Many stakeholders are involved in the financing of the treatment and care of Alzheimer’s, who often have decision-making powers on the amount of the sickness benefit. These are both private and public entities, which include public health and social care departments of regions and cities, which decide on the financing of beds and the creation and financing of capacities in residential facilities, but also for outpatient services. The combination of both health and social care in a single facility (whether social as the home for the elderly) and the follow-up funding make the situation very confusing. This is evidenced by the fact that at present, there is still no unified evidence of costs for AD patients in the Czech Republic or Europe.
Figure 6 provides an idea of how financial resources are linked to key players in the context of economic development. In the case of increasing productivity in the economy, the level of GDP is rising, so there is a potential for raising financial resources for health and social care. This, in turn, increases the quality of life not only for the patients themselves, but also for their caregivers, which in turn translates into productivity growth in the system.
The need to declare cost items and their interconnection with financial resources is a fundamental basis for decision-making of competent persons, and the BPMN tool that describes in detail the individual activities is suitable for this purpose.
4.1. Alzheimer’s Disease Cost Model
The Alzheimer’s disease process is, in this section, only focused on those AD components that allocate costs. The resulting process is rather a description and analysis of activities aimed at slowing down the progress of the disease or making the patient’s daily life easier. Due to the primary BPMN notation used, Alzheimer’s disease treatment can be defined as a process that typically consists of parallel activities. The decision on how the remedy is deployed is then based on a cyclically-performed MMSE test, whose score in this paper is a key indicator of the severity of the disease. The severity of Alzheimer’s disease is divided into three stages, as shown below:
The crucial second attribute for process management is the time point that is related to determining the stage of the disease. The determination of the stage also determines the remedies. In the real world, discovering the stage and severity of the illness is a complex problem that significantly draws from soft data and experience. For this paper, therefore, the decisive criterion for the transition between the stages will be time. Each disease phase takes a certain amount of time that is being considered in the process.
Due to the technical limitations of Enterprise Architect, the length of each phase of the disease was fixed. A specific element of coincidence in this model is the element of the AD drop. Any MMSE test may cause the MMSE score to drop. When this decrease is high, the pharmacological treatment of cognition is terminated. After the transition to the next phase, cognitive therapy is restarted and its effectiveness controlled. Each of the Alzheimer’s disease phases then has a differently defined complexity of individual activities. Typically, medication is changing along with the illness progression, and the nature of patient care changes as well. In the initial stages, attention is focused to the fullest extent possible on slowing down the illness progression and preserving the patient’s healthy lifestyle. In the following phases, care is focused more on minimizing the impacts of the disease and retaining the patient’s quality of life. This is illustrated, for example, in
Figure 7 and
Figure 8.
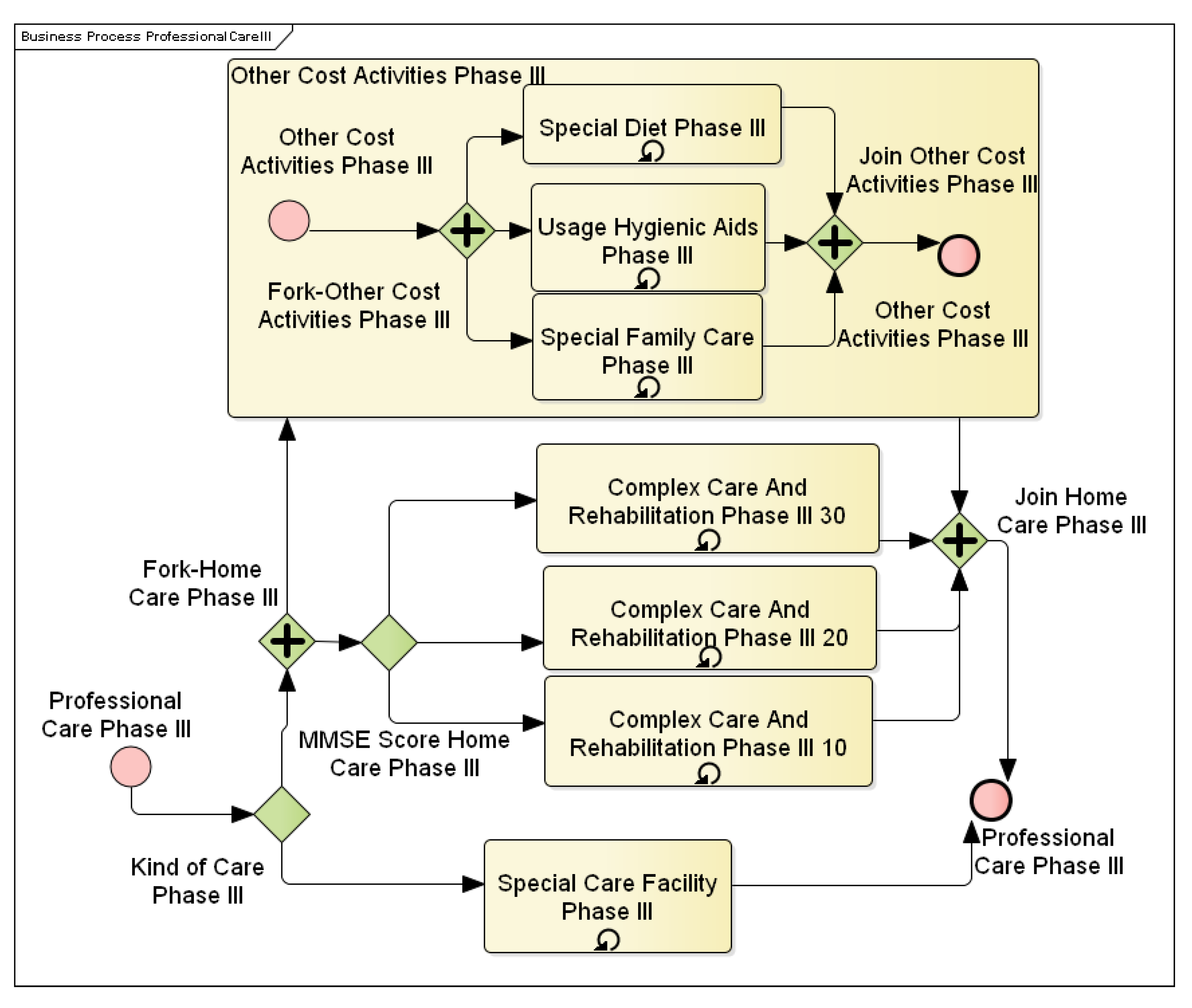
Figure 7 shows patient care activities in the first stage of the disease. This includes activation activities and psychotherapy with the patient. These activities aim to keep the patient mentally active and creative. On the other hand,
Figure 8 shows the third phase of AD, where the patient is already entirely dependent on other people, and therefore, care is reduced only to comprehensive medical care as such.
Most activities also take place over one phase repeatedly. Unless otherwise specified in the process, activities were repeated in monthly intervals. Typical representatives of events with a different range are medical examinations. Where the conversion of days to months was used in the process, it was assumed that the month was 30 days.
