Investigations on Novel Ternary Green Polymer Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.2. Studies of Thermal and Crystallization Behaviors
2.3. Morphological Observations and Characterizations
2.4. Discussions on Crystalline Structures
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, N.K.; Singh, S.K.; Dash, D.; Gonugunta, P.; Misra, M.; Maiti, P. CNT Induced β-phase in polylactide: Unique crystallization, biodegradation, and biocompatibility. J. Phys. Chem. C 2013, 117, 10163–10174. [Google Scholar] [CrossRef]
- Ni’mah, H.; Woo, E.M. A novel hexagonal crystal with a hexagonal star-shaped central core in poly(l-lactide) (PLLA) induced by an ionic liquid. Crystengcomm 2014, 16, 4945–4949. [Google Scholar] [CrossRef]
- Hughes, A.; Tai, H.; Tochwin, A.; Wang, W. Biodegradable and biocompatible PDLLA-PEG1k-PDLLA diacrylate macromers: Synthesis, characterisation and preparation of soluble hyperbranched polymers and crosslinked hydrogels. Processes 2017, 5, 18. [Google Scholar] [CrossRef]
- Pawar, R.P.; Tekale, S.U.; Shisodia, S.U.; Totre, J.T.; Domb, A.J. Biomedical applications of poly(lactic acid). Recent Pat. Regen. Med. 2014, 4, 40–51. [Google Scholar] [CrossRef]
- Vink, E.T.H.; Rábago, K.R.; Glassner, D.A.; Springs, B.; O’Connor, R.P.; Kolstad, J.; Gruber, P.R. The sustainability of NatureWorks™ polylactide polymers and Ingeo™ polylactide fibers: An update of the future. Macromol. Biosci. 