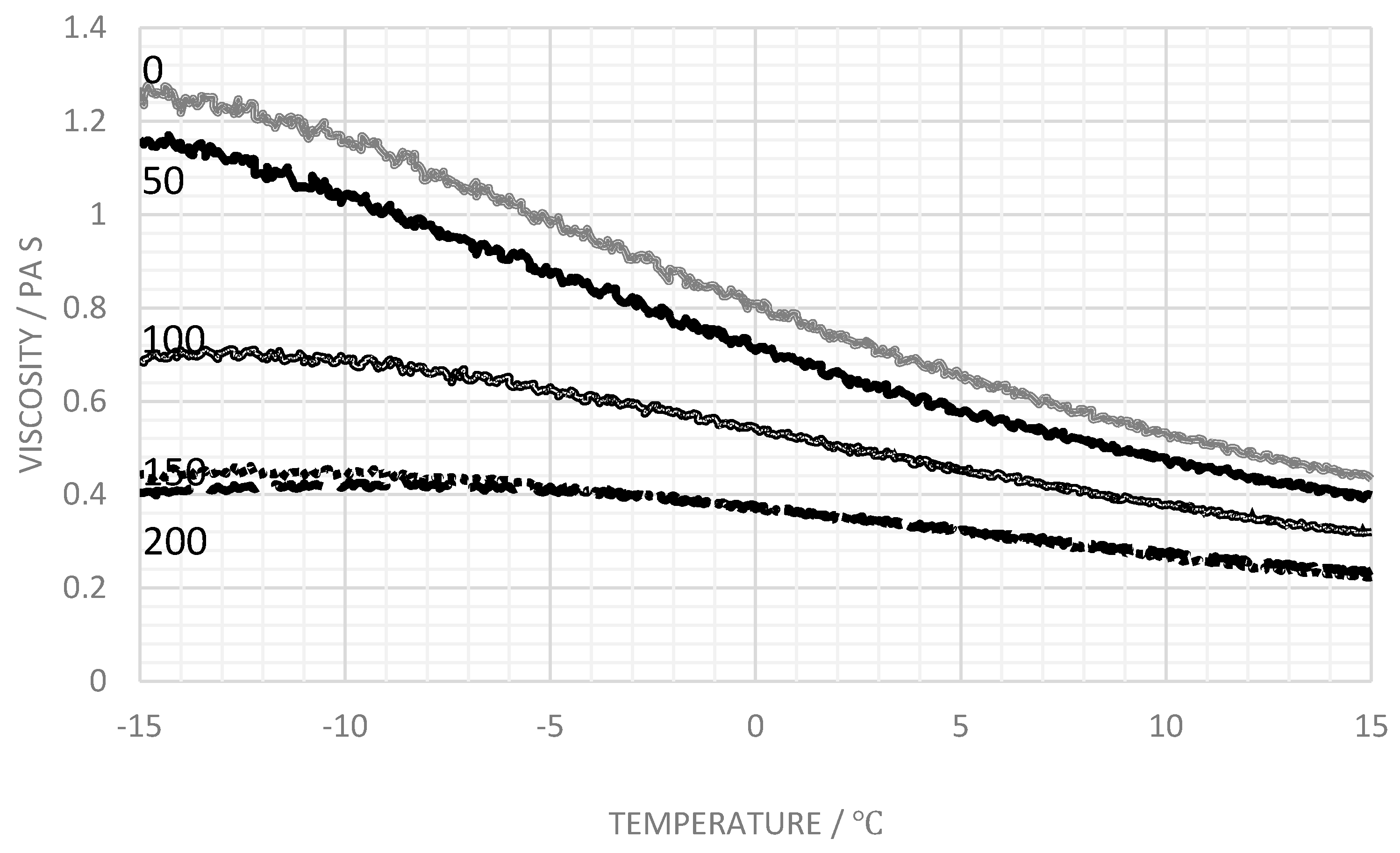
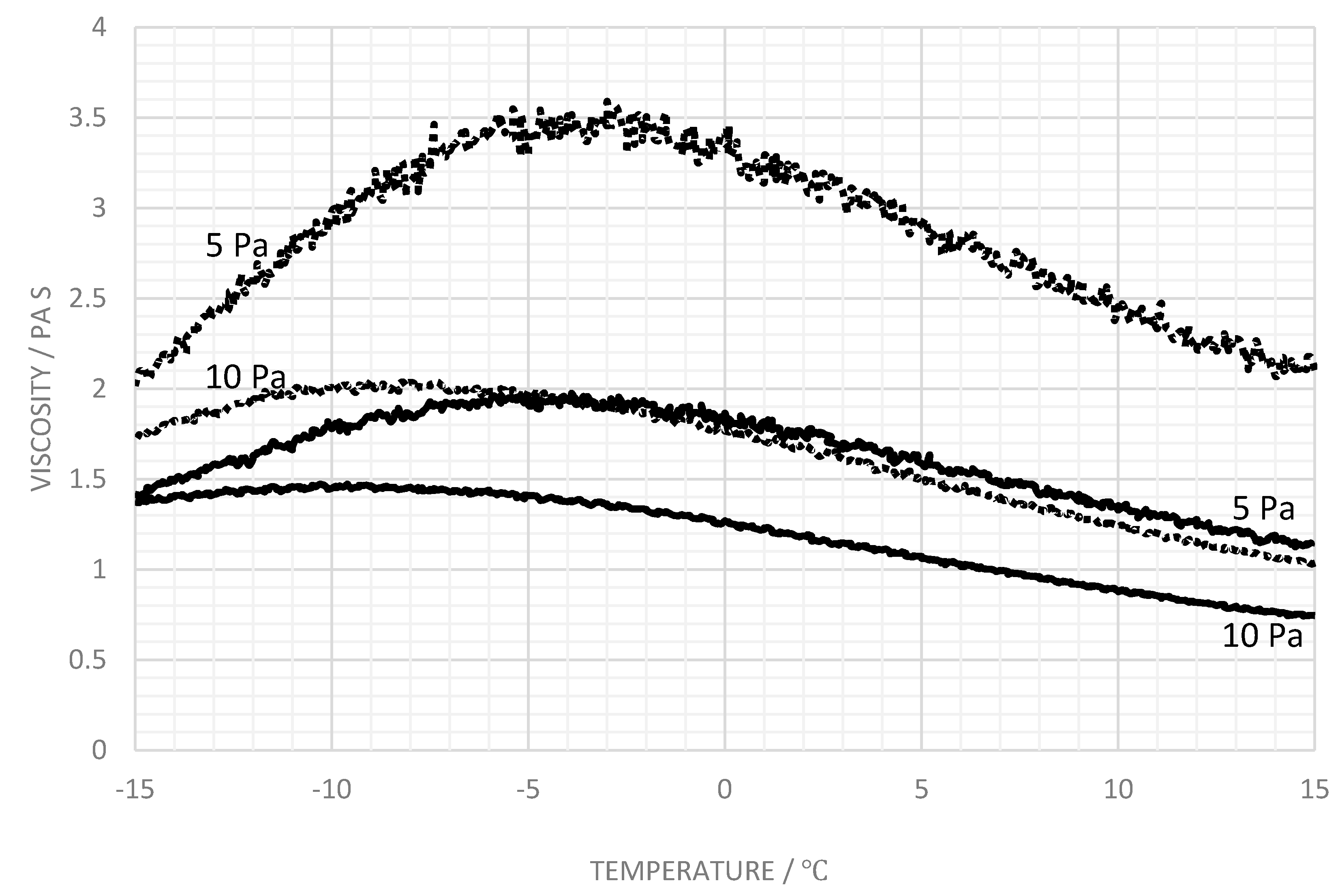
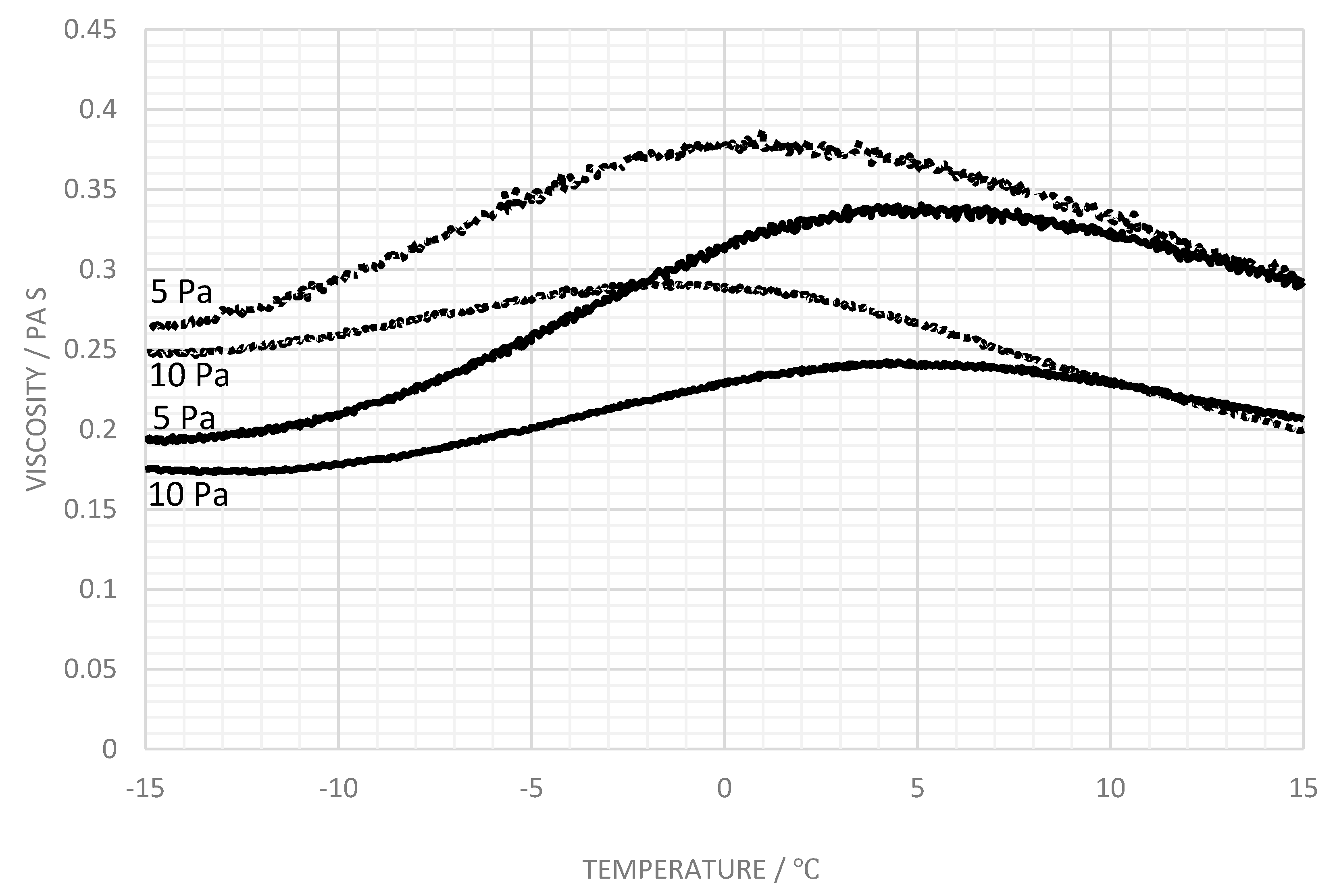
3.3. Influence of Salt
4. Discussion
- (i)
- The monovalent cations can bind to the carboxyl groups of the polymer, but in doing so they create an ion pair which blocks the possibility of hydrogen bonding with the groups on the same molecule or between adjacent polymer molecules. The net effect is an inhibition of stable gel formation.
- (ii)
- The presence of the sodium or potassium formate will influence the ionic strength of the ‘solution’, and this normally has the effect of lowering the viscosity and hence inhibiting polymer–polymer interactions.