Remedies for dealing with AD are classified in different ways; the main ones are pharmacological treatment and formal care. Parallel with these activities, regular medical examinations are still carried out. These examinations are intended to monitor the condition of the patient and, if appropriate, to adjust the terms of care or pharmacological treatment. Specialized aids from areas of care for the patient’s daily life or therapeutic aids are also included in the care, developing the cognitive and motor functions of the patient. As an alternative to patient care, work is then selected in a specialized facility where it is assumed that all operations are secured directly in the facility and do not generate any additional costs.
4.2. Alzheimer’s Disease Cost Calculation
For the individual activities described in the previous section, it was necessary to assign cost variables and their units. For the whole model, the Czech crown was used as the currency, mainly due to the use of current prices in online resources such as the State Institute for Drug Control and, where appropriate, price-lists of care services or homes. Quantities taken from research and articles where they were denominated in euros were translated at the current exchange rate. This conversion caused values to be distorted, but this was not a significant issue in terms of the calculation mechanism. The outputs referred to in this article were then converted and denominated in euros.
Medical performances were valued based on the point value of individual actions. Each operation had a certain number of points indicating the intensity of the activity, and one point had its value in crowns. Given that Decree No. 134/1998 Coll. is very complex and in order to arrive at the real point value, it was necessary to combine several tasks at once, and the point evaluation of individual acts was taken from the article [
57].
The cost of pharmaceutical treatment was based on the recommended dosage of medication per day. The dosing was then compared to the indicated package size and package prices stated on the website of the State Institute for Drug Control [
42] in the “Indicative Selling Price” box.
To define the cost of care, the primary source of the public price list was the Sunflower and Home at the Fountain website. The Sunflower Agency provides specialized patient care at home, and Fountain Home is a dedicated facility for seniors and people living with dementia. A partial source for the definition of care costs was [
56], which presented the prices of musical therapy and activation activities.
A specific area for costing was the cost of tools, meals, or increased family care. To determine the price of food, Decree 505/2006 Coll. was used. The value of the maximum amount of reimbursement for the provision of care services and the like according to the VZP Payment Card was used for the determination of the cost of hygiene aids. The cost of increased family care was calculated as the cost of the sacrificial opportunity, taking into account the average wage in the Czech Republic. Assay calculations were based on published research in the thesis [
59].
The starting point for determining costs was to divide Alzheimer’s disease into a total of four central units. These units were then submerged into subprocesses, depending on how the cost would be calculated. The subprocesses were then broken down into individual sub-activities for which specific costs had already been defined. The following sections describe the unique activities and assign numerical cost values to them.
Identifying specific values for individual activities was very difficult, as not all information was directly traceable from public sources. Alternatively, resources vary greatly (e.g., client care hours). For this reason, the subjects were chosen in the article, and their level of appreciation corresponded to the breakdown of individual processes in this article.
4.2.1. Cost of Alzheimer’s Disease Diagnostics
The subprocess only occurred once in the process. Given that this subprocess had just one occurrence in the model, at the beginning of the model, it was decided to treat it as a separate unit. The cost of this subprocess consisted of medical examinations of a patient suspected of having AD. Each exam had a point value [
57]. The exam fee was then determined by multiplying this point value and point price. The point price was taken from the Ministry of Health’s response [
60], where the value of one point was approximately 0.02 €. The costs of individual actions are shown in
Table 1.
