Effect of Heating Oxidation on the Surface/Interface Properties and Floatability of Anthracite Coal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Heating Oxidation Process
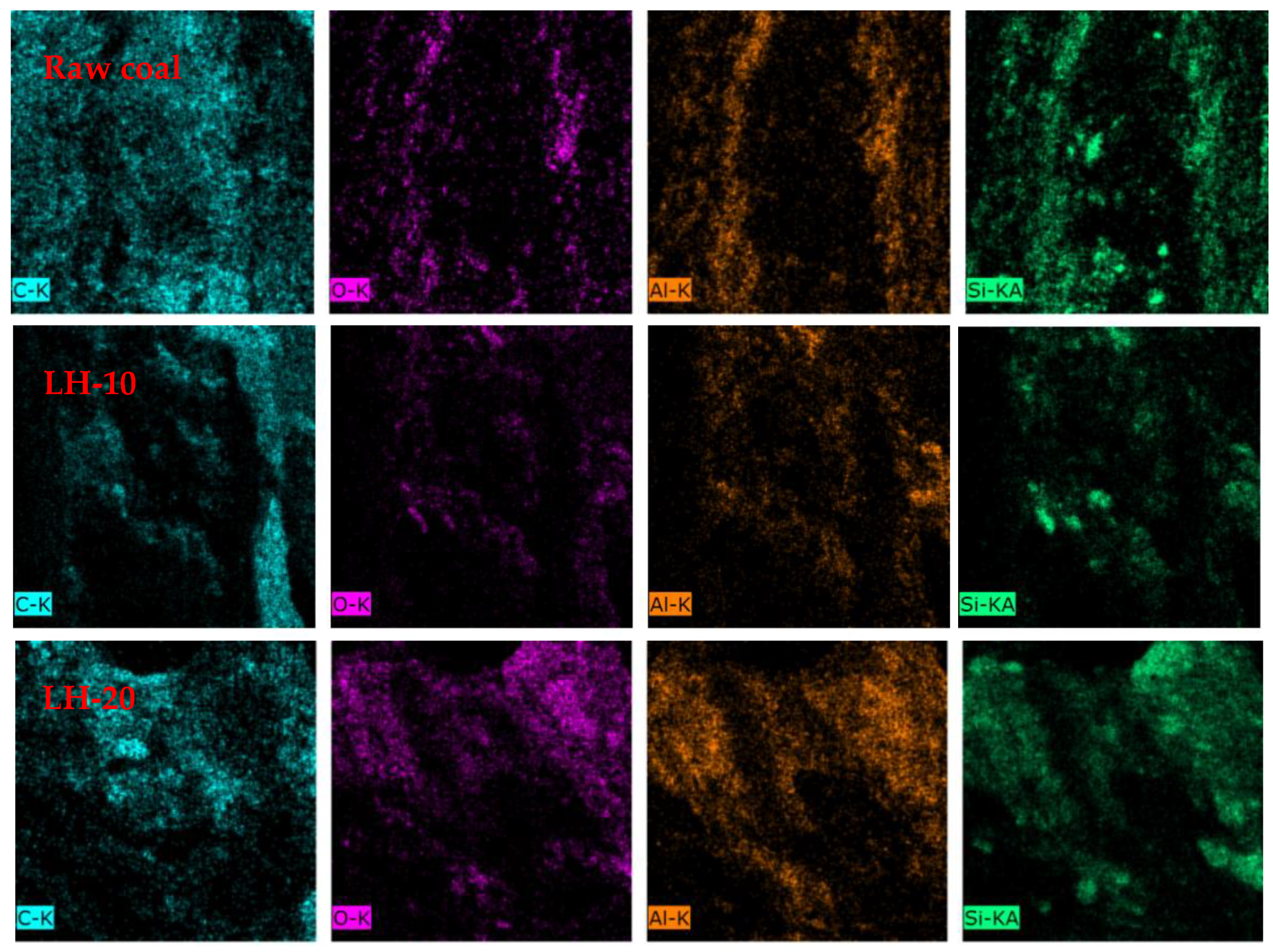
2.2. SEM
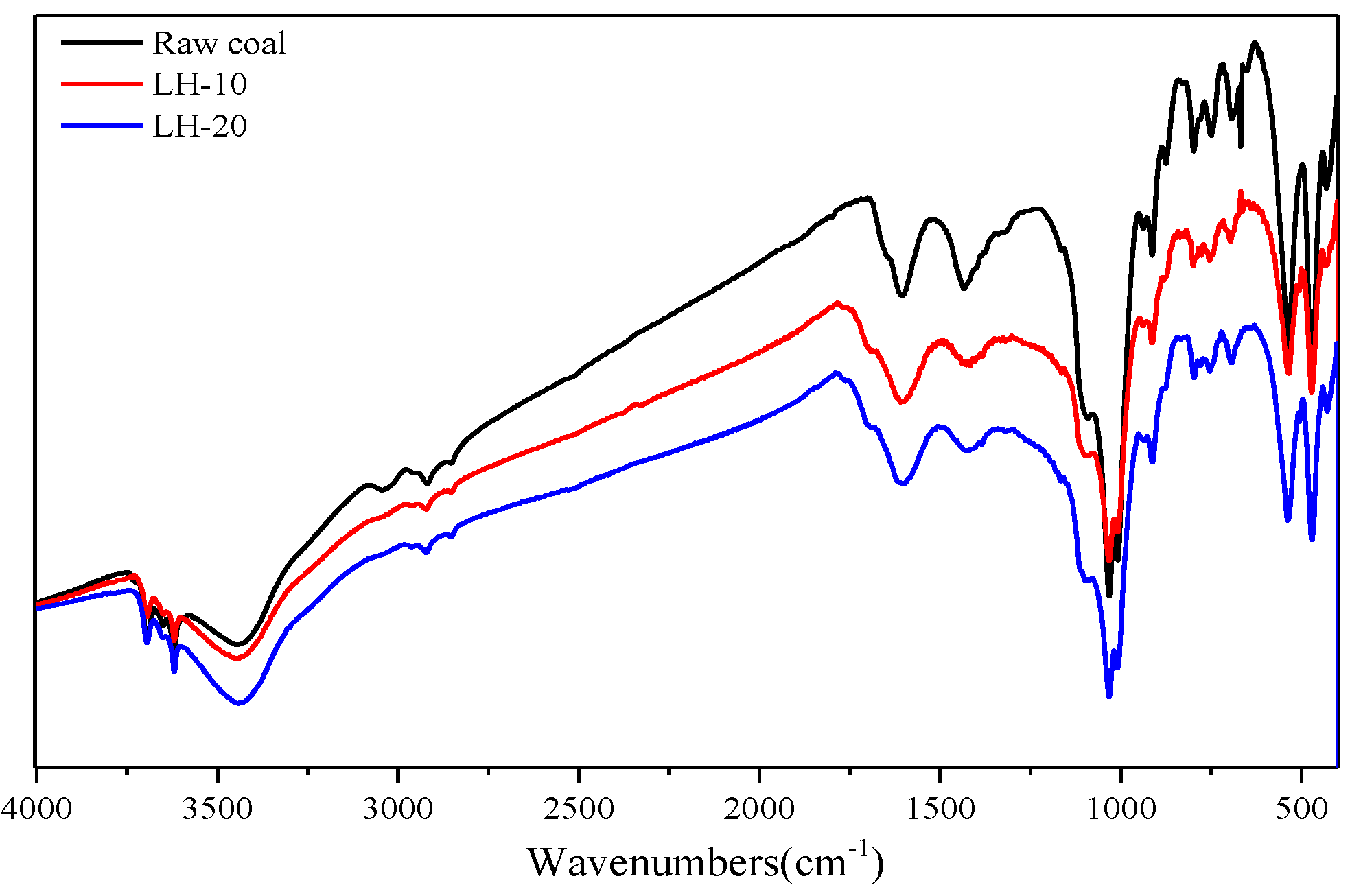
2.3. FTIR
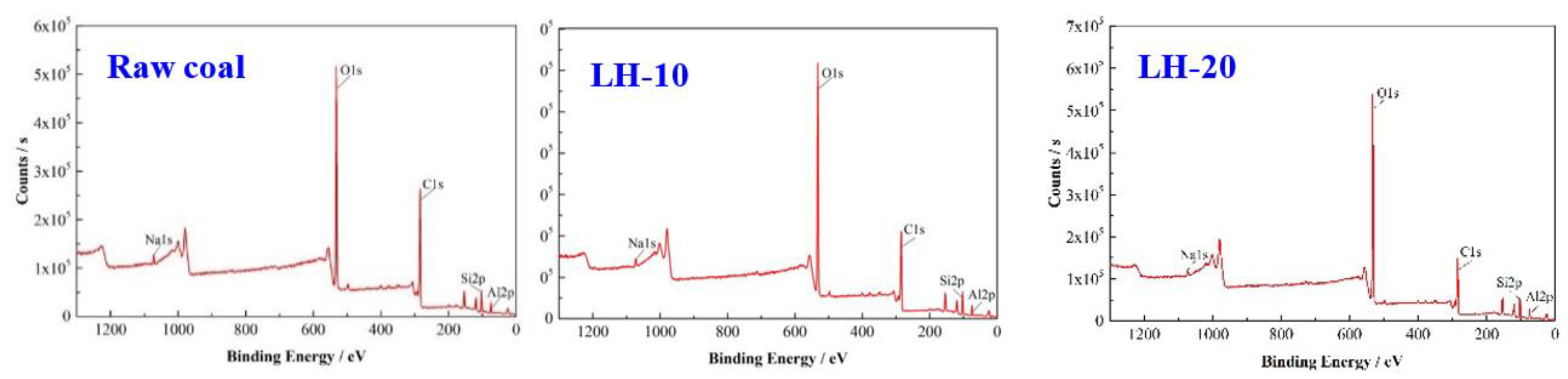
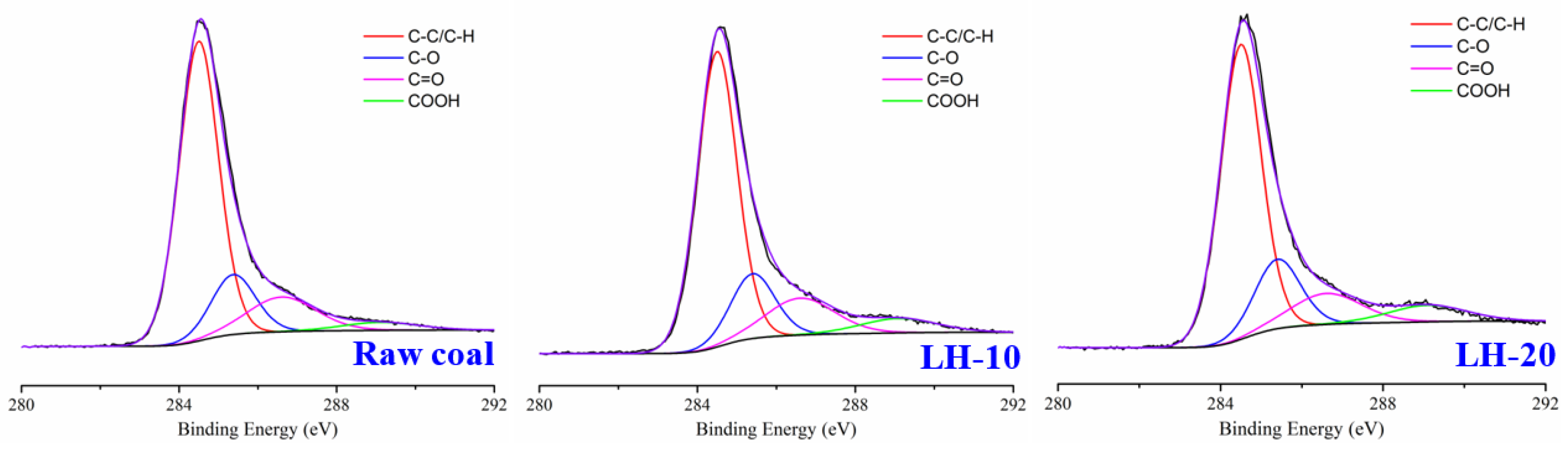
2.4. XPS
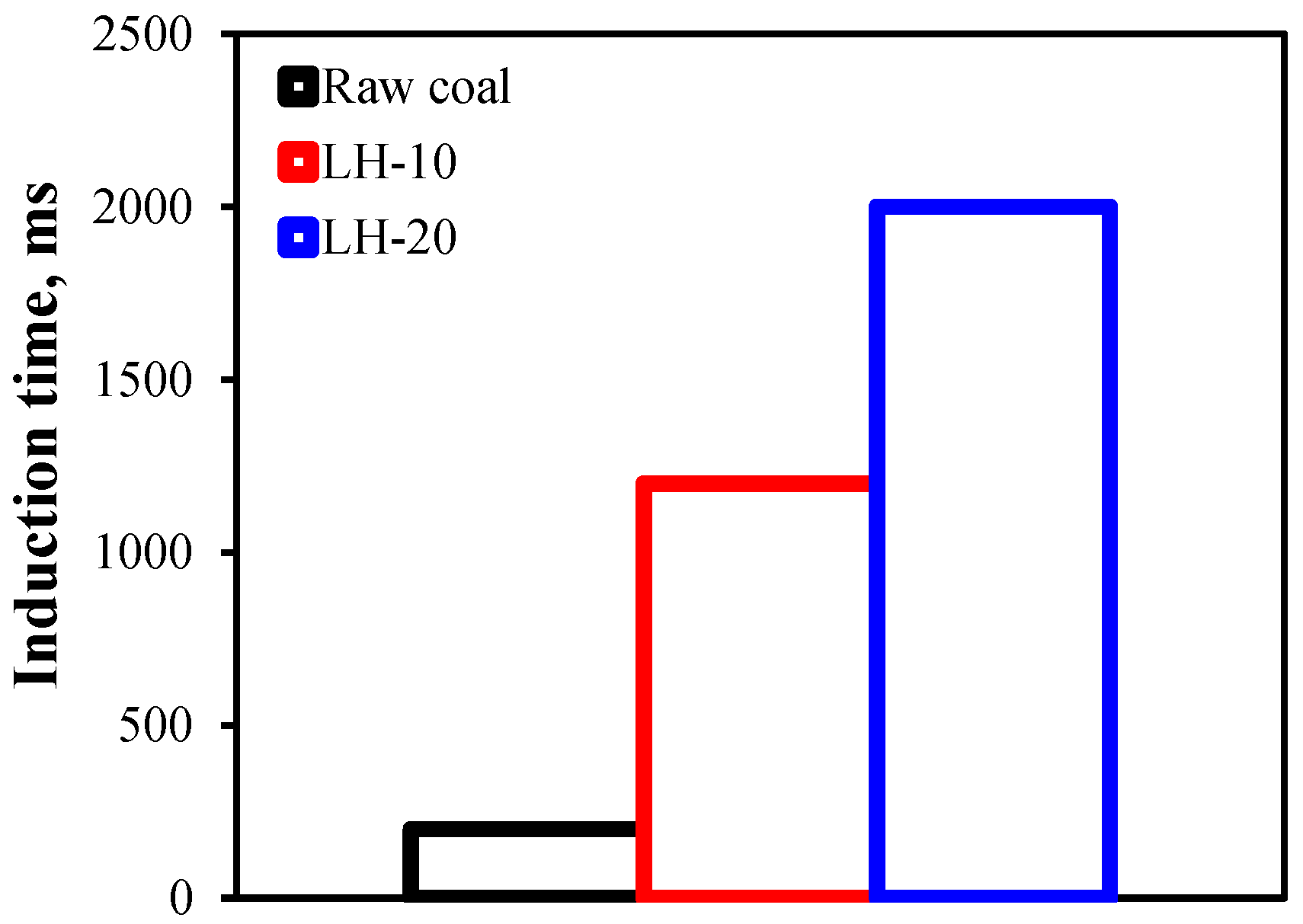
2.5. Induction Time Test
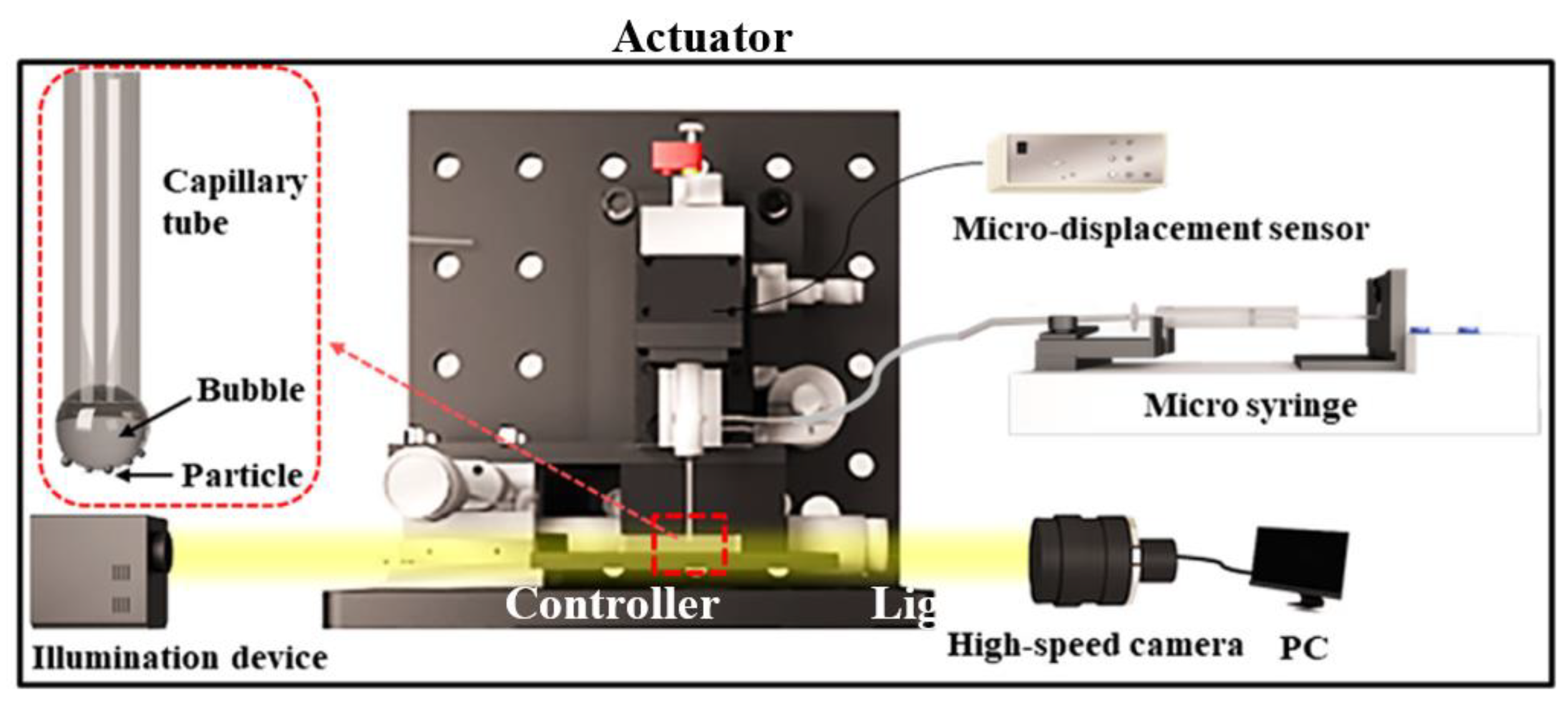
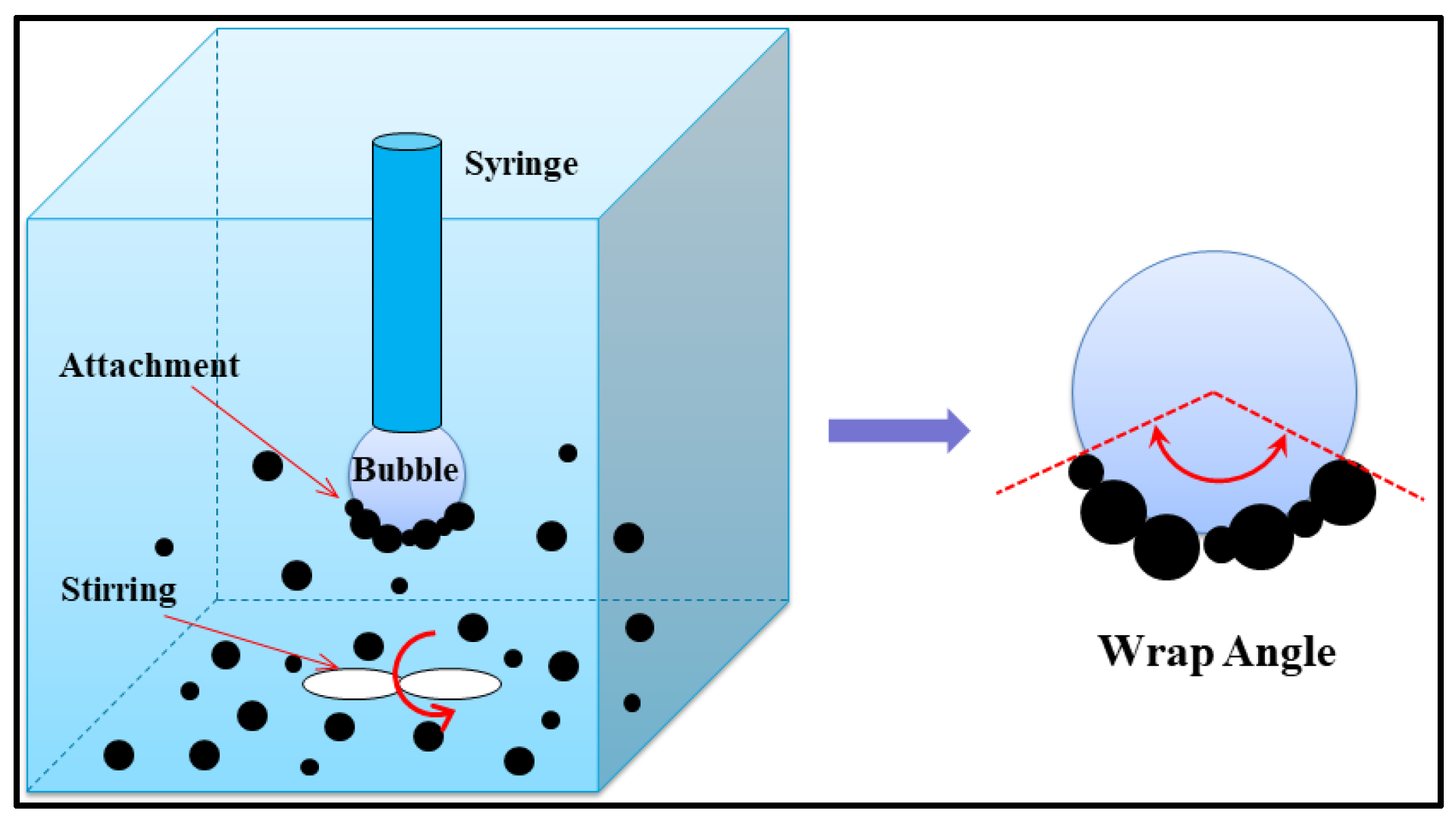
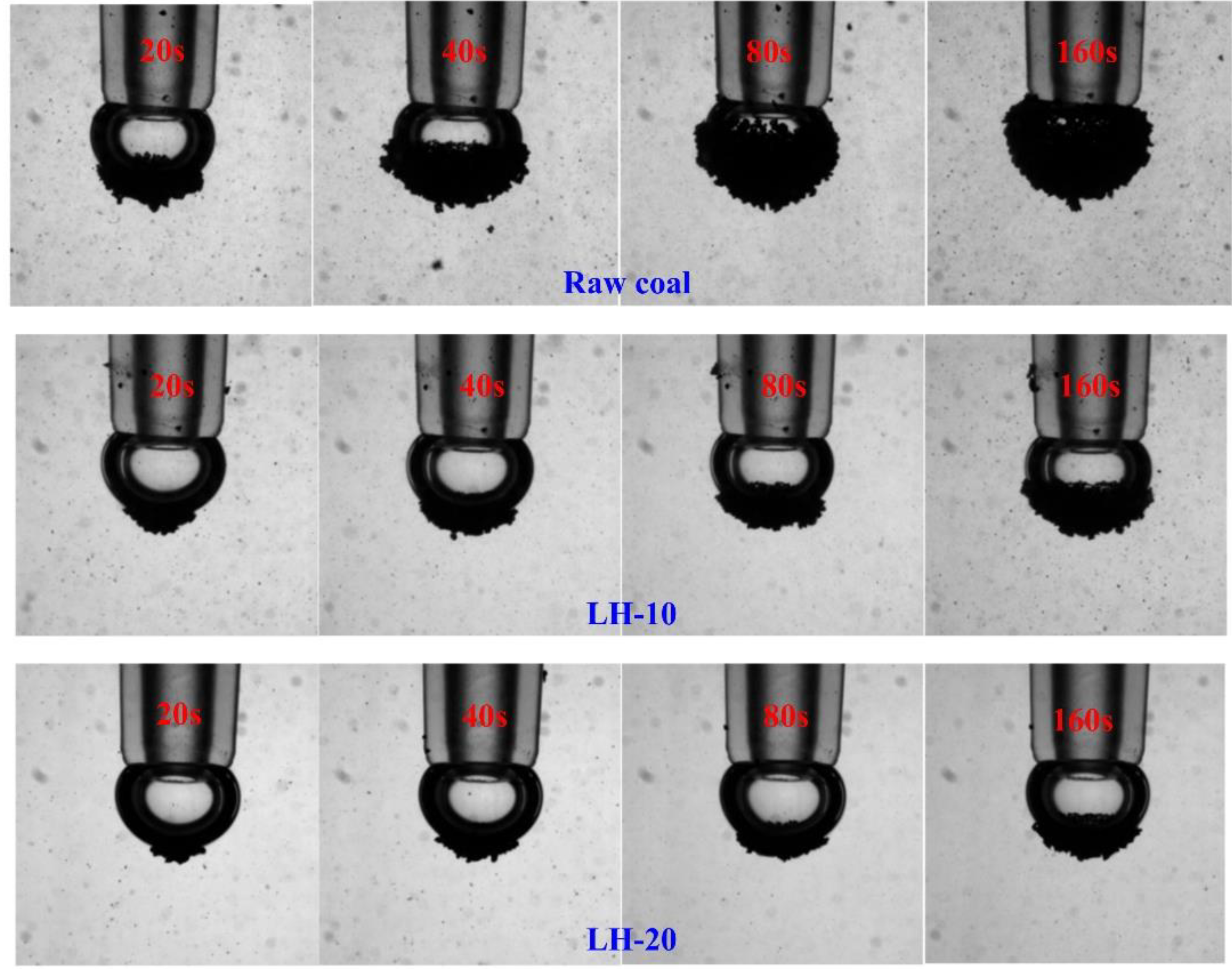
2.6. Bubble-Particle Wrap Angle Experiment
3. Results and Discussion
3.1. Effect of Heating Oxidation at 200 °C on Surface Morphology and Functional Groups of Anthracite Coal
3.2. Effect of Heating Oxidation at 200 °C on the Floatability of Anthracite Coal