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Dedication
References
- Wang, Y.; Pethrick, R.A.; Hudson, N.E.; Schaschke, C.J. Rheology of poly (acrylic acid) in water/glycol/salt mixtures. Ind. Eng. Chem. Res. 2013, 52, 594–602. [Google Scholar] [CrossRef]
- Blau, K. Recommendations for De-Icing/Anti-Icing Aeroplanes on the Ground, 30th ed.; AEA Publications: Nashville, TN, USA, 2015. [Google Scholar]
- D’Avirro, J.; Chaput, M.D.; Board NECTAR. Optimizing the Use of Aircraft De-icing and Anti-Icing Fluids; Transportation Research Board: Airport Cooperative Research Program (ACRP); United States Fire Administration (USFA): Washington, DC, USA, 2011. [Google Scholar]
- Sapienza, R.; Johnson, A.R.; Ricks, W.F. Environmentally Benign Anti-Icing or De-Icing Fluids. U.S. Patent No. 6,506,318, 14 January 2003. [Google Scholar]
- Hille, J. De-Icing and Anti-Icing Fluid Residues, Aero; Boeing Commercial Airplanes Group: Seattle, WA, USA, 2007; pp. 14–21. [Google Scholar]
- Buttner, K. German Federal Bureau of Aircraft Accidents Investigation; Bundesstelle für Flugunfalluntersuchung: Braunschweig, Germany, 2005. [Google Scholar]
- Cal, A. Training Recommendations and Background Information for De-Icing/Anti-Icing of Aircraft on the Ground, 5th ed.; AEA Publications: Pittsburgh, PA, USA, 2008. [Google Scholar]
- Beisswenger, A.; Laforte, J.-L.; Trenblay, M.-M.; Perron, J. Investigation of a New Reference Fluid for Use in Aerodynamic Acceptance Evaluation of Aircraft Ground De-Icing and Anti-Icing Fluid; Federal Aviation Administration: Washington, DC, USA, 2007. [Google Scholar]
- Simonot, M.; Mischka, G.; Holland, N. Recommedations for De-Icing/Anti-Icing of Aircraft on the Ground, 19th ed.; AEA Publications: Pittsburgh, PA, USA, 2004. [Google Scholar]
- Jenkins, R.D.; Bassett, D.R.; Lightfoot, R.H.; Boluk, M.Y. Aircraft Anti-icing Fluids Thickened by Associative Polymers. WO Patent No. 9,324,543, 1993. [Google Scholar]
- Seiler, M.; Bernhardt, S. De-Icing Agent and/or Anti-Icing Agent, C09K 3/18 ed.; USPTO, Ed.; Evonik Degussa GmbH: Essen, Germany, 2011. [Google Scholar]
- Dow, J.P. Understanding the stall-recovery procedure for turboprop airplanes in icing conditions. In Flight Safety Digest; Flight Safety Foundation, Inc.: Alexandria, VA, USA, 2005; pp. 1–17. [Google Scholar]
