Abstract
The purpose of this study was to attempt the encapsulation of lemongrass (Cymbopogon citratus) essential oil utilizing spray drying technique. An array of process parameters including concentration of wall (15–30%), type of wall materials (maltodextrin, maltodextrin and gum Arabic mixture), and concentration of essential oil (0.5–2.0%) were thoroughly investigated. The results show that the use of sole maltodextrin as encapsulant gave microcapsules characteristics comparable to that of powder produced using maltodextrin and gum Arabic mixture. The encapsulation process that was performed with maltodextrin at the concentration of 30% as wall material and lemongrass essential oil at the concentration of 1.5% as core material showed highest drying yield (84.49%), microencapsulation yield (89.31%) and microencapsulation efficiency (84.75%). Encapsulated essential oils retained most of their major constituents in comparison with the bare essential oils without any significant compromise in product quality.
1. Introduction
Essential oils, due to their benefits, have been extensively utilized in various industries, including manufacture of antimicrobial agents, medicines and food additives, to meet the recently emerging demand for products of natural origin [1,2]. Lemongrass (Cymbopogon citratus), which belongs to the Cymbopogon family [3], consists of about 55 species and is cultivated mainly in tropical and subtropical countries. Lemongrass is a highly organoleptic spice in culinary applications and a common medicinal herb which is extensively used in folk medicine [4,5]. The lemongrass essential oil accounts for more than 75% of the weight of the plant. Lemongrass essential oils have been reported to play an important role in the treatment of different diseases such as oily skin, scabies, acne, antibacterial, and antimicrobial [6]. Among the biological activities exhibited by lemongrass essential oil, antimicrobial activity figures prominently, possibly due to the presence of geraniol and citral isomers. Nevertheless, the highly volatile nature and easily degradable properties of the oil are the main disadvantages limiting the use of lemongrass essential oil in the food and pharmaceutical sectors [1].
One of the solutions that was devised to address such issues is microencapsulation. Microencapsulation refers to the process in which active substances (core materials), like food oils, are packaged by secondary (wall) material. In pharmaceutical applications, microencapsulation could bring about controlled release of a drug, afford gastro-resistant microparticles, alter drug solubility and prevent pharmaceutical incompatibilities [7,8]. In food applications, microencapsulation is feasible to improve the stability of essential oils. Microcapsule walls commonly consist of polymeric materials, which are prepared by different chemical and physical methods, such as spray drying [9], precipitation [10], coacervation [11], dissolving [12] and solid complexation [13]. Among them, spray drying is the most usually applied encapsulation technique for food products. However, efficient spray drying encapsulation depends on the high retention of the core materials [14].
To date, only four investigations that involve encapsulation of lemongrass essential oil via spray-drying have been attempted. The most recent study aimed to justify the use of four different wall materials including gum Arabic, corn maltodextrin, cassava maltodextrin and modified starch with octenyl succinic anhydride in encapsulation of lemongrass essential oils [15]. A wide range of product characteristics, such as oil retention, volatile profiles and morphology, were considered, and the combination of gum Arabic and modified starch was identified as the optimal feed emulsion for spray drying. However, process parameters and assessment of encapsulation efficiency were not elaborated in this study. The second study, which explored the encapsulation of lemongrass essential oil with a new cross-linked sodium trimetaphosphate-gum Arabic, found that the modified gum Arabic could achieve a very high encapsulation efficiency and maintain a good volatile profile of the obtained product [16]. In the study of Phunpee et al., binding affinities of three cyclodextrins toward two isomers of citral were investigated, showing good citral retention of beta- and alpha-cyclodextrin [17]. This result was experimentally confirmed by a spray-drying attempt with lemongrass essential oil. In the fourth study, the retention rate of different volatiles of lemongrass oil was explored with respect to the use of two wall materials (beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin) [18]. The results suggest the use of beta-cyclodextrin as a suitable wall material for lemongrass essential oil encapsulation.
Generally, the four aforementioned studies lack determination of effects of feed emulsion parameters (such as concentration of wall and core materials) on product characteristics. In addition, microencapsulation efficiencies with common wall materials have not been adequately reported. Therefore, the present study aimed to fulfil two main objectives. First, we attempted the manufacture of microcapsulates containing lemongrass essential oil via spray-drying using different combinations of wall materials. Secondly, different process parameters, including encapsulant combination, concentration of wall material and core material, were investigated. Encapsulation outcomes that were examined include product moisture, drying yield (DY), microencapsulation yield (MEY), microencapsulation efficiency (MEE) and volatile profile of lemongrass essential oil. The study result is expected to aid in further effort in seeking new encapsulant materials and in reducing cost for preservation of volatile and thermally sensitive compounds.
2. Materials and Methods
2.1. Materials
Lemongrass (Cymbopogon citratus) essential oil, purchased from the AOTANICA company, located in Tien Giang province, Viet Nam was used as the core material. Gum Arabic and maltodextrin from maize starch with DE 12 obtained from Sigma-Aldrich (St. Louis, MO, USA) were used as the wall materials. Tween 80 (99%, Xilong, Shantou, China) was used as an emulsifier. n-pentane (99%, Xilong, Shantou China) was used to determine the microencapsulation efficiency.
2.2. Microencapsulation of Essential Oil by Using Spray-Drying
Firstly, wall materials consisting of maltodextrin and gum Arabic at a specified ratio (either 1:0 or 4:1 (w/w)) were mixed with 500 mL of distilled water under stirring. Produced mixtures were allowed to stand overnight at room temperature. Then, the lemongrass essential oil was mixed with the wall material solution and Tween 80 with amount equal to 5% weight of essential oil under stirring at 6000 rpm for 20 min, using a rotor-stator blender to form an emulsion. Following that, approximately 800 mL of the mixture was taken to spray drying in a lab-scale instrument (YC-015 spray dryer, c). The wall material concentration and the amount of lemongrass essential oil were 30% and 1.5% of the mass of the solutions, respectively. The operational conditions included an inlet temperature of 140 °C and feed rate of 120 mL h−1. The obtained dried powder was kept in the sealed glass bottle at 25 °C until further analysis. Table 1 and Table 2 summarize feed emulsion compositions used in experiments.

