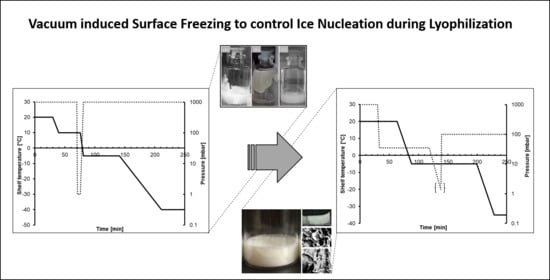
Controlling Ice Nucleation during Lyophilization: Process Optimization of Vacuum-Induced Surface Freezing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Analytical Characterization
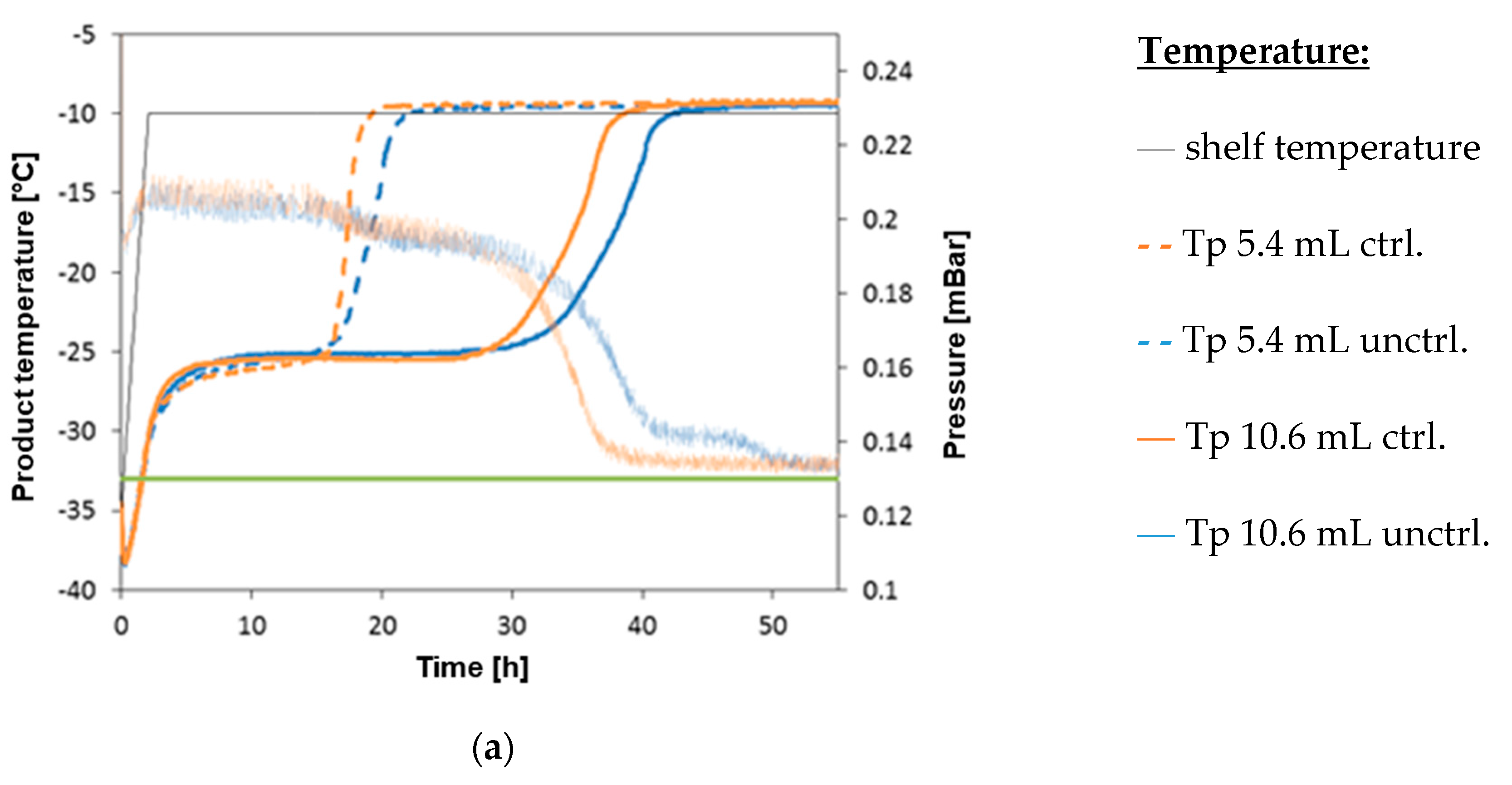
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Analytical Panel
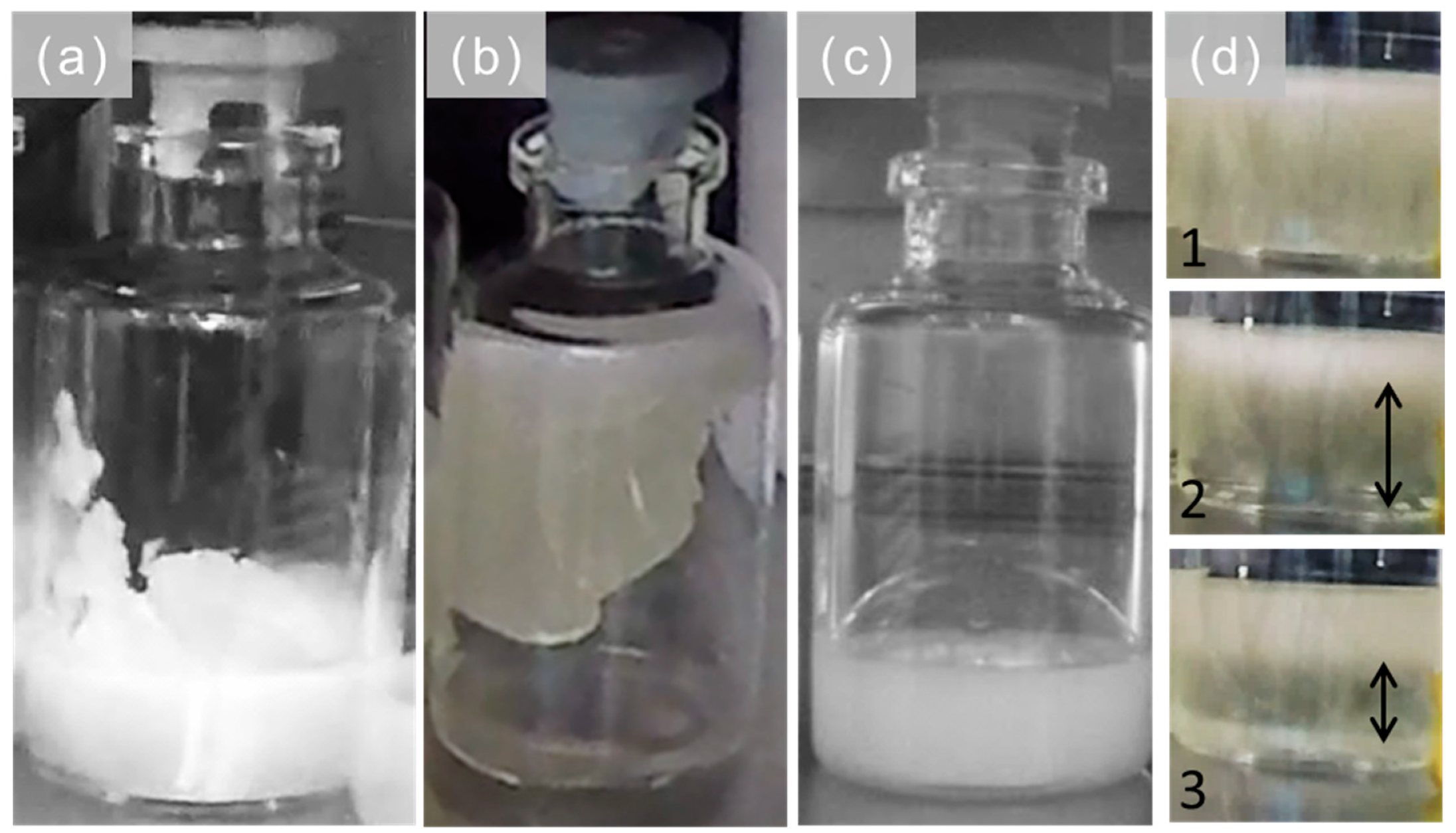
Appendix A.1. Visual Inspection
Appendix A.2. Polydimethylsiloxane (PDMS) Embedding
Appendix A.3. Scanning Electron Microscopy (SEM)
Appendix A.4. Karl Fischer Titration
Appendix A.5. Specific Surface Area
Appendix A.6. Reconstitution Time
Appendix B


References
- Manning, M.C.; Chou, D.K.; Murphy, B.M.; Payne, R.W.; Katayama, D.S. Stability of protein pharmaceuticals: An update. Pharm. Res. 2010, 27, 544–575. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, B.; Tchessalov, S. Advances in Freeze Drying of Biologics and Future Challenges and Opportunities. In Drying Technologies for Biotechnology and Pharmaceutical Applications, 1st ed.; Satoshi Ohtake, S., Izutsu, K.I., Lechuga-Ballesteros, D., Eds.; Wiley-VCH: Weinheim, Germany, 2020; pp. 137–177. [Google Scholar]
- Kasper, J.C.; Winter, G.; Friess, W. Recent advances and further challenges in lyophilization. Eur. J. Pharm. Biopharm. 2013, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.C.; Friess, W. The freezing step in lyophilization: Physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur. J. Pharm. Biopharm. 2011, 78, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Viverette, T.; Virgin, M.; Anderson, M.; Paresh, D. A study of the impact of freezing on the lyophilization of a concentrated formulation with a high fill depth. Pharm. Dev. Technol. 