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xing, Y.; Gui, X.; Cao, Y.; Wang, D.; Zhang, H. Clean low-rank-coal purification technique combining cyclonic-static microbubble flotation column with collector emulsification. J. Clean. Prod. 2017, 153, 657–672. [Google Scholar] [CrossRef]
- Ozkan, S.G. Further investigations on simultaneous ultrasonic coal flotation. Minerals 2017, 7, 177. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Pan, L.; Pinchasik, B.E.; Cao, Y.; Liu, J.; Kappl, M.; Butt, H.J. Recent experimental advances for understanding bubble–particle attachment in flotation. Adv. Colloid Interface Sci. 2017, 246, 105–132. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, B.; Zhao, P.; Duan, C.; Zhao, Y.; Zhang, Z.; Yan, G.; Zhu, X.; Ding, W.; Rao, Z. Upgrading low-quality oil shale using high-density gas-solid fluidized bed. Fuel 2019, 252, 666–674. [Google Scholar] [CrossRef]
- Li, E.; Lu, Y.; Cheng, F.; Wang, X.; Miller, J.D. Effect of oxidation on the wetting of coal surfaces by water: Experimental and molecular dynamics simulation studies. Physicochem. Probl. Miner. Process. 2018, 54, 1039–1051. [Google Scholar]
- Armed, H.A.; Drzymala, J. Effect of flotation procedure and composition of reagents on yield of a difficult-to-float coal. Physicochem. Probl. Miner. Process. 2004, 38, 53–63. [Google Scholar]
- Bolat, E.; Saglam, S.; Piskin, S. The effect of oxidation on the flotation properties of a Turkish bituminous coal. Fuel Process. Technol. 1998, 55, 101–105. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Diao, J. Characterization of coal oxidation and coal wetting behavior by film flotation. Coal Prep. 1992, 10, 1–17. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Yang, G.; Laskowski, J.S. Oxidation phenomena in coal flotation part I. Correlation between oxygen functional group concentration, immersion wettability and salt flotation response. Coal Prep. 1987, 4, 161–182. [Google Scholar] [CrossRef]