Table 1.
Feed emulsion compositions in the first investigation.

Table 2.
Feed emulsion compositions in the second investigation.
2.3. Characterisation of the Microcapsules
2.3.1. Determination of Moisture Content
The determination of moisture content of the product followed the AOAC International (AOAC, 2007) method. To be specific, the weight loss percentage (%) of the product, obtained after oven-drying at 105 °C until constant weight, was used to calculate the moisture content (%).
2.3.2. Determination of Drying Yield (DY)
The DY, or powder recovery, is defined as the mass of the obtained spray-dried product over quantity of feed solution, calculated on the dry basis [19].
where m1 is mass of the feed emulsion (g), m2 is the mass of the powder product, x is the solid percentage (%) and y is the moisture of the obtained product.
2.3.3. Determination of Microencapsulation Yields (MEY)
The microencapsulation yield, or oil retention, is defined as the quantity of essential oil in the obtained product over the quantity of essential oil in the feed solution, calculated on the dry basis. The procedure is as follows [20]. Firstly, 30 g of the microencapsulated powder was added in 150 mL of distilled water and stirred. The solution was then hydrodistilled in a Clevenger-type apparatus for 4 h. Following that, the oil phase that floated on top of the aqueous phase in the collector was measured for volume. MEY was calculated as follows.
2.3.4. Determination of Microencapsulation Efficiency (MEE) and Surface Oil (SO)
Microencapsulation efficiency was determined as the quantity of encapsulated oil over the total oil existing in the obtained spray-dried product, calculated on the dry basis. The procedure for the determination of the mass of encapsulated essential oils is as follows. First, 30 g of the obtained microcapsulate powder was placed in a beaker, followed by the addition of 150 mL of n-pentane. The mixture was periodically stirred every 10 min for 1 h. n-pentane was then separated from the mixture by filtering through a Whatman no. 1 filter paper [21]. The amount of powder that was retained in the filter was dried in the oven for 5 h at 60 °C. The obtained dried product was then subjected to hydrodistillation in Clevenger-type apparatus for 4 h. The obtained essential oil was then used to calculate MEE as follows.
The surface oil was determined as SO = (1 − MEE) × 100%.
2.3.5. Gas Chromatography–Mass Spectrometry
A Gas Chromatography–Mass Spectrometry (GC–MS) was applied to investigate the composition of the essential oil samples prior to and after encapsulation [22]. An amount of 25 µL of the sample of essential oil was mixed in 1.0 mL n-hexane. The instrument used was GC Agilent 6890N coupled with MS 5973 inert with an HP5-MS column. The head column pressure was set to 9.3 psi. The C–MS system operates under the following conditions: carrier gas was He; flow rate of 1.0 mL/min; split ratio of 1:100; injection volume of 1.0 µL; injection temperature at 250 °C; oven temperature progress included an initial hold at 50 °C for 2 min, then increased by 2 °C/min to 80 °C, and increased by 5 °C/min to 150 °C, risen to 200 °C at 10 °C/min and finally risen to 300 °C at 20 °C/min for 5 min.
2.3.6. Particle Morphology
All spray-dried powders were observed under a scanning electron microscope (JSM 6300 SEM). Samples were directly deposited on carbon conductive tape on aluminium SEM stubs and were coated with a thin gold layer using gold sputtering.
2.4. Statistical Analysis
Each measurement was conducted at least three times. Statgraphics statistics software (version 20, Statpoint Technologies, Inc., Warrenton, VA, USA) was used to evaluate the data. Analysis of variance (ANOVA) and the least significant difference (LSD) were performed to compare the mean value of the film’s properties with a significance of 0.05.
3. Results
3.1. Composition of the Lemongrass Essential Oil
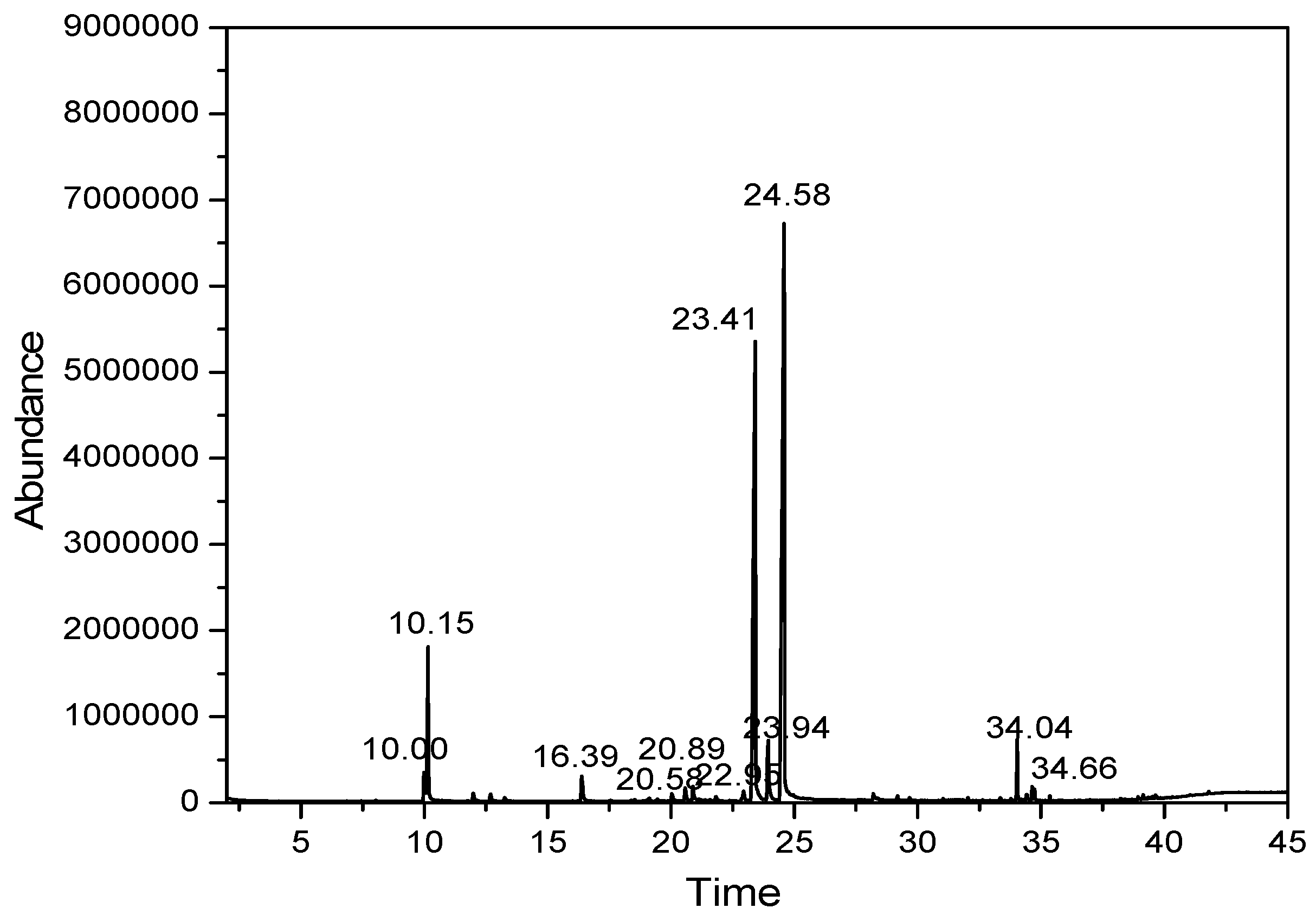
The core material of lemongrass essential oil was first determined for chemical composition by gas chromatography–mass spectrometry (GC–MS). The GC–MS spectrum of essential oil samples is presented in Figure 1.