2005, 10, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Searles, J.A.; Carpenter, J.F.; Randolph, T.W. The ice nucleation temperature determines the primary drying rate of lyophilization for samples frozen on a temperature-controlled shelf. J. Pharm. Sci. 2001, 90, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Geidobler, R.; Winter, G. Controlled ice nucleation in the field of freeze-drying: Fundamentals and technology review. Eur. J. Pharm. Biopharm. 2013, 85, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, A.K.; Kuu, W.; Otten, L.; Nail, S.L.; Sever, R.R. Controlled nucleation in freeze-drying: Effects on pore size in the dried product layer, mass transfer resistance, and primary drying rate. J. Pharm. Sci. 2011, 100, 3453–3470. [Google Scholar] [CrossRef] [PubMed]
- Rambhatla, S.; Ramot, R.; Bhugra, C.; Pikal, M.J. Heat and mass transfer scale-up issues during freeze drying: II. Control and characterization of the degree of supercooling. AAPS PharmSciTech 2004, 5, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awotwe-Otoo, D.; Agarabi, C.; Read, E.K.; Lute, S.; Brorson, K.A.; Khan, M.A.; Shah, R.B. Impact of controlled ice nucleation on process performance and quality attributes of a lyophilized monoclonal antibody. Int. J. Pharm. 2013, 450, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Luoma, J.; Magill, G.; Kumar, L.; Yusoff, Z. Controlled Ice Nucleation Using ControLyo® Pressurization-Depressurization Method. In Lyophilization of Pharmaceuticals and Biologicals—New Technologies and Approaches, 1st ed.; Ward, K., Matejtschuk, P., Eds.; Humana Press: New York, NY, USA, 2019; pp. 57–77. [Google Scholar]
