The Potential Use of Zeolite, Montmorillonite, and Biochar for the Removal of Radium-226 from Aqueous Solutions and Contaminated Groundwater
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Samples Measurement
2.3. Adsorption Isotherm Analysis
2.4. Adsorption Kinetic Analysis
3. Results and Discussion
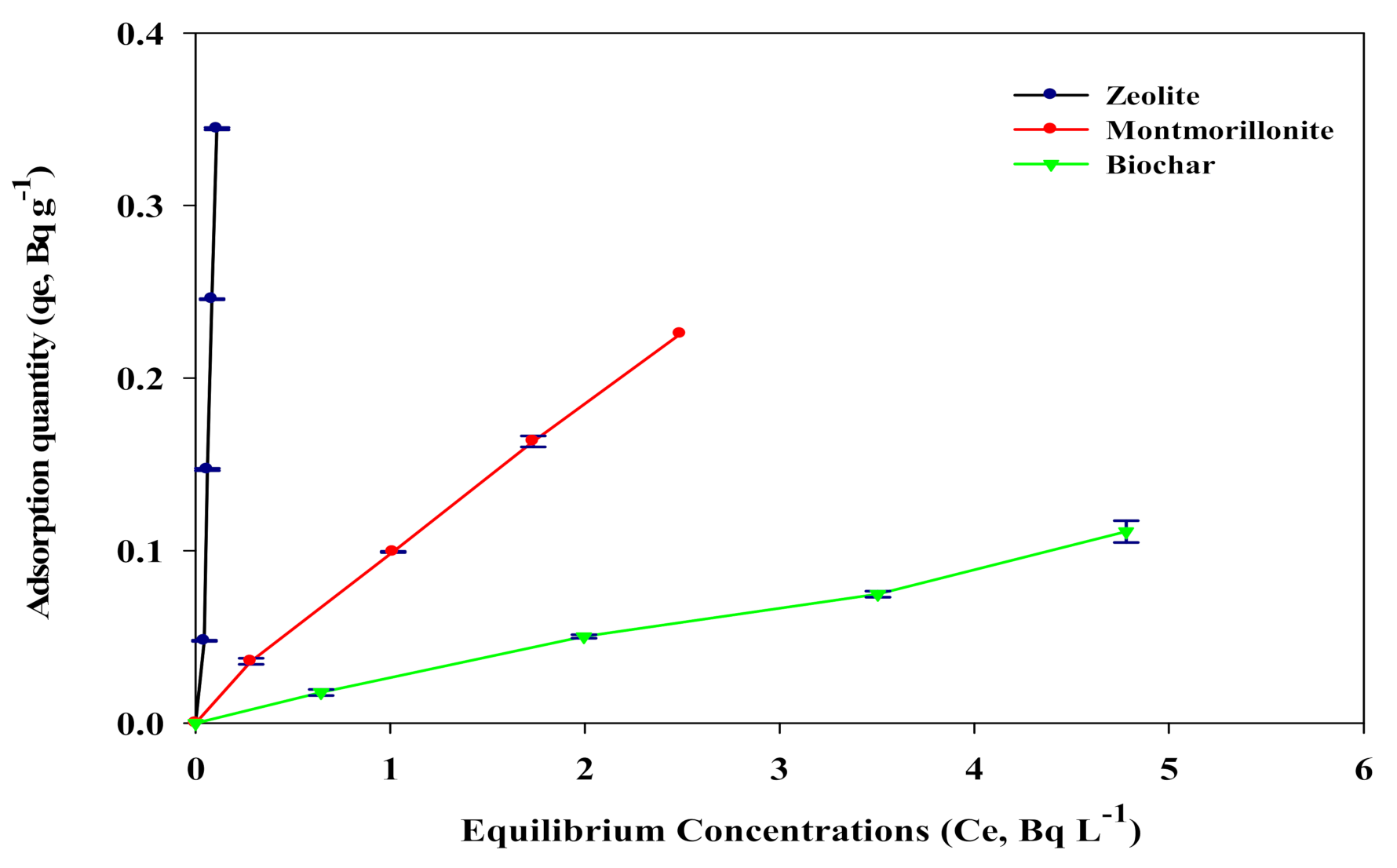
3.1. Adsorption Isotherms of 226Ra onto Sorbents
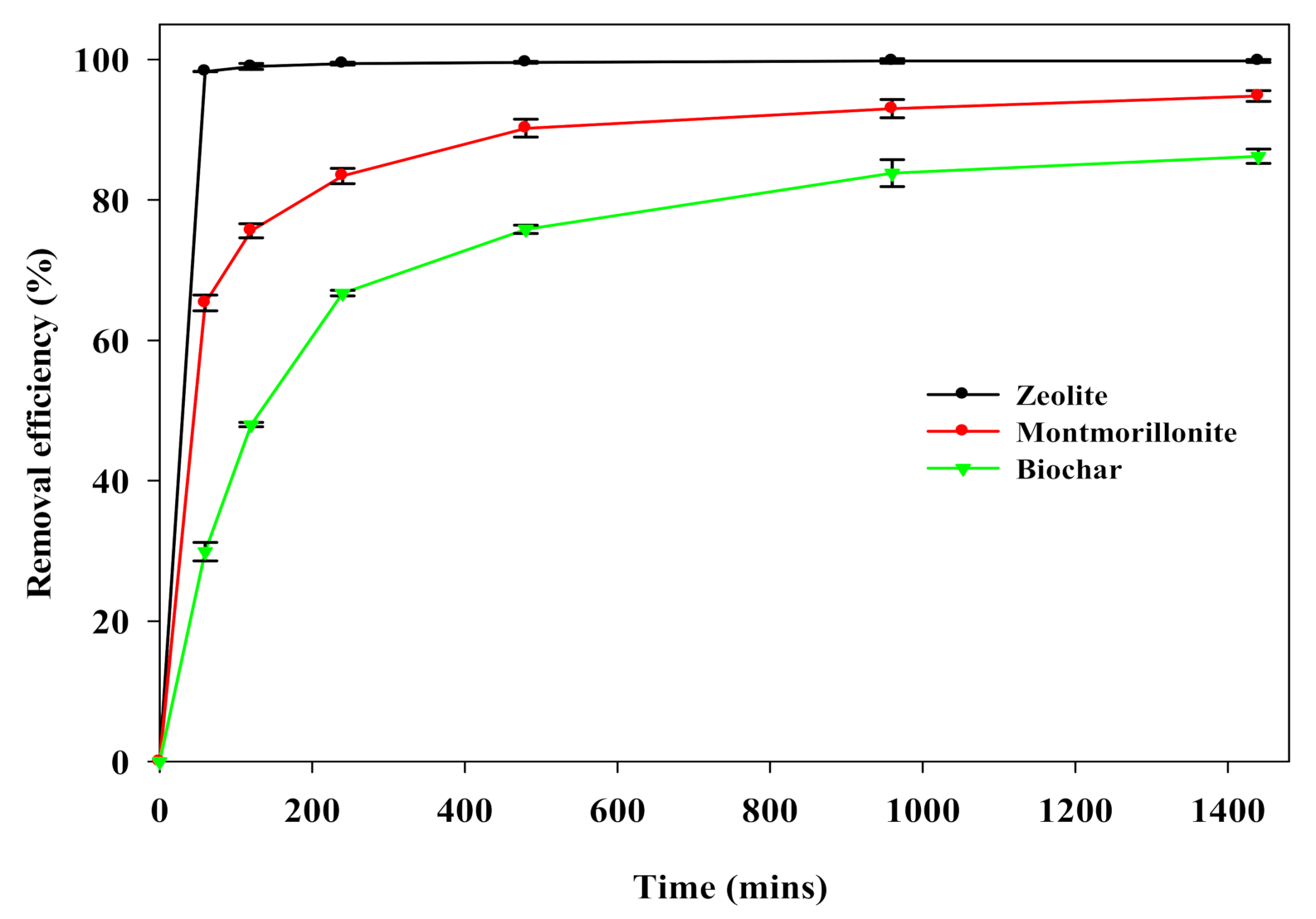
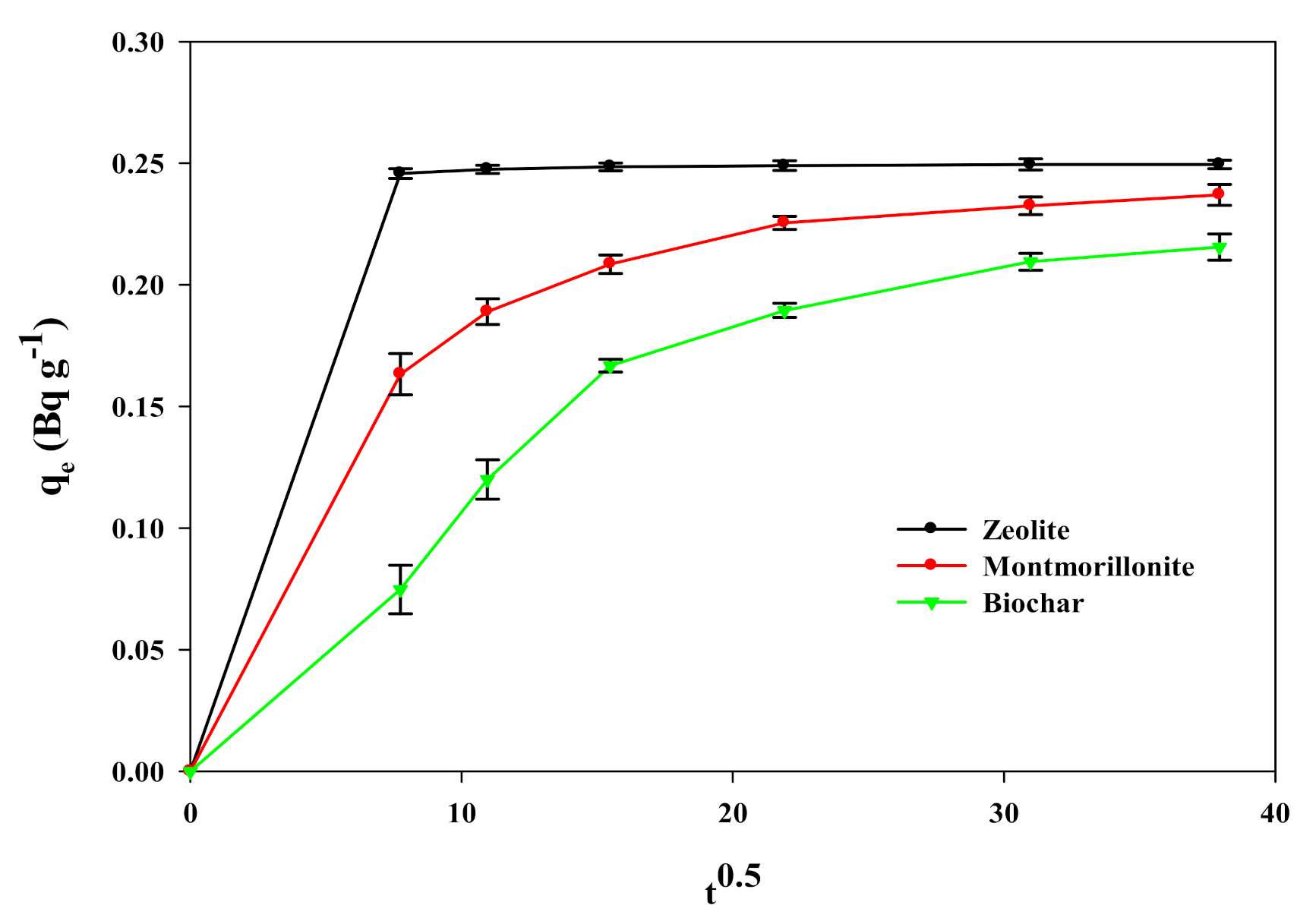