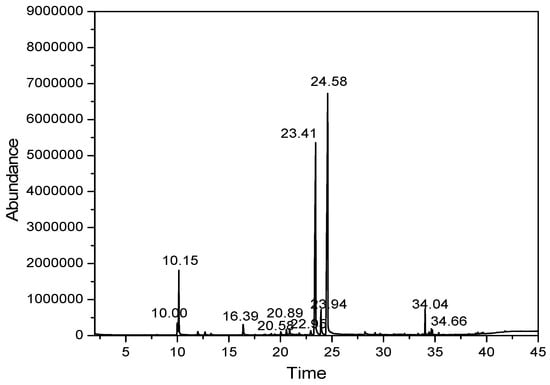
Figure 1.
The spectrum of lemongrass essential oil samples.
At first glance, two peaks located at 23.41 min and 24.59 min were found to have the greatest intensities. Mass spectrometry approximated the molecular formula and weight of the two substances as m/z = 137 and m/z = 152, respectively. This was affirmed by the compositional results 1 displaying the structural formulas and identification of active ingredients existing in lemongrass essential oil. In total, there were nine identified components, accounting for approximately 89.984% of the total essential oil content. The major components were citral (46.603%), myrcene (34.983%), geraniol (3.556%), Farnesol (1.896%), citronella (1.525%) and 6-methyl-5-hepten-2-one (1.421%). The abundance of citral components is in line with other previously reported results [23]. Comparing the current results with the published data, similarities to those of Indian essential oils were found, having similar contents of citral and geraniol, but a higher myrcene content. Boukhatem et al. (2013) also shown similar results when isolating essential oils from lemongrass leaves, showing main constituents of neral (24.30%), geranial (28.93%) and myrcene (23.92%) [24,25].
3.2. Effects of Concentration of Wall Material
Two wall materials were used in the present study: maltodextrin and the mixture of maltodextrin and gum Arabic. The latter was used with a maltodextrin:gum ratio of 4:1 [26]. Due to the high molecular weight, the highly branched nature, and the composition that includes various acidic polysaccharides of gum Arabic, the mixture that is composed of gum Arabic and maltodextrin showed higher observable viscosity than that of the maltodextrin solution. Conversely, maltodextrin with a concentration of up to 70% (w/w) is water-soluble and has a good membrane-forming capability due to its medium molecular weight, suggesting its use in encapsulation for prolonging storage time of food [27,28]. Table 3 shows the visual characteristics and moisture contents of the microcapsulates in powder form obtained using different wall materials at varying concentrations. In comparison with the use of sole maltodextrin, the combination of maltodextrin and gum Arabic seemed to result in the product with a lower moisture content. This is possibly due to the higher viscosity of the combination mixture in comparison with that of the maltodextrin solution. These results necessitate the elevation of inlet/outlet air temperatures when using the combination mixture to ensure the low moisture content of the obtained product.

Table 3.
Moisture content and texture of microencapsulation powder produced at different concentrations of the wall material.