- Sarikaya, M. Flotation test as a method for studying coal weathering. Int. J. Miner. Process. 1995, 43, 31–35. [Google Scholar] [CrossRef]
- Xia, W.; Xie, G. Changes in the hydrophobicity of anthracite coals before and after high temperature heating process. Powder Technol. 2014, 264, 31–35. [Google Scholar] [CrossRef]
- Chang, Z.; Chen, X.; Peng, Y. Understanding and improving the flotation of coals with different degrees of surface oxidation. Powder Technol. 2017, 321, 190–196. [Google Scholar] [CrossRef]
- Gui, X.; Xing, Y.; Wang, T.; Cao, Y.; Miao, Z.; Xu, M. Intensification mechanism of oxidized coal flotation by using oxygen-containing collector α-furanacrylic acid. Powder Technol. 2017, 305, 109–116. [Google Scholar] [CrossRef]
- Jia, R.; Harris, G.H.; Fuerstenau, D.W. An improved class of universal collectors for the flotation of oxidized and/or low-rank coal. Int. J. Miner. Process. 2000, 58, 99–118. [Google Scholar] [CrossRef]
- Ozdemir, O.; Ersoy, O.F.; Guven, O.; Turgut, H.; Cinar, M.; Çelik, M.S. Improved flotation of heat treated lignite with saline solutions containing mono and multivalent ions. Physicochem. Probl. Miner. Process. 2018, 54, 1070–1082. [Google Scholar]
- Sokolovic, J.M.; Stanojlovic, R.D.; Markovic, Z.S. Activation of oxidized surface of anthracite waste coal by attrition. Physicochem. Probl. Miner. Process. 2012, 48, 5–18. [Google Scholar]
- Xia, W. The effect of heating on the wettability of lignite. Energy Sources Part A 2016, 38, 3521–3526. [Google Scholar] [CrossRef]
- Çinar, M. Floatability and desulfurization of a low-rank (Turkish) coal by low-temperature heat treatment. Fuel Process. Technol. 2009, 90, 1300–1304. [Google Scholar] [CrossRef]