- Patel, S.M.; Bhugra, C.; Pikal, M.J. Reduced pressure ice fog technique for controlled ice nucleation during freeze-drying. AAPS PharmSciTech 2009, 10, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Ling, W. Controlled Nucleation during Freezing Step of Freeze Drying Cycle Using Pressure Differential Ice Fog Distribution. U.S. Patent 8839528B2, 29 April 2011. [Google Scholar]
- Geidobler, R.; Mannschedel, S.; Winter, G. A new approach to achieve controlled ice nucleation of supercooled solutions during the freezing step in freeze-drying. J. Pharm. Sci. 2012, 101, 4409–4413. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, B.S.; Sever, R.R.; Hunek, B.; Gasteyer, T.H. Freeze-Dryer and Method of Controlling the Same. U.S. Patent 8240065B2, 14 August 2012. [Google Scholar]
- Kramer, M.; Sennhenn, B.; Lee, G. Freeze-drying using vacuum-induced surface freezing. J. Pharm. Sci. 2002, 91, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Oddone, I.; Van Bockstal, P.J.; De Beer, T.; Pisano, R. Impact of vacuum-induced surface freezing on inter- and intra-vial heterogeneity. Eur. J. Pharm. Biopharm. 2016, 103, 167–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hof, H.G.; Schilder, G. Verfahren zur Gefriertrockung eines mit einem Lösungsmittel versehenen, feuchten Produktes. EP2728287A2, 24 August 2013. [Google Scholar]
- Allmendinger, A.; Schilder, G.; Mietzner, R.; Butt, Y.L.; Luemkemann, J.; Lema Martinez, C.; Controlled Nucleation during Freeze Drying Using Vacuum-Induced Surface Freezing. Invention disclosure rd633018. 2017. Available online: http://www.researchdisclosure.com (accessed on 24 July 2020).