- Mohiley, A.; Franzaring, J.; Calvo, O.C.; Fangmeier, A. Potential toxic effects of aircraft de-icers and wastewater samples containing these compounds. Environ. Sci. Pollut. Res. 2015, 22, 13094–13101. [Google Scholar] [CrossRef] [PubMed]
- Kent, R.A.; Andersen, D.; Caux, P.-Y.; Teed, S. Canadian Water Quality Guidelines for Glycols, an Ecotoxicological Review of Glycols and Associated Aircraft Anti-Icing and Deicing Fluids. Environ. Toxicol. 1999, 14, 481–522. [Google Scholar] [CrossRef]
- Corsi, S.R.; Geis, S.W.; Loyo-Rosales, J.E.; Rice, C.P.; Sheesley, R.I.; Failey, G.G.; Cancilla, D.A. Characterization of Aircraft Deicer and Anti-Icer Components and Toxicity in Airport Snowbanks and Snowmelt Runoff. Environ. Sci. Technol. 2006, 40, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.E.; Smith, K.W. De-Icing, C09K003/18 ed.; USPTO, Ed.; Clearwater, Inc.: Richmond, VA, USA, 2003. [Google Scholar]
- Ritter, S. What’s that stuff? Chem. Eng. News Arch. 2001, 79, 30. [Google Scholar] [CrossRef]
- Paredes, X.; Pensado, A.S.; Comunas, M.A.J.P.; Fernandez, J. Pressure effects the Dynamic Viscosities of Two Poly(propylene glycol) Dimethyl Ether Lubricants. J. Chem. Eng. Data 2010, 55, 4088–4094. [Google Scholar] [CrossRef]
- Coffey, D.A.; Nieh, E.C.Y.; Armstrong, R.A. Aircraft Deicing Fluid with Thermal and pH-table Wetting Agent; USPTO, Ed.; Texaco Inc.: Beaumont, TX, USA, 1995. [Google Scholar]
- Jenkins, R.D.; Bassett, D.R.; Lightfoot, R.H.; Boluk, M.Y. Aircraft Anti-Icing Fluids, C08K 505 ed.; USPTO, Ed.; Union Carbide Chemicals & Plastics Technology Corporation: Wilmington, DE, USA, 1995. [Google Scholar]
- Jenkins, R.D.; Bassett, D.R.; Lightfoot, R.H.; Boluk, M.Y. Aircraft Anti-Icing Fluids Thickened by Associative Polymers, C08K 505 ed.; USPTO, Ed.; Union Carbide Chemicals & Plastics Technology Corporation: Wilmington, DE, USA, 1997. [Google Scholar]
- Ross, F. Aircraft De-Icing/Anti-Icer; USPTO, Ed.; Kilfrost Limited: Newcastle Upon Tyne, UK, 2011. [Google Scholar]
- Bollinger, T.K.; Mineau, P.; Wickstrom, M.L. Toxicity of sodium chloride to house sparrows (Passer domesticus). J. Wildl. Dis. 2005, 41, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Hudson, N.E.; Ferguson, J.; Warren, B.C.H. Polymer complexation effects in extensional flows. J. Non-Newton Fluid Mech. 1988, 30, 251–266. [Google Scholar] [CrossRef]