2004, 4, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Czekalski, J.; Krucińska, I.; Kowalska, S.; Puchalski, M. Effect of twist stabilisation and dyeing on the structural and physical properties of agricultural strings. Fibres Text. East. Eur. 2013, 21, 39–44. [Google Scholar]
- Xiang, S.; Feng, L.; Bian, X.; Zhang, B.; Sun, B.; Liu, Y.; Li, G.; Chen, X. Toughening modification of PLLA with PCL in the presence of PCL-b-PLLA diblock copolymers as compatibilizer. Polym. Adv. Technol. 2019, 30, 963–972. [Google Scholar] [CrossRef]
- Zaldua, N.; Mugica, A.; Zubitur, M.; Iturrospe, A.; Arbe, A.; Re, G.L.; Raquez, J.M.; Dubois, P.; Müller, A.J. The role of PLLA-g-montmorillonite nanohybrids in the acceleration of the crystallization rate of a commercial PLA. Crystengcomm 2016, 18, 9334–9344. [Google Scholar] [CrossRef]
- Singh, S.; Maspoch, M.L.; Oksman, K. Crystallization of triethyl-citrate-plasticized poly(lactic acid) induced by chitin nanocrystals. J. Appl. Polym. Sci. 2019, 136, 47936. [Google Scholar] [CrossRef]
- Kodal, M.; Sirin, H.; Ozkoc, G. Non-isothermal crystallization kinetics of PEG plasticized PLA/G-POSS nanocomposites. Polym. Compos. 2017, 38, 1378–1389. [Google Scholar] [CrossRef]
- Chen, P.Y.; Lian, H.Y.; Shih, Y.F.; Chen Wei, S.M.; Jeng, R.J. Preparation, characterization and crystallization kinetics of Kenaf fiber/multi-walled carbon nanotube/polylactic acid (PLA) green composites. Mater. Chem. Phys. 2017, 196, 249–255. [Google Scholar] [CrossRef]