- Lam, P.; Patapoff, T.W. An improved method for visualizing the morphology of lyophilized product cakes. PDA J. Pharm. Sci. Technol. 2011, 65, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Haeuser, C.; Goldbach, P.; Huwyler, J.; Friess, W.; Allmendinger, A. Be Aggressive! Amorphous Excipients Enabling Single-Step Freeze-Drying of Monoclonal Antibody Formulations. Pharmaceutics 2019, 11, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
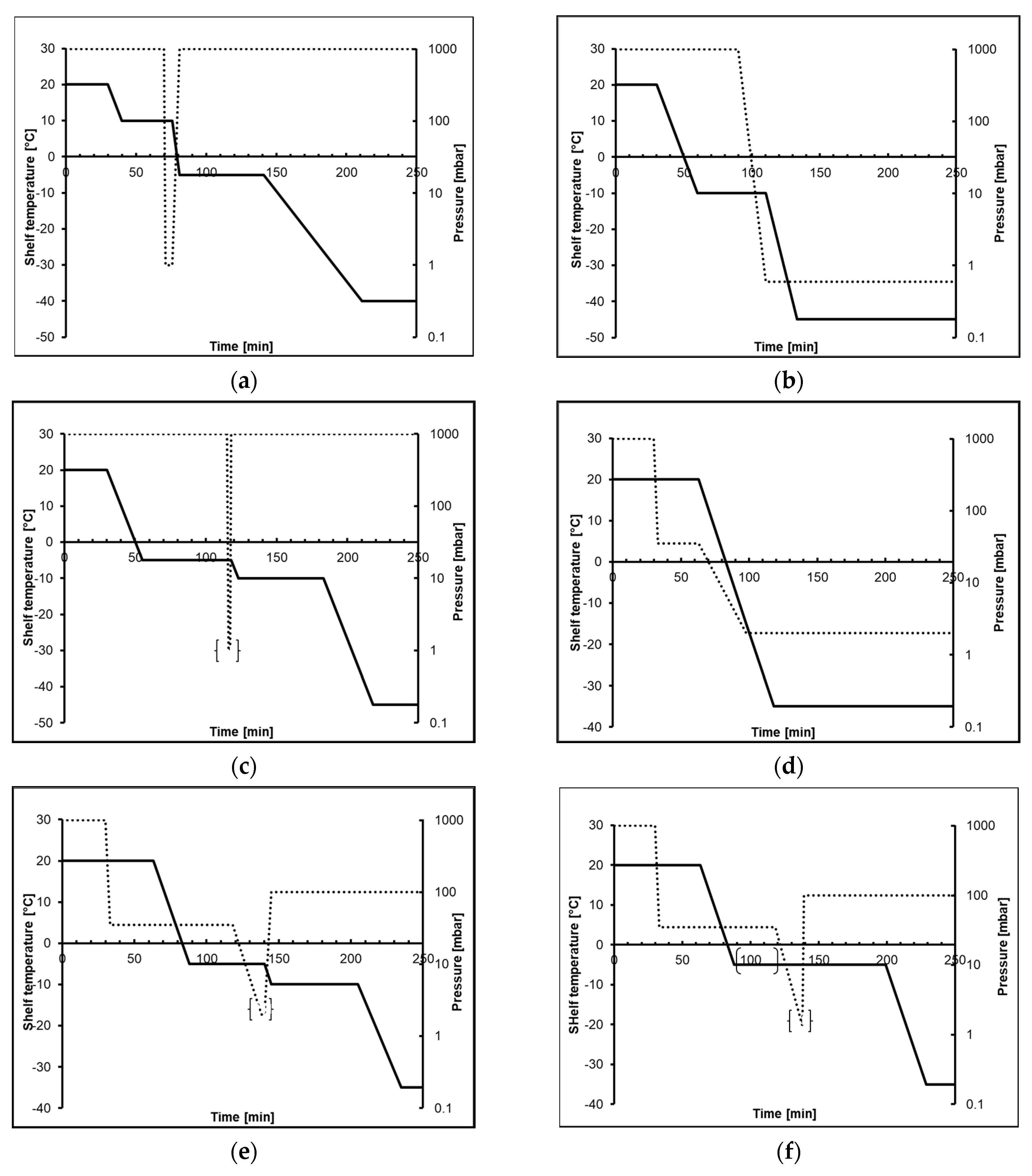
| Uncontrolled Cycle | Controlled Cycle | Purpose | |||||
|---|---|---|---|---|---|---|---|
| Cycle Step | Time [hh:mm] | Temp [°C] | Pressure [mbar] | Time [hh:mm] | Temp [°C] | Pressure [mbar] | |
| Loading | 01:00 | 20 | 1000 | 01:00 | 20 | 1000 | Preparation |
| Freezing | - | Cooling of Condenser | |||||
| 00:03 | 20 | 35 | Pressure Ramp 1 | ||||
| 00:30 | 20 | 35 | Degassing | ||||
| 00:25 | −5 | 35 | Temperature Ramp | ||||
| 00:30–03:00 2 | −5 | 35 | Temperature Equilibration | ||||
| 00:20 | −5 | 1.35 | Induction of Ice Nucleation | ||||