- Miquelard-Garnier, G.; Demoures, S.; Creton, C.; Hourdet, D. Synthesis and rheological behavior of new hydrophobically modified hydrogels with tunable properties. Macromolecules 2006, 39, 8128–8139. [Google Scholar] [CrossRef]
- Ikegami, A.; Imai, N. Precipitation of polyelectrolytes by salts. J. Polym. Sci. 1962, 56, 133–152. [Google Scholar] [CrossRef]
- Lubrizol Advanced Materials, I. Minimizing Influence of Salts in Presence of Carbopol Polymers; TDS-542002; Cleveland, OH, USA.
- Louchez, P.R.; Laforte, J.L.; Bouchard, G.; Farzaneh, F. Laboratory Evaluation of Aircraft Ground De/Antiicing Products, 4th ed.; Chung, J.S., Karal, K., Koteryama, W., Eds.; International Society Offshore & Polar Engineers: Cupertino, CA, USA, 1994; pp. 479–483. [Google Scholar]
- Louchez, P.; Laforte, J.L.; Bouchard, G. Boundary Layer Evaluation of Anti-Icing Fluids for Commuter Aircraft; University of Quebec at Chicoutimi (UQAC): Chicoutimi, QC, Canada, 1994. [Google Scholar]
- Louchez, P.; Laforte, J.L.; Bernardin, S. A Proposal of Standard Evaluation of Aerodynamic Performance of De-Icing and Anti-Icing Fluids Applied on Commuter Type Aircraft; Universite du Quebec a Chicoutimi: Chicoutimi, QC, Canada, 1994. [Google Scholar]
- Renton Division Aerodynamics Engineering. Aerodynamic Acceptance Test for Aircraft Ground De-Icing/Anti-Icing Fluids; Boeing: Chicago, IL, USA, 1992. [Google Scholar]
- SAE. SAE AS5901B Water Spray and High Humidity Endurance Test Methods for SAE AMS1424 and SAE AMS1428 Aircraft De-Icing/Anti-Icing Fluids; SAE International: Warrendale, PA, USA, 2010. [Google Scholar]
- Chu, J.S.; Yu, D.M.; Amidon, G.L.; Weiner, N.D.; Goldberg, A.H. Viscoelastic Properties of Polyacrylic-Acid Gels in Mixed-Solvents. Pharm. Res. 1992, 9, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, V.N.; Lyubina, S.Y.; Barskaya, T.V. The flow birefringence and viscosity of poly (acrylic acid) solutions. Polym. Sci. USSR 1964, 6, 886–892. [Google Scholar] [CrossRef]
- Takahashi, A.; Nagasawa, M. Excluded Volume of Polyelectrolyte in Salt Solutions. J. Am. Chem. Soc. 1964, 86, 543–548. [Google Scholar] [CrossRef]
- Yaoita, T.; Isaki, T.; Masubuchi, Y.; Watanabe, H.; Ianniruberto, G.; Marrucci, G. Primitive Chain Network Simulation of Elongational Flows of Entangled Linear Chains: Role of Finite Chain Extensibility. Macromolecules 2011, 44, 9675–9682. [Google Scholar] [CrossRef]