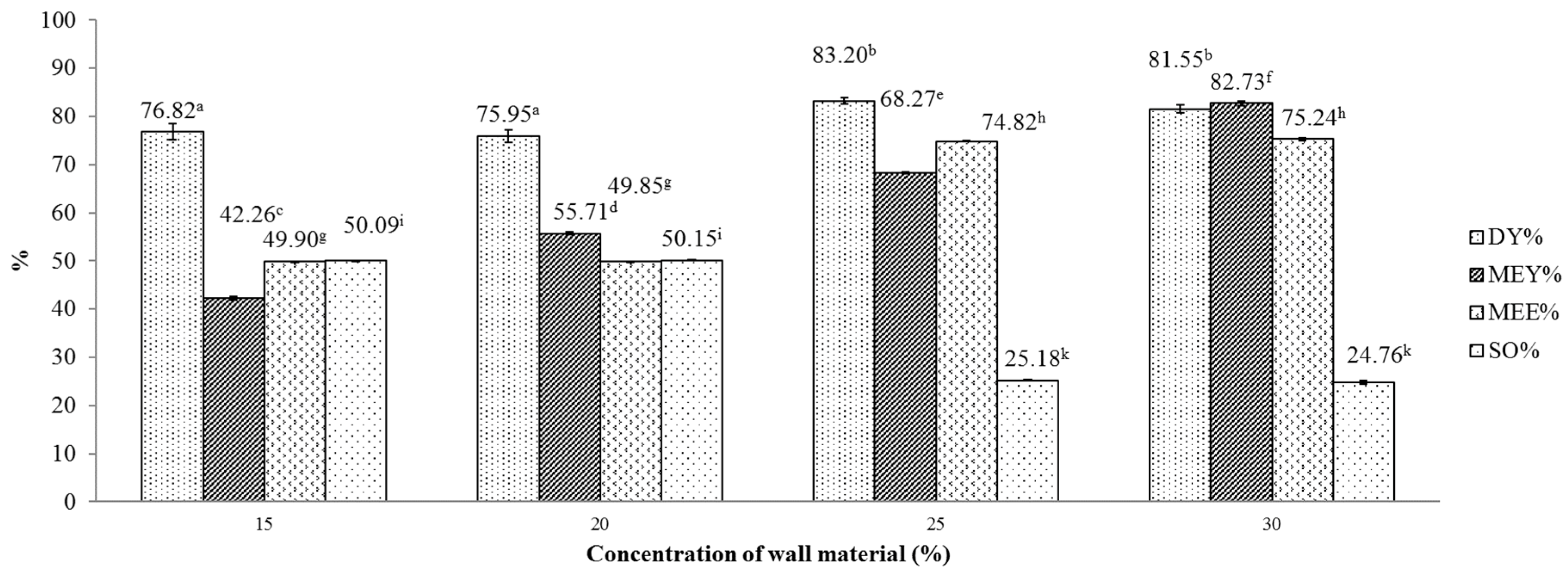
We then examined these combinations of wall materials with respect to encapsulation outcome, as shown in Figure 2 and Figure 3. Figure 2 illustrates the effects of the concentration of the maltodextrin wall matrix on several indicators of obtained microencapsulates. One-way ANOVA revealed that these effects on MEY, MEE and SO were statistically significant (p < 0.05). Generally, as the concentration rises from 15% to 30%, both MEY and MEE indices were improved. This is possibly due to the influence of surface-active carbohydrates of wall materials that include maltodextrin and gum Arabic. Since surface-active carbohydrates could bind with volatile constituents existing in essential oils [27], a higher concentration of wall materials improves the retention of volatiles in the obtained product. For the DY indicator, this indicator peaked at a 25% concentration, achieving a yield of approximately 83.2%. Further testing with LSD multiple range test for DY and MEE revealed that the differences between the outcomes that were obtained at 15% and at 20% concentration were marginal and insignificant. However, in comparison with DY and MEE obtained at 25% and 30% concentrations, DY and MEE obtained at lower concentrations (15% and 20%) were clearly lower. For MEY, the multiple range test indicates clear differences between the yields obtained at the four specified concentrations. The highest MEY (80.74 ± 0.003) was achieved at the 30% concentration maltodextrin.
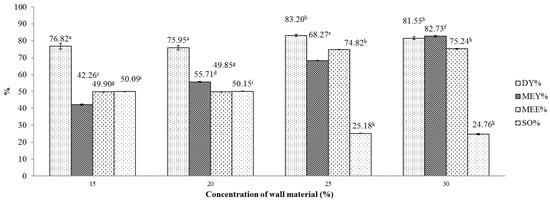
Figure 2.
Effect of maltodextrin concentration on various indicators of the microencapsulation process. Figures with same letters indicate statistical indifference.
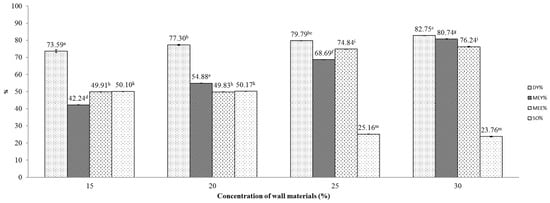
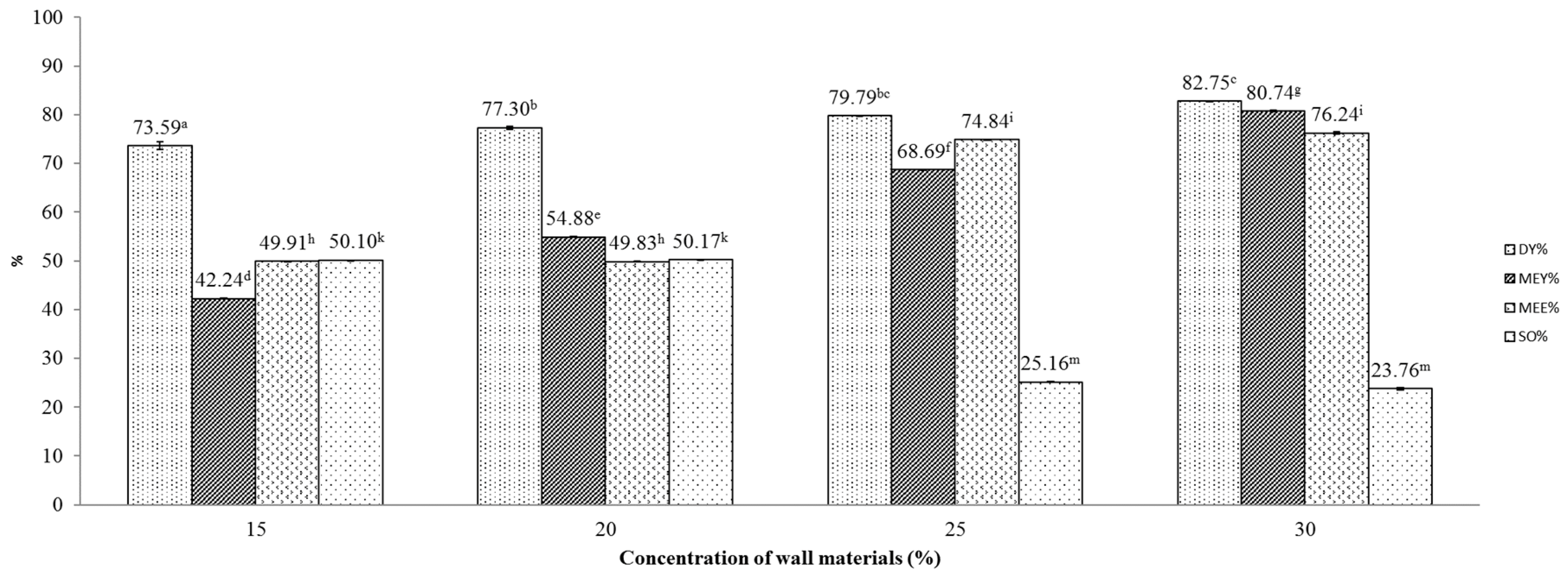
Figure 3.