| 00:01 | −5 | 100 | Aeration to Avoid Boiling | ||||
| Close Isolation Valve | |||||||
| 01:00 | −5 | 100 | Holding Step for Ice Crystal Growth | ||||
| 03:03 | −35 | 1000 | 01:40 | −35 | 100 | Freezing Ramp | |
| 03:00 | −35 | 1000 | 03:00 | −35 | 100 | Complete Freezing | |
| Primary Drying | 00:05 | −35 | 0.13 | 00:05 | −35 | 0.13 | Evacuation 3 |
| 02:05 | −10 | 0.13 | 02:05 | −10 | 0.13 | Temperature Ramp | |
| TBD | −10 | 0.13 | TBD | −10 | 0.13 | Primary Drying Hold Time | |
| Secondary Drying | 02:55 | 25 | 0.13 | 02:55 | 25 | 0.13 | Temperature Ramp |
| 08:00 | 25 | 0.13 | 08:00 | 25 | 0.13 | Secondary Drying Hold Time | |
| Stoppering | - | 25 | 700 | - | 25 | 700 | Stoppering |
| Storage | - | 5 | 1000 | - | 5 | 1000 | Storage |
| Uncontrolled Nucleation | Controlled Nucleation | ||||
|---|---|---|---|---|---|
| 5.4 mL | 10.6 mL | 5.4 mL | 10.6 mL | ||
| (a) Cake Appearance |  |  |  |  | |
| (b) Macroscopic Cake Structure | 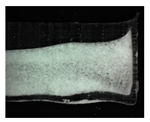 |  |  |  | |
| (c) Pore Structure. | Top |  |  |  |  |