- Chen, J.; Xu, C.; Wu, D.; Pan, K.; Qian, A.; Sha, Y.; Wang, L.; Tong, W. Insights into the nucleation role of cellulose crystals during crystallization of poly(β-hydroxybutyrate). Carbohydr. Polym. 2015, 134, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.-T.; Hsu, C.-Y.; Hung, S.-P. Promoted crystallization kinetics of biodegradable poly(butylene succinate) by a nucleation agent of green chemical. J. Polym. Res. 2019, 26, 255. [Google Scholar] [CrossRef]
- Okamoto, K.; Ichikawa, T.; Yokohara, T.; Yamaguchi, M. Miscibility, mechanical and thermal properties of poly(lactic acid)/polyester-diol blends. Eur. Polym. J. 2009, 45, 2304–2312. [Google Scholar] [CrossRef]
- Wu, J.H.; Yen, M.S.; Kuo, M.C.; Chen, B.H. Physical properties and crystallization behavior of silica particulates reinforced poly(lactic acid) composites. Mater. Chem. Phys. 2013, 142, 726–733. [Google Scholar] [CrossRef]
- Wu, T.; Tong, Y.; Qiu, F.; Yuan, D.; Zhang, G.; Qu, J. Morphology, rheology property, and crystallization behavior of PLLA/OMMT nanocomposites prepared by an innovative eccentric rotor extruder. Polym. Adv. Technol. 2018, 29, 41–51. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; You, C.; Chen, M. Effects of MgO whiskers on mechanical properties and crystallization behavior of PLLA/MgO composites. Mater. Des. 2016, 89, 573–581. [Google Scholar] [CrossRef]