Effect of maltodextrin:gum Arabic mixture (4:1) concentration on various indicators of the microencapsulation process. Figures with same letters indicate statistical indifference.
The effect of maltodextrin: gum Arabic mixture (4:1) concentration on DY, MEY, MEE and SO is displayed as in Figure 3. At first glance, these results are similar to those in the former investigation, except for results of DY, where a positive relationship between concentration and DY was observed. In this investigation, the concentration of the wall material mixture of 30% seemed to induce the highest DY, MEE and MEY, attaining 82.75 ± 2.06, 80.74 ± 0.003 and 76.24 ± 0.18, respectively.
Comparing the DY in the two investigations, we found that the use of maltodextrin gave better yields than the use of combined wall materials. Although the average DY reached 82.75% in the experiment that utilized combined wall materials at a concentration of 30%, that figure was highly variable, as reflected by the large standard deviation. On the other hand, the use of sole maltodextrin produced consistently high DY regardless of maltodextrin concentration, ranging from 76.82 to 83.20%. This could be explained by the higher required air temperature for encapsulation with the maltodextrin:gum Arabic walling mixture. To be specific, the high inlet air temperature (200 °C) may cause a caramelization reaction to occur, producing furans, furanones, pyrones and carbocyclics and reducing drying yields, as suggested by a previous study [29].
Considering the lower cost of maltodextrin in comparison with that of gum Arabic and MEY and MEE results, sole maltodextrin at the concentration of 30% would be used in subsequent experiments.
3.3. Effect of Concentration of the Core Material
Different concentrations of lemongrass essential oil were investigated with respect to moisture content first. The results are presented in Table 4. Visually, higher concentrations seem to lead to a darker color and less agglomeration of obtained particles. In addition, the moisture content of the product also positively correlates with essential oil concentration. To elaborate, as the concentration of the essential oils increases, the viscosity of the solution would be aggravated, impeding the droplet atomization process. As a result, the required drying time will be longer and bonding and covering of the wall on core materials will occur more slowly, leading to a higher moisture content of the obtained powder.

Table 4.
Moisture content and texture of microencapsulation powder produced at different core materials concentrations.
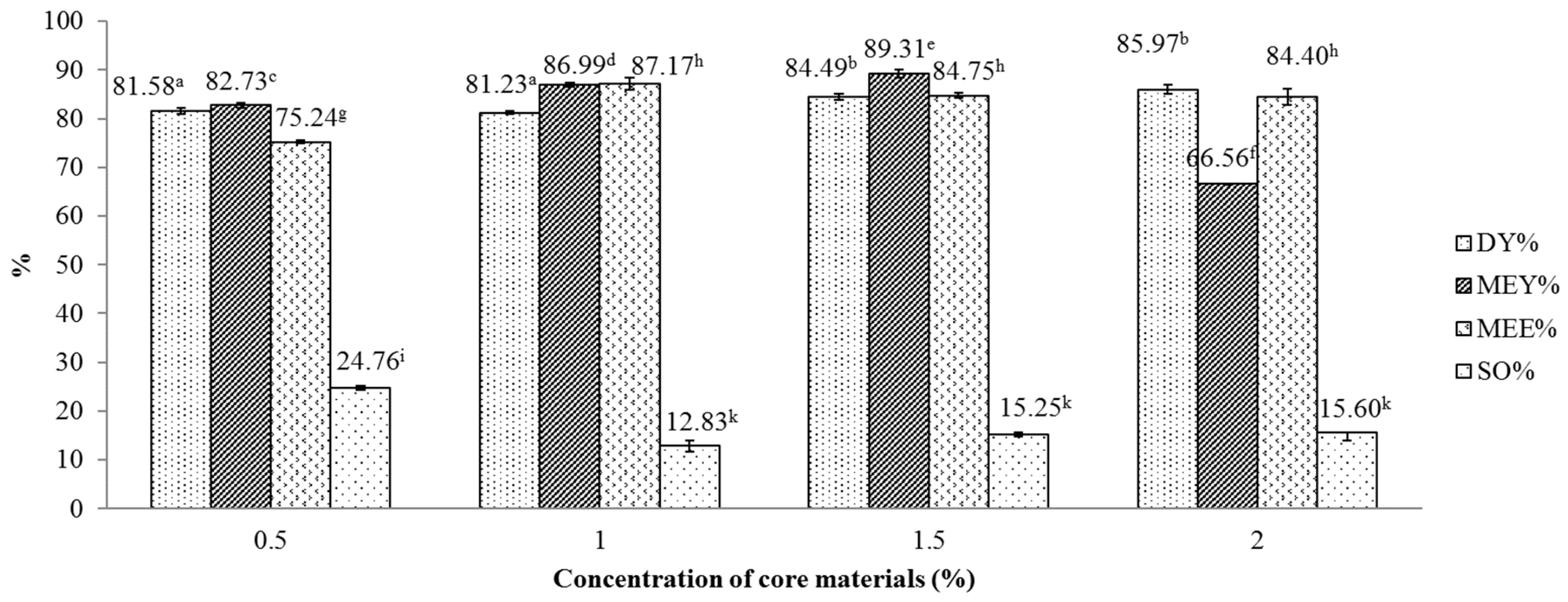
Figure 4 displays the influence of the essential oil concentration on several indicator of the spray drying process, including DY, MEY, MEE and SO. One-way ANOVA revealed that concentration of core material exerted significant effects (p < 0.05) on the MEY, MEE and SO index of the encapsulated powder. Overall, the MEE and DY index showed an increasing trend with the concentration of essential oils which ranged from 0.5% to 2%. However, there was no difference observed between MEE values attained at 1%, 1.5% and 2% concentration and between DY values attained at 1.5% and 2% concentration. For MEY, the indicator peaked at the oil concentration of 1.5%, reaching 89.31% and decreased thereafter to 66.56% when rising the concentration to 2%. This trend is in line with the study of Hogan et al. where a sharp decline in MEE from 89.2% to 18.8% was observed when increasing the soy oil/sodium caseinate ratio from 0.25 to 3.0 [30]. The current peak MEY is higher than a previously reported MEY (81.2%) [15], which was obtained using a combination of gum Arabic and modified starch as wall materials. The current results suggest that the core material concentration of 1.5% gave the optimal DY, MEY and MEE. The reduction in MEE in the current study is explained by the removal of hydroxyl groups (-OH) from hydrophobic compounds, such as citral, from the linkage network of maltodextrin under an excessive high concentration of essential oils [31].