| Bottom | 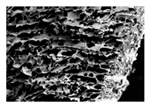 |  |  |  | |
(d) Product Attributes controlled controlled uncontrolled uncontrolled | 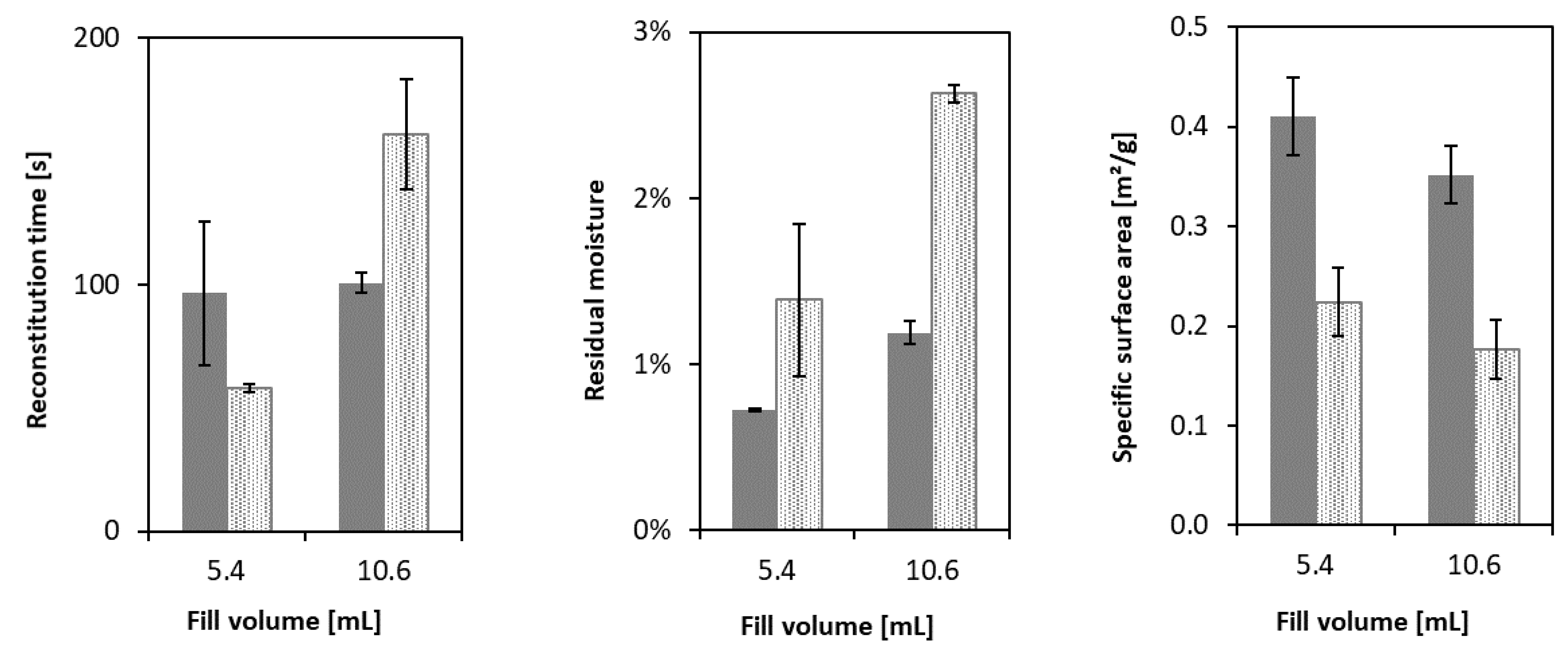 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allmendinger, A.; Butt, Y.L.; Mietzner, R.; Schmidt, F.; Luemkemann, J.; Lema Martinez, C. Controlling Ice Nucleation during Lyophilization: Process Optimization of Vacuum-Induced Surface Freezing. Processes 2020, 8, 1263. https://doi.org/10.3390/pr8101263
Allmendinger A, Butt YL, Mietzner R, Schmidt F, Luemkemann J, Lema Martinez C. Controlling Ice Nucleation during Lyophilization: Process Optimization of Vacuum-Induced Surface Freezing. Processes. 2020; 8(10):1263. https://doi.org/10.3390/pr8101263
Chicago/Turabian StyleAllmendinger, Andrea, Yuen Li Butt, Raphael Mietzner, Felix Schmidt, Joerg Luemkemann, and Carmen Lema Martinez. 2020. "Controlling Ice Nucleation during Lyophilization: Process Optimization of Vacuum-Induced Surface Freezing" Processes 8, no. 10: 1263. https://doi.org/10.3390/pr8101263