4.2.2. Costs of Pharmacological Treatment
Costs of pharmacological treatment included repeatedly occurring subprocesses from each of the three phases of the disease. Some medications are always administered, regardless of the severity of Alzheimer’s disease. Other drugs are only given at a certain hardness of the disease or only at a specific trend of disease development. Specifically, this applies to donepezil, which is only used for moderate Alzheimer’s disease.
Where more possible drugs could be chosen from available sources and it was not possible to simulate their combination (e.g., substitution for poor drug tolerance), the most commonly-used drug was chosen. A similar approach was used if only the active substance were mentioned in the article, and several drugs could be traced under different brand names.
Assignment of drug groups to the different stages of the disease was based on consultations with MUDr. Jiří Kuchyňka, and only specific names of drugs and their prices were sought; see
Table 2.
Donepezil: This is an active substance that is represented by a drug with the marketing name Aricept. This medicine is intended for patients with an MMSE score higher than 20. This medicine belongs to the cognitive group.
Memantine: This is another cognitive drug intended for patients with an MMSE score of fewer than 20 points. The name of the active substance is the same as the name of the medicine. For both of these drugs, it is common that the effectiveness of the treatment is evaluated every three months. When the treatment is not effective, drug administration is discontinued.
Prozac: This is an antidepressant administered in all three phases of the disease to alleviate the effects of depressive states. In the modeled process, it is still prescribed at the same dose.
Tiapride: This is a neuroleptic/antipsychotic drug for the second stage of Alzheimer’s disease. During the entire second phase, it is administered as a single dose.
Risperdal: This is a neuroleptic/antipsychotic drug for the third phase of Alzheimer’s disease. During the entire third phase, it is administered as a single dose.
4.2.3. Formal Care
This subprocess reappears in each of the three phases of the disease. In terms of patient care, two different approaches are possible, which will be reflected in the cost calculation. The first is patient care in the place of the patient’s residence. In this case, all care items must be calculated individually, including extra costs for “material”. The material covers hygienic and therapeutic aids and catering costs. The concept of care includes activities that are carried out by specialized personnel. These involve various therapies and activation activities. It also covers general comprehensive care and mobility-based exercises provided by an assistance service at the place of residence to compensate for the patient’s inability to perform normal activities. For example, this includes assistance with dressing, and so on. Specific in this care is the cost arising from the need for the family to provide increased attention to the patient.
The cost of assistance services was calculated per hour of work. The price list of the Sunflower Agency was used for pricing, and regular care for seniors was expected at an hourly rate of 6.25 €. The cost of activation activities was taken from the article [
56], where the values were given per month. The price of psychotherapy was determined based on a price list on the Internet, and two psychiatric visits per month were expected.
The number of hours spent on patient care was obtained from the diploma thesis [
59], where the average values for formal and informal care and the severity of illness were stated. This article assumed that care was divided equally between the assistance service and the family. The value of the care provided by the assistance service was further adjusted according to the stage of the illness. It was assumed that a patient in the first stage will require less care than a patient in the last stage. Family care was supposed to be the same throughout the illness.
The last kind of formal care was the placement of the patient in a home for the elderly. In the calculation, the price list of Home at the Fountain was used. Placement costs included payment for accommodation in a single-occupancy room with five meals per day. Besides, the cost of care was calculated as a cost depending on the degree of dementia. The models then used the total price per month. The exact calculation is shown in
Table 5.
4.2.4. Other Expenditure Activities
This subprocess was linked to activities that generate costs associated with formal and informal care at home, but the nature of these activities cannot be included in formal care. Other costs are specific to each stage of the disease, and where activities are repeated at different stages, they can be valued at different rates.
Table 6 shows the cost valued into various stages of the disease.
Another specific feature of this subprocess is that each activity was valued differently. Material aids in this subprocess were evaluated based on the Methodology for VZP Medical Devices. This methodology specifies a group of incontinence aids, indicating the maximum amount of reimbursement based on the severity of the disease.
Caregivers are also included in this category. For the sake of simplicity, it was valued hourly, and one hour of the family member’s work was recalculated from the average Czech wage. According to the Czech Statistical Office, the average gross salary in the fourth quarter of 2017 was . The average gross hourly wage was determined as a gross wage divided by 160, i.e., per hour after rounding.
The food costs in this paper were based on Decree 505/2006 Coll., which sets the maximum amount of food costs. For this paper, only lunch was calculated in the first phase, while in the second and third stages, full-day meals were calculated. The price of one lunch was set at 3 €, and full-day meals cost .
The cost of providing special tools was taken from the diploma thesis [
59,
61], which showed the average price per month for medical aids. Costs were differentiated according to the severity of the disease. The value of rehabilitation aids was derived from this amount.
In
Table 7, the number of units entered in the BPMN simulation and activity costs were entered. Subsequently, the total cost was divided by the number of units, and unit costs were obtained that matched the costs entered into the EA simulation engine.