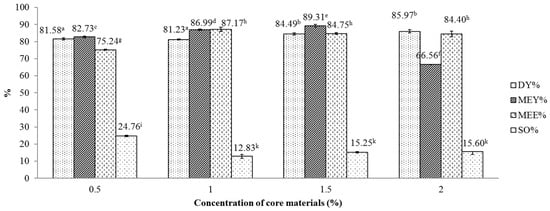
Figure 4.
Effect of lemongrass essential oil concentration on various indicators of the microencapsulation process. Figures with same letters indicate statistical indifference.
3.4. Chemical Composition of Encapsulated Essential Oils
Table 5 compares constituents in the lemongrass oil and their contents before and after microencapsulation. Noticeable compositional changes that were introduced during spray-drying include moderate reduction in myrcene content (from 34.983% to 30.492%) and the improvement in citral content (from 46.603% to 49.076%). The myrcene reduction could be attributable to the volatility of monoterpenes. To be specific, due to their low molecular weight, monoterpenes tend to exit droplets at elevated temperatures, leading to a lower myrcene concentration. The lower myrcene content could also in part explain the rise in citral, geraniol and citronella concentration through rearrangement processes [32]. The appearance of piperitone and limonene, which had been not previously detected in bare essential oils, is due to the inability of the technique to detect constituents at very low concentrations. After spray-drying, the reduction of myrcene contributed to the increased concentration of these compounds. Therefore, encapsulation of lemongrass essential oil via spray-drying might maintain the content of major components without significantly compromising oil quality.

Table 5.
Comparison of lemongrass essential oil compositions before and after encapsulation.
3.5. Powder Morphology
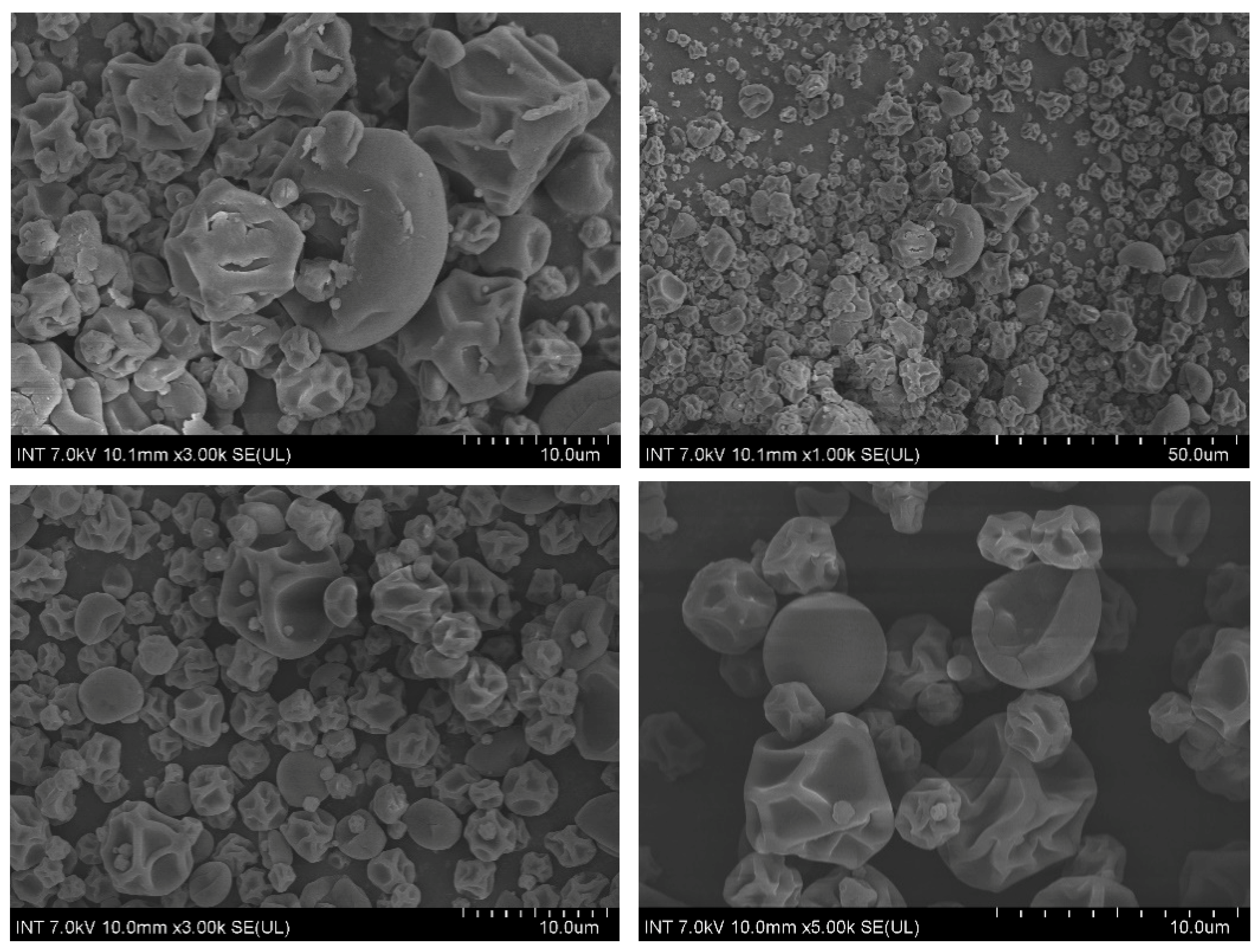
Figure 5 illustrates the SEM microphotograph of microcapsulates fabricated at 140 °C with an oil concentration of 1.5% (w/w) and a maltodextrin concentration of 30% (w/w). The particles exhibited a relatively uniform morphology and no apparent fissures or cracks were found, suggesting good core retention and protection of capsulates. Regarding particle shape, under current drying temperature, most of the obtained particles were predominantly spherical with external surface being shriveled and concave, which is indicative of low drying temperatures and better protection of core material [33,34]. However, there exist some particles with a smooth and hard crust. This is possibly due to faster evaporation.