- Li, J.; Qiu, Z. Significantly enhanced crystallization of poly(l-lactide) by the synergistic effect of poly(diethylene glycol adipate) and cellulose nanocrystals in their fully biodegradable ternary composite. Ind. Eng. Chem. Res. 2019, 58, 15526–15532. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, C.; Wang, X. One-pot solvothermal synthesis of water-soluble boron nitride nanosheets and fluorescent boron nitride quantum dots. Mater. Lett. 2019, 234, 306–310. [Google Scholar] [CrossRef]
- Puente, J.A.S.; Esposito, A.; Chivrac, F.; Dargent, E. Effect of boron nitride as a nucleating agent on the crystallization of bacterial poly(3-hydroxybutyrate). J. Appl. Polym. Sci. 2013, 128, 2586–2594. [Google Scholar] [CrossRef]
- Sijla Rosely, C.V.; Shaiju, P.; Bhoje Gowd, E. Poly(l-lactic acid)/boron nitride nanocomposites: Influence of boron nitride functionalization on the properties of poly(l-lactic acid). J. Phys. Chem. B 2019, 123, 8599–8606. [Google Scholar] [CrossRef] [PubMed]
- Sijla Rosely, C.V.; Nagendra, B.; Sivaprasad, V.P.; Bhoje Gowd, E. Influence of boron nitride nanosheets on the crystallization and polymorphism of poly(l-lactide). J. Phys. Chem. B 2018, 122, 6442–6451. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Zhu, B.; Kai, W.; Dong, T.; Inoue, Y. Polymorphic transition in disordered poly(l-lactide) crystals induced by annealing at elevated temperatures. Macromolecules 2008, 41, 4296–4304. [Google Scholar] [CrossRef]