3.2. Adsorption Kinetics of 226Ra onto Sorbents
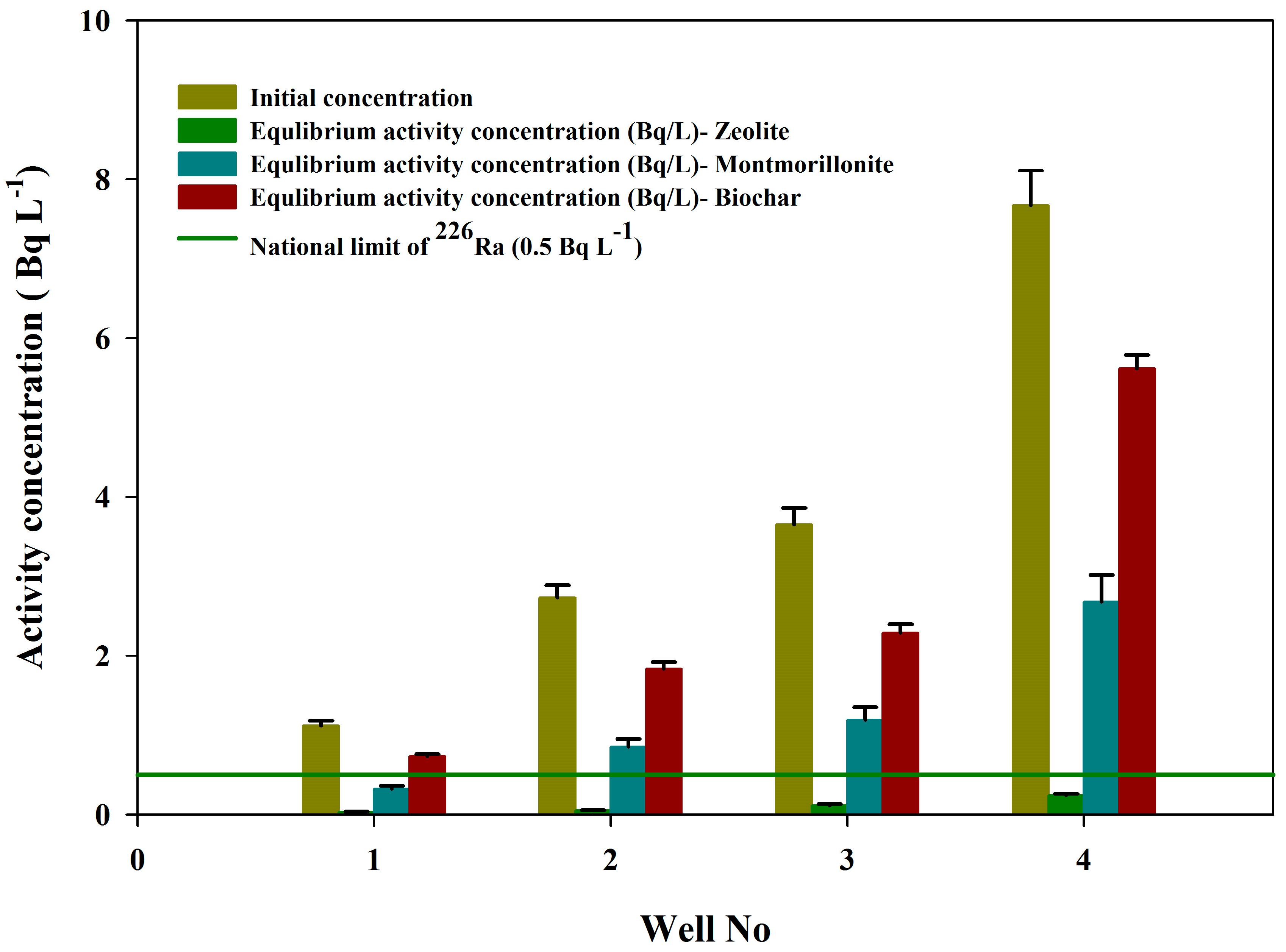
3.3. The Sorbent Potential for Groundwater Remediation
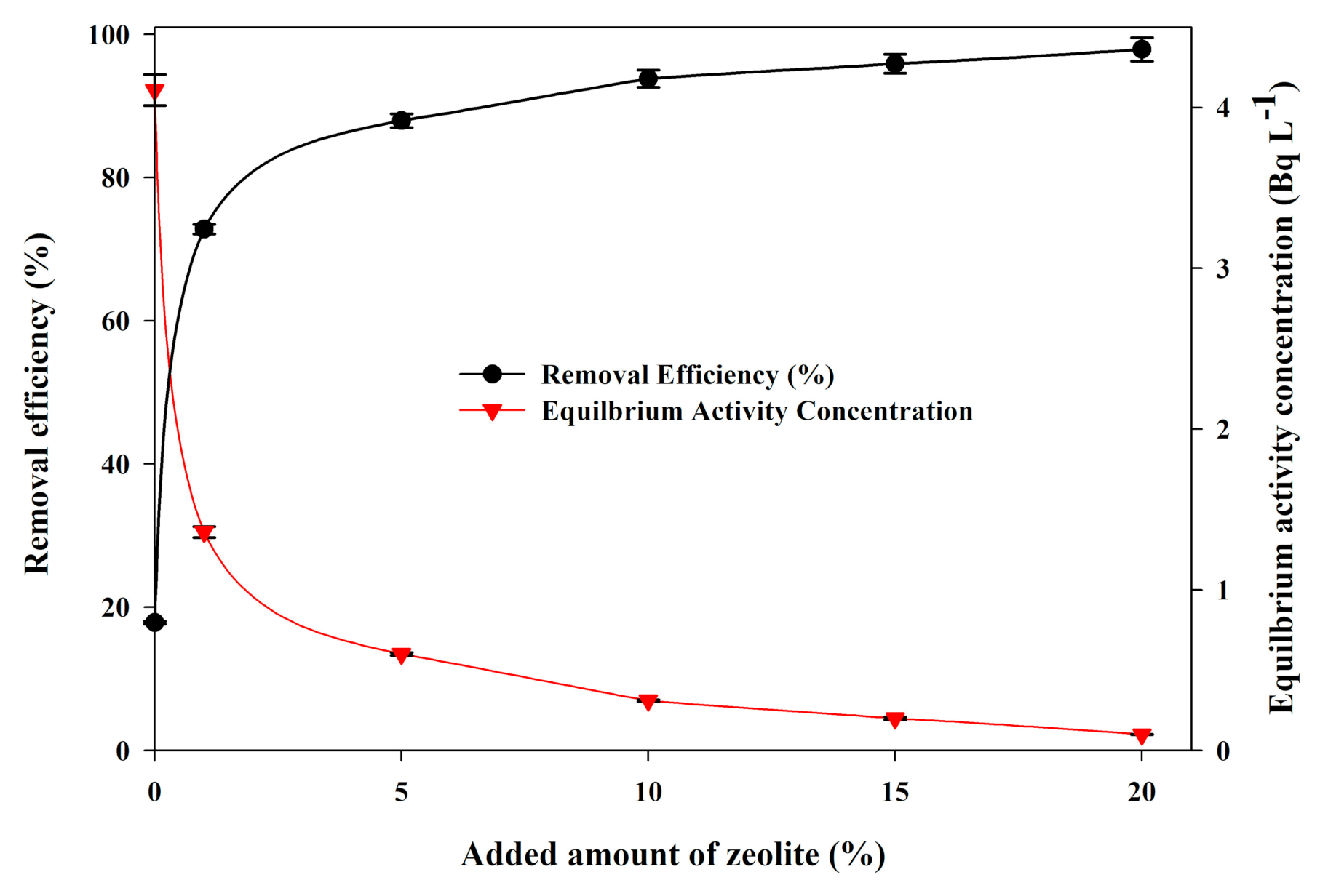
3.4. 226Ra Adsorption by Sandy Soil Amended with Zeolite
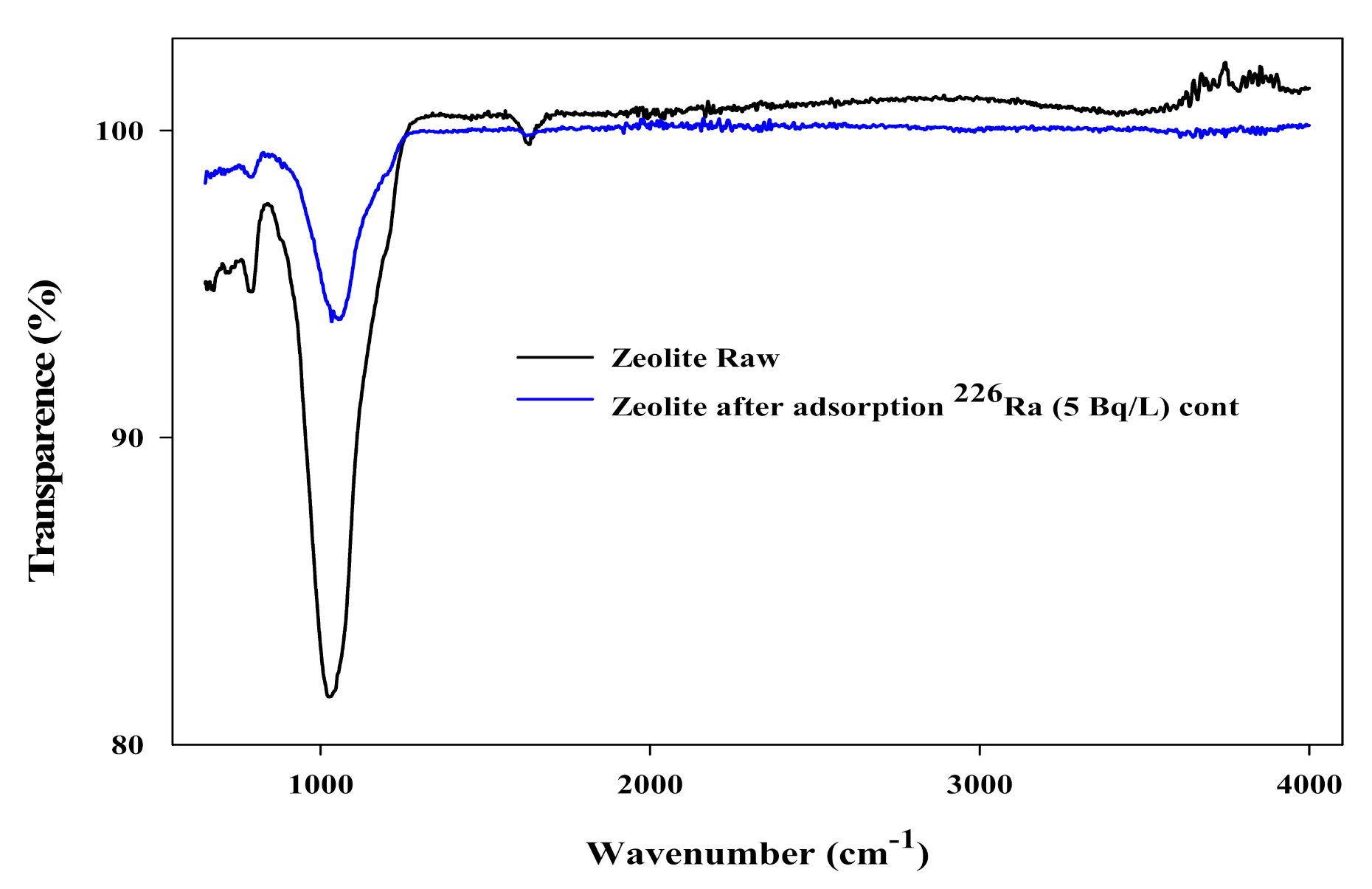
3.5. Mechanism of 226Ra Adsorption onto Natural Zeolite (Clinoptilolite)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Condomines, M.; Rihs, S.; Lloret, E.; Seidel, J. Determination