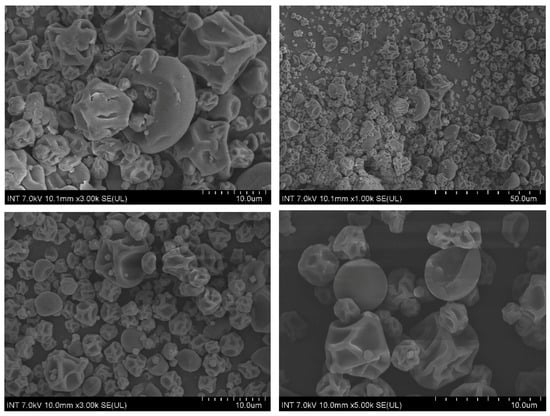
Figure 5.
Scanning electron micrographs of the particles containing lemongrass essential oil using maltodextrin as wall material.
The current results share some similarities with the results of Alamilla-Beltrán et al. where the morphology of maltodextrin-coated microcapsules was investigated with respect to different temperatures [34]. To be specific, our obtained particles correspond with particles dried under temperature of 110 °C, which shrank and exhibited a shriveled surface. Elevating the temperature to 170 °C seemed to produce a greater number of smooth but fractured particles and is unfavorable for volatile retention. In addition, a low drying temperature may lower efficiency due to the high moisture. Therefore, an inlet temperature of 140 °C was selected as the optimal condition for the encapsulation of lemongrass essential oil using maltodextrin.
4. Conclusions
The current study successfully attempted the encapsulation of lemongrass essential oils via spray-drying using maltodextrin and its combination with gum Arabic as wall materials. Phytochemical screening of essential oils revealed the presence of citral, myrcene, geraniol, farnesol, citronella and 6-methyl-5-hepten-2-oneas major compounds in lemongrass essential oil. After spray-drying, the volatile composition of encapsulated essential oil was generally well maintained with an increased concentration of citral content. Moisture content, drying yield, microencapsulation yield and efficiency of the spray-dried microcapsulate powder were determined with respect to different concentrations and compositions of wall and core materials. Optimal spray-drying parameters included maltodextrin as wall material with a concentration of 30% (w/w) and an essential oil concentration of 1.5% (w/w). These parameters corresponded with optimal microencapsulation efficiency, oil retention and a drying yield of 84.75%, 89.31% and 84.49%, respectively. Obtained microcapsulates were spherical with shriveled and concave surface. The presented results suggest the use of maltodextrin as a potential replacement for gum Arabic in microencapsulation of essential oils via spray-drying.
Author Contributions
Investigation, N.P.T.N., V.T.T., T.D.L., N.C.H. and T.T.T.; Supervision, M.H.C., L.T.H.N., Q.T.T. and L.G.B.; Writing—original draft, N.P.T.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Tien Giang Department of Science and Technology, Tien Giang province, Vietnam under the grant number 103/HĐ-QPTKH&CN.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Del Nobile, M.A.; Lucera, A.; Costa, C.; Conte, A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 287. [Google Scholar]
- Rungqu, P. Isolation, Characterisation, and Biological Activity Evaluation of Essential Oils of Cymbopogon validus (Stapf) Stapf ex Burtt Davy and Hyparrhenia hirta (L.) Stapf. Ph.D. Thesis, University of Fort Hare, Alice, South Africa, 2015. [Google Scholar]
- Chaisawadi, S.; Thongbute, D.; Methawiriyasilp, W.; Pitakworarat, N.; Chaisawadi, A.; Jaturonrasamee, K.; Khemkhaw, J.; Tanuthumchareon, W. Preliminary Study Of Antimicrobial Activities On Medicinal Herbs of Thai Food Ingredients. Acta Hortic. 2005, 675, 111–114. [Google Scholar] [CrossRef]
- Nguyen, T.V.L.; Nguyen, M.D.; Nguyen, D.C.; Bach, L.G.; Lam, T.D. Model for Thin Layer Drying of Lemongrass (Cymbopogon citratus) by Hot Air. Processes 2019, 7, 21. [Google Scholar] [CrossRef]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Ranade, V.V.; Cannon, J.B. Drug Delivery Systems; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9780429130694. [Google Scholar]
- Rawat, S.; Jain, S.K. Solubility enhancement of celecoxib using β-cyclodextrin inclusion complexes. Eur. J. Pharm. Biopharm. 2004, 57, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Raffin, R.P.; Colomé, L.M.; Schapoval, E.E.S.; Pohlmann, A.R.; Guterres, S.S. Increasing sodium pantoprazole photostability by microencapsulation: Effect of the polymer and the preparation technique. Eur. J. Pharm. Biopharm. 2008, 69, 1014–1018. [Google Scholar] [CrossRef]
- Bhandari, B.R.; D’Arc, B.R.; Thi Bich, L.L. Lemon Oil to β-Cyclodextrin Ratio Effect on the Inclusion Efficiency of β-Cyclodextrin and the Retention of Oil Volatiles in the Complex. J. Agric. Food Chem. 1998, 46, 1494–1499. [Google Scholar] [CrossRef]
- Oyedele, A.O.; Gbolade, A.A.; Sosan, M.B.; Adewoyin, F.B.; Soyelu, O.L.; Orafidiya, O.O. Formulation of an effective mosquito-repellent topical product from Lemongrass oil. Phytomedicine 2002, 9, 259–262. [Google Scholar] [CrossRef]
- Holvoet, C.; Vander Heyden, Y.; Plaizier-Vercammen, J. Influence of preparation method on itraconazole oral solutions using cyclodextrins as complexing agents. Pharmazie 2007, 62, 510–514. [Google Scholar]
- Loftsson, T.; Sigurđsson, H.H.; Másson, M.; Schipper, N. Preparation of solid drug/cyclodextrin complexes of acidic and basic drugs . Available online: https://www.ingentaconnect.com/content/govi/pharmaz/2004/00000059/00000001/art00005 (accessed on 12 November 2019).