- Marrucci, G.; Bhargava, S.; Cooper, S.L. Models of shear-thickening behaviour in physically cross-linked networks. Macromolecules 1993, 26, 6483–6488. [Google Scholar] [CrossRef]
- Wall, F.T.; Grieger, P.F.; Huizenga, J.R.; Doremus, R.H. Electrolytic Properties of Aqueous Solutions of Polyacrylic Acid and Sodium Hydroxide. III. The Rate of Sodium Ion Exchange between Polyacrylate and Free Sodium Ions. J. Chem. Phys. 1952, 20, 1206–1211. [Google Scholar] [CrossRef]
- Miyajima, T.; Mori, M.; Ishiguro, S.-I. Analysis of Complexation Equilibria of Polyacrylic Acid by a Donnan-Based Concept. J. Colloid Interface Sci. 1997, 187, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Pochard, I.; Foissy, A.; Couchot, P. Conductometric and microcalorimetric analysis of the alkaline-earth/alkali-metal ion exchange onto polyacrylic acid. Colloid Polym. Sci. 1999, 277, 818–826. [Google Scholar] [CrossRef]
- Schweins, R.; Huber, K. Collapse of sodium polyacrylate chains in calcium salt solutions. Eur. Phys. J. 2001, 5, 117–126. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Zhang, M.; Luo, W.; Yang, J.; Zhu, F. Macromolecular Aggregation of Aqueous Polyacrylic Acid in the Presence of Surfactants Revealed by Resonance Rayleigh Scattering. Macromolecules 2008, 41, 4873–4880. [Google Scholar] [CrossRef]
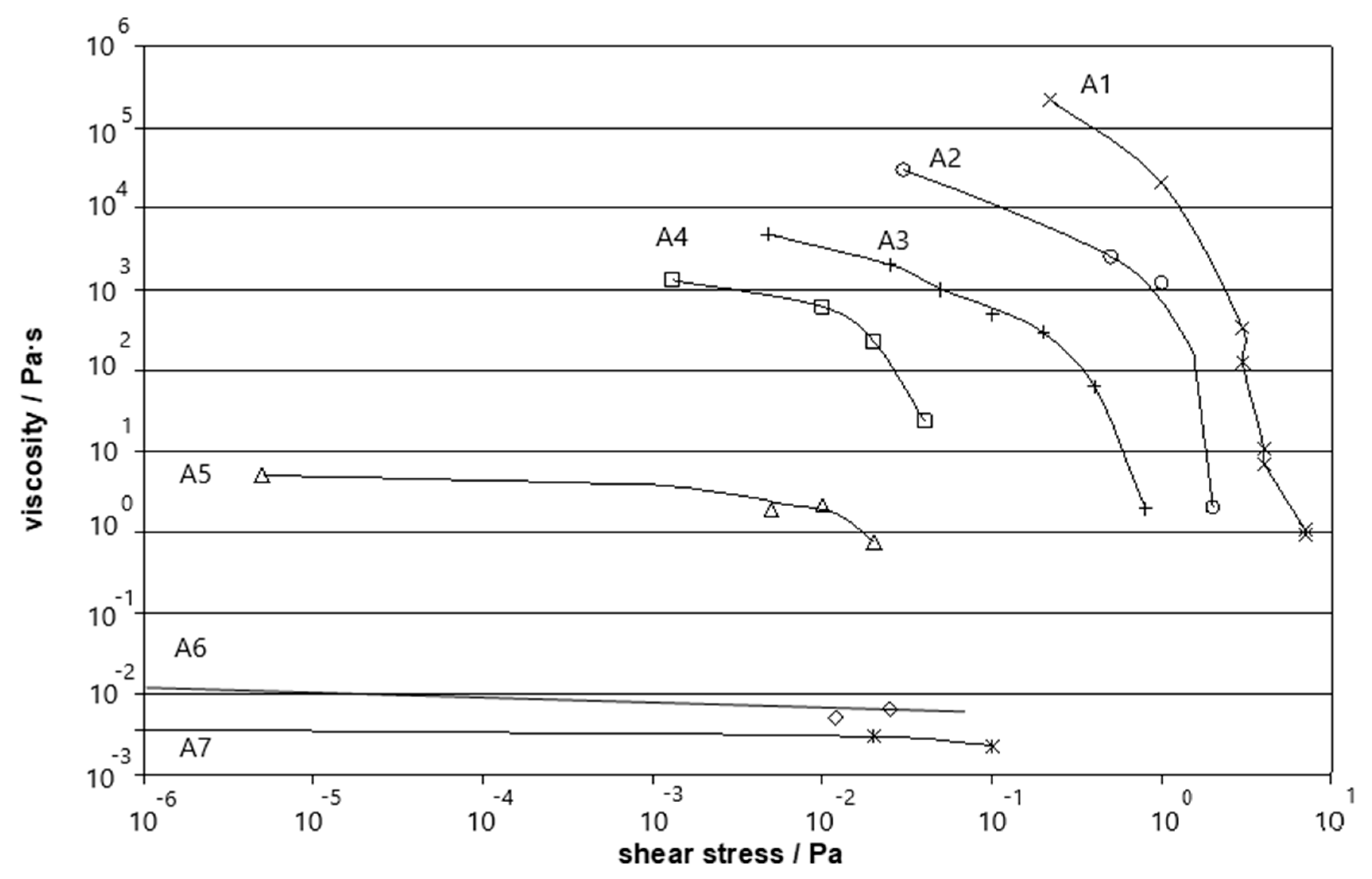

| Fluid Name | Carbomer A Concentraton (g dL−1) |
|---|---|
| A1 | 0.2145 |
| A2 | 0.1608 |
| A3 | 0.1072 |
| A4 | 0.0804 |
| A5 | 0.0536 |
| A6 | 0.0268 |
| Fluid Name | Cation Concentration (%) |
|---|---|
| A1K | 0.03% K+ |
| A1N | 0.03% Na+ |
| A2K | 0.06% K+ |
| A2N | 0.06% Na+ |
| A3K | 0.03% Na+ and 0.045% K+ |
| A3N | 0.075% Na+ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hudson, N.E.; Pethrick, R.A.; Schaschke, C.J. Electrolyte Effects on Poly (Acrylic Acid)-Based Aircraft De-icing Fluids. Processes 2019, 7, 332. https://doi.org/10.3390/pr7060332
Wang Y, Hudson NE, Pethrick RA, Schaschke CJ. Electrolyte Effects on Poly (Acrylic Acid)-Based Aircraft De-icing Fluids. Processes. 2019; 7(6):332. https://doi.org/10.3390/pr7060332
Chicago/Turabian StyleWang, Yuchen, Nicholas E. Hudson, Richard A. Pethrick, and Carl J. Schaschke. 2019. "Electrolyte Effects on Poly (Acrylic Acid)-Based Aircraft De-icing Fluids" Processes 7, no. 6: 332. https://doi.org/10.3390/pr7060332