- Ye, Y.; Jin, R.; Miller, J.D. Thermal treatment of low-rank coal and its relationship to flotation response. Int. J. Coal Prep. Util. 1988, 6, 1–16. [Google Scholar] [CrossRef]
- Çelik, M.S.; Seyhan, K. Effect of heat treatment on the flotation of Turkish lignites. Int. J. Coal Prep. Util. 1995, 16, 65–79. [Google Scholar] [CrossRef]
- Xu, M.; Xing, Y.; Cao, Y.; Gui, X. Effect of dodecane and oleic acid on the attachment between oxidized coal and bubbles. Minerals 2018, 8, 29. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, R.; Xing, Y.; Gui, X. Improving the adsorption of oily collector on the surface of low-rank coal during flotation using a cationic surfactant: An experimental and molecular dynamics simulation study. Fuel 2019, 235, 687–695. [Google Scholar] [CrossRef]
- Chu, P.; Mirnwzami, M.; Finch, J.A. Quantifying particle pick up at a pendant bubble: A study of non-hydrophobic particle–bubble interaction. Miner. Eng. 2014, 55, 162–164. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, L.; Zhang, R.; Yang, Z.; Xing, Y.; Gui, X.; Cao, Y.; Sun, W. Enhancement of flotation response of fine low-rank coal using positively charged microbubbles. Fuel 2019, 245, 505–513. [Google Scholar] [CrossRef]
- Wang, B.; Peng, Y.; Vink, S. Diagnosis of the surface chemistry effects on fine coal flotation using saline water. Energy Fuels 2013, 27, 4869–4874. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Li, G.; Liao, Y.; Xing, Y.; Gui, X. Combined effect of chemical composition and spreading velocity of collector on flotation performance of oxidized coal. Powder Technol. 2018, 325, 1–10. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Zhang, R.; Xing, Y.; Gui, X. Enhancement of the surface hydrophobicity of low-rank coal by adsorbing DTAB: An experimental and molecular dynamics simulation study. Fuel 2019, 239, 145–152. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, G.; Xu, M.; Wang, D.; Gui, X.; Xing, Y. Effect of Heating Oxidation on the Surface/Interface Properties and Floatability of Anthracite Coal. Processes 2019, 7, 345. https://doi.org/10.3390/pr7060345
Rong G, Xu M, Wang D, Gui X, Xing Y. Effect of Heating Oxidation on the Surface/Interface Properties and Floatability of Anthracite Coal. Processes. 2019; 7(6):345. https://doi.org/10.3390/pr7060345
Chicago/Turabian StyleRong, Guoqiang, Mengdi Xu, Dongyue Wang, Xiahui Gui, and Yaowen Xing. 2019. "Effect of Heating Oxidation on the Surface/Interface Properties and Floatability of Anthracite Coal" Processes 7, no. 6: 345. https://doi.org/10.3390/pr7060345
APA StyleRong, G., Xu, M., Wang, D., Gui, X., & Xing, Y. (2019). Effect of Heating Oxidation on the Surface/Interface Properties and Floatability of Anthracite Coal. Processes, 7(6), 345. https://doi.org/10.3390/pr7060345