- Li, F.J.; Zhang, S.D.; Liang, J.Z.; Wang, J.Z. Effect of polyethylene glycol on the crystallization and impact properties of polylactide-based blends. Polym. Adv. Technol. 2015, 26, 465–475. [Google Scholar] [CrossRef]
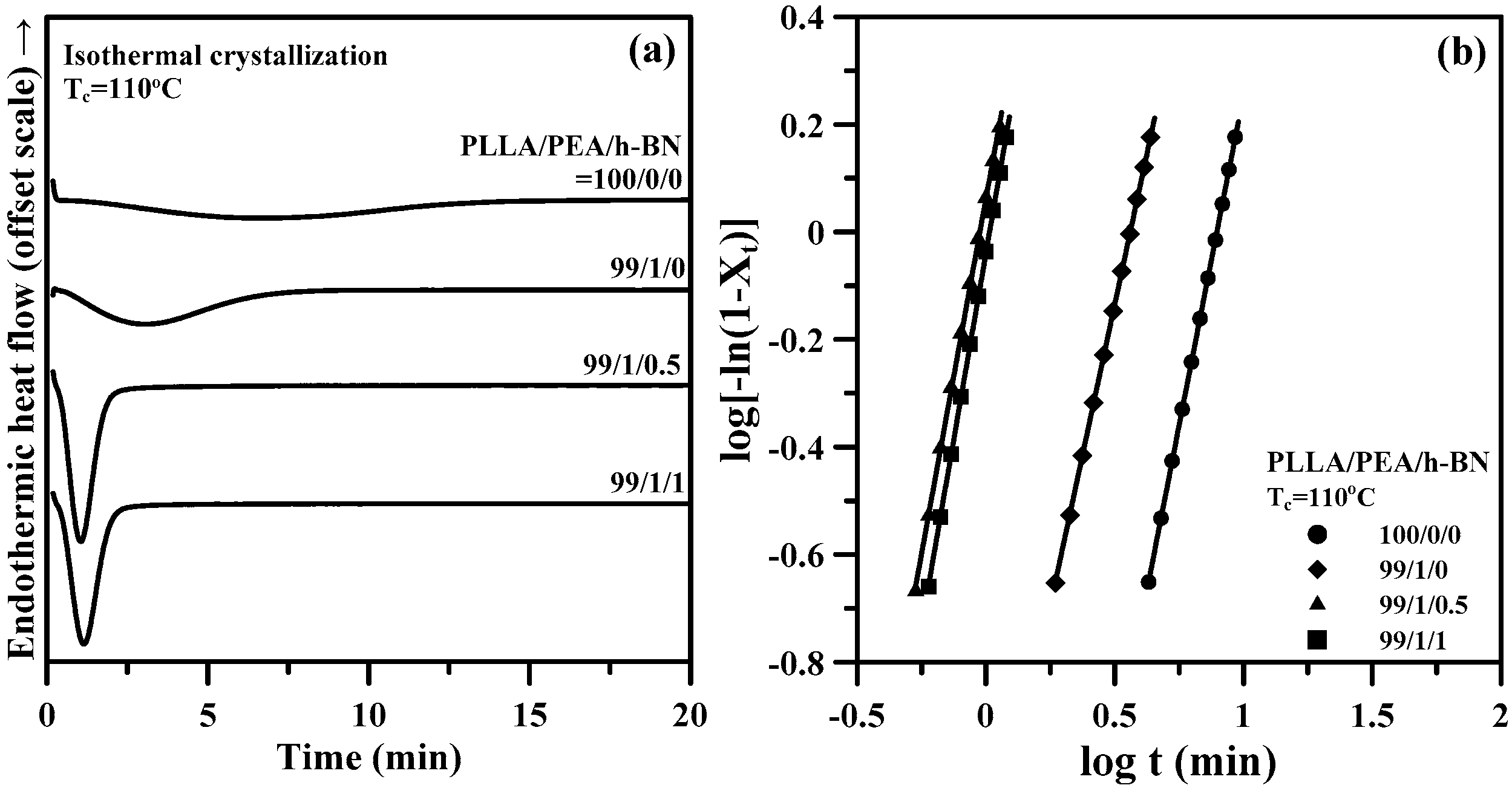
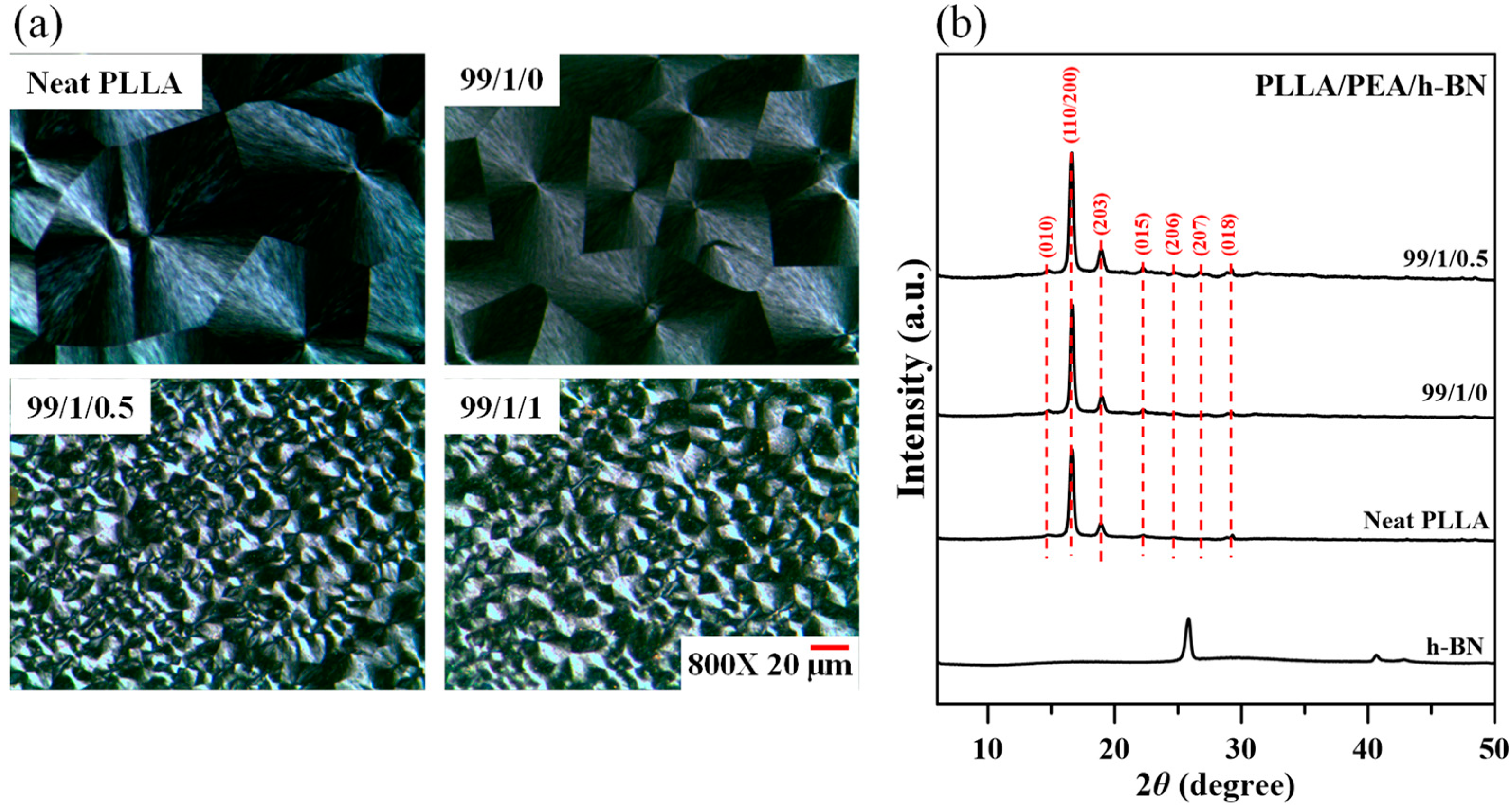
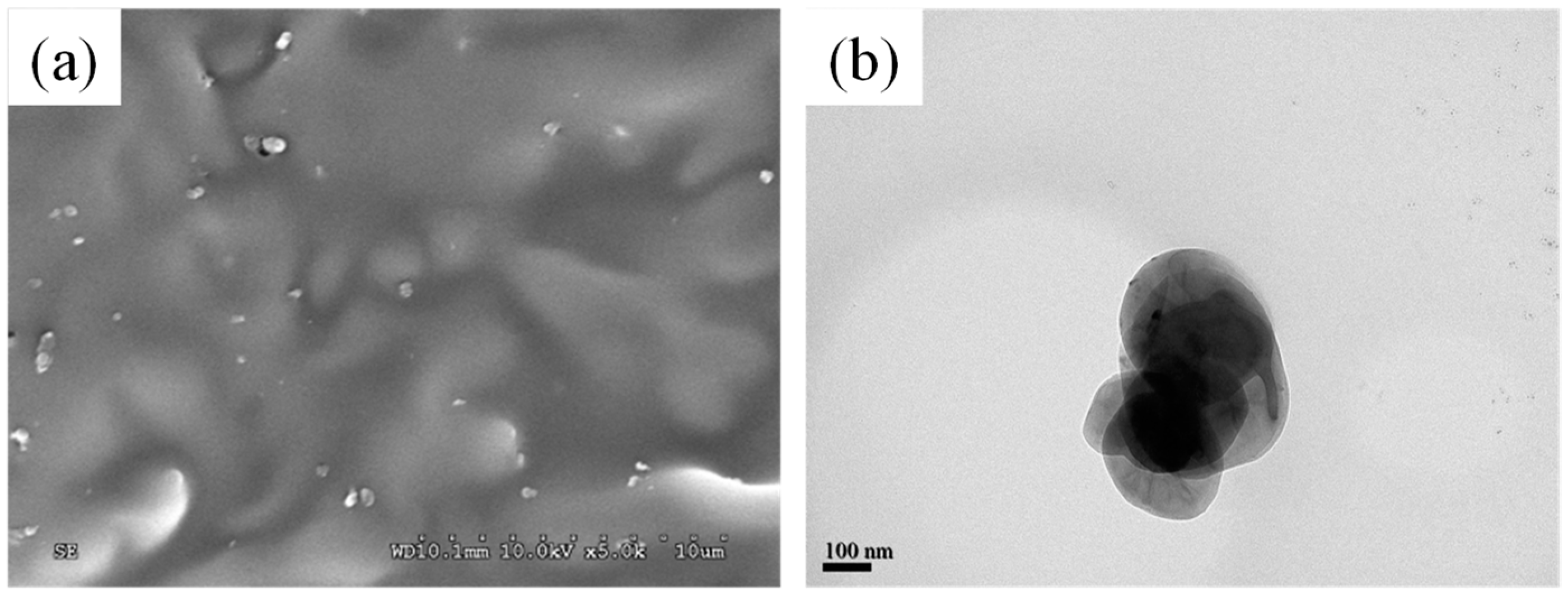
| PLLA/PEA/h-BN (Relative Weight Ratio) | Tc (°C) | n | K (min−n) | t0.5 (min) | 1/t0.5 (min−1) |
|---|---|---|---|---|---|
| 100/0/0 | 110 | 2.46 | 6.268 × 10−3 | 6.79 | 0.147 |
| 99/1/0 | 110 | 2.24 | 5.56 × 10−2 | 3.08 | 0.324 |
| 99/1/0.5 | 110 | 2.64 | 1.153 | 0.82 | 1.213 |
| 99/1/1 | 110 | 2.78 | 9.137 × 10−1 | 0.91 | 1.105 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, T.-C.; Lee, L.-T.; Wu, X.-Y. Investigations on Novel Ternary Green Polymer Composite. Processes 2020, 8, 31. https://doi.org/10.3390/pr8010031
Hsu T-C, Lee L-T, Wu X-Y. Investigations on Novel Ternary Green Polymer Composite. Processes. 2020; 8(1):31. https://doi.org/10.3390/pr8010031
Chicago/Turabian StyleHsu, Ting-Chia, Li-Ting Lee, and Xin-Yun Wu. 2020. "Investigations on Novel Ternary Green Polymer Composite" Processes 8, no. 1: 31. https://doi.org/10.3390/pr8010031
APA StyleHsu, T.-C., Lee, L.-T., & Wu, X.-Y. (2020). Investigations on Novel Ternary Green Polymer Composite. Processes, 8(1), 31. https://doi.org/10.3390/pr8010031