of the four natural Ra isotopes in thermal waters by gamma-ray spectrometry. Appl. Radiat. Isot. 2010, 68, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Moise, T.; Starinsky, A.; Katz, A.; Kolodny, Y. Ra isotopes and Rn in brines and ground waters of the Jordan-Dead Sea Rift Valley: Enrichment, retardation, and mixing. Geochim. Cosmochim. Acta 2000, 64, 2371–2388. [Google Scholar] [CrossRef]
- Al-Masri, M.; Suman, H. NORM waste management in the oil and gas industry: The Syrian experience. J. Radioanal. Nucl. Chem. 2003, 256, 159–162. [Google Scholar] [CrossRef]
- IAEA. Radiation Protection and the Management of Radioactive Waste in the Oil and Gas Industry International Atomic Energy Agency; Safety Reports Series No.34; IAEA: Vienna, Austria, 2003. [Google Scholar]
- Testa, C.; Desideri, D.; Meli, M.; Roselli, C.; Bassignani, A.; Finazzi, P. Determination of uranium, thorium and radium in waters, soils and muds around a uranium mine in decommissioning. Appl. Radiat. Isot. 1994, 45, 394. [Google Scholar] [CrossRef]
- Barišić, D.; Lulić, S.; Miletić, P. Radium and uranium in phosphate fertilizers and their impact on the radioactivity of waters. Water Res. 1992, 26, 607–611. [Google Scholar] [CrossRef]
- UNSCEAR. Sources and Effects of Ionizing Radiation: Sources; United Nations Publications: New York, NY, USA, 2000; Volume 1. [Google Scholar]
- USEPA. Guidelines for Developmental Toxicity Risk Assessment; Risk Assessment Forum, U.S. Environmental Protection Agency: Washington, DC, USA, 1991.
- USEPA. United States Environmental Protection Agency: Drinking Water Regulations and Health Advisories; EPA 822-B-96-002; Office of Water, U.S. EPA: Washington, DC, USA, 1996.
- Alkhomashi, N.; Al-Hamarneh, I.F.; Almasoud, F. Determination of natural radioactivity in irrigation water of drilled wells in