- Leimann, F.V.; Gonçalves, O.H.; Machado, R.A.F.; Bolzan, A. Antimicrobial activity of microencapsulated lemongrass essential oil and the effect of experimental parameters on microcapsules size and morphology. Mater. Sci. Eng. C 2009, 29, 430–436. [Google Scholar] [CrossRef]
- Carvalho, G.R.; de Barros Fernandes, R.V.; e Silva, P.D.C.; de Abreu Dessimoni, A.L.; Oliveira, C.R.; Borges, S.V.; Botrel, D.A. Influence of modified starches as wall materials on the properties of spray-dried lemongrass oil. J. Food Sci. Technol. 2019, 56, 4972–4981. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.W.M.; Laurentino, L.D.S.; Alves, C.R.; Bastos, M.D.S.R.; Costa, J.M.C.D.; Canuto, K.M.; Furtado, R.F. Chemical modification of gum arabic and its application in the encapsulation of Cymbopogon citratus essential oil. J. Appl. Polym. Sci. 2015, 132, 41519. [Google Scholar] [CrossRef]
- Phunpee, S.; Ruktanonchai, U.R.; Yoshii, H.; Assabumrungrat, S.; Soottitantawat, A. Encapsulation of lemongrass oil with cyclodextrins by spray drying and its controlled release characteristics. Biosci. Biotechnol. Biochem. 2017, 81, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Weisheimer, V.; Miron, D.; Silva, C.B.; Guterres, S.S.; Schapoval, E.E.S. Microparticles containing lemongrass volatile oil: Preparation, characterization and thermal stability. Pharmazie 2010, 65, 885–890. [Google Scholar] [PubMed]
- Jafari, S.M.; Ghalegi Ghalenoei, M.; Dehnad, D. Influence of spray drying on water solubility index, apparent density, and anthocyanin content of pomegranate juice powder. Powder Technol. 2017, 311, 59–65. [Google Scholar] [CrossRef]
- Fernandes, R.V.D.B.; Borges, S.V.; Botrel, D.A.; Silva, E.K.; Costa, J.M.G.D.; Queiroz, F. Microencapsulation of Rosemary Essential Oil: Characterization of Particles. Dry. Technol. 2013, 31, 1245–1254. [Google Scholar] [CrossRef]
- Bae, E.K.; Lee, S.J. Microencapsulation of avocado oil by spray drying using whey protein and maltodextrin. J. Microencapsul. 2008, 25, 549–560. [Google Scholar] [CrossRef]
- Alves, S.F.; Borges, L.L.; dos Santos, T.O.; de Paula, J.R.; Conceição, E.C.; Bara, M.T.F. Microencapsulation of Essential Oil from Fruits of Pterodon emarginatus Using Gum Arabic and Maltodextrin as Wall Materials: Composition and Stability. Dry. Technol. 2014, 32, 96–105. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Liquid and vapour-phase antifungal activities of selected essential oils against candida albicans: Microscopic observations and chemical characterization of Cymbopogon citratus. BMC Complementary Altern. Med. 2010, 10, 65. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Kameli, A.; Ferhat, M.A.; Saidi, F.; Tayebi, K. The food preservative potential of essential oils: Is lemongrass the answer? J. Verbr. Lebensm. 2014, 9, 13–21. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Ferhat, M.A.; Kameli, A.; Saidi, F.; Kebir, H.T. Lemon grass (Cymbopogon citratus) essential oil as a potent anti-inflammatory and antifungal drugs. Libyan J. Med. 2014, 9, 25431. [Google Scholar] [CrossRef] [PubMed]
- Simon-Brown, K.; Solval, K.M.; Chotiko, A.; Alfaro, L.; Reyes, V.; Liu, C.; Dzandu, B.; Kyereh, E.; Goldson Barnaby, A.; Thompson, I.; et al. Microencapsulation of ginger (Zingiber officinale) extract by spray drying technology. LWT 2016, 70, 119–125. [Google Scholar] [CrossRef]
- Bylaitë, E.; Rimantas Venskutonis, P.; Maþdþierienë, R. Properties of caraway (Carum carvi L.) essential oil encapsulated into milk protein-based matrices. Eur. Food Res. Technol. 2001, 212, 661–670. [Google Scholar]
- Huber, K.C.; Embuscado, M.E. (Eds.) Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; ISBN 9780387928234. [Google Scholar]
- Nunes, I.L.; Mercadante, A.Z. Encapsulation of lycopene using spray-drying and molecular inclusion processes. Braz. Arch. Biol. Technol. 2007, 50, 893–900. [Google Scholar] [CrossRef]
- Hogan, S.A.; McNamee, B.F.; O’Riordan, E.D.; O’Sullivan, M. Emulsification and microencapsulation properties of sodium caseinate/carbohydrate blends. Int. Dairy J. 2001, 11, 137–144. [Google Scholar] [CrossRef]
- Rungsardthong Ruktanonchai, U.; Srinuanchai, W.; Saesoo, S.; Sramala, I.; Puttipipatkhachorn, S.; Soottitantawat, A. Encapsulation of Citral Isomers in Extracted Lemongrass Oil with Cyclodextrins: Molecular Modeling and Physicochemical Characterizations. Biosci. Biotechnol. Biochem. 2011, 75, 2340–2345. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Impact of different storage conditions on the quality of selected essential oils. Food Res. Int. 2012, 46, 341–353. [Google Scholar] [CrossRef]
- Rubiano, K.D.; Cárdenas, J.A.; Ciro, V.; Héctor, J. Encapsulation of d-limonene flavors using spray drying: Effect of the addition of emulsifiers. Ing. y Compet. 2015, 17, 77–89. [Google Scholar]
- Alamilla-Beltrán, L.; Chanona-Pérez, J.J.; Jiménez-Aparicio, A.R.; Gutiérrez-López, G.F. Description of morphological changes of particles along spray drying. J. Food Eng. 2005, 67, 179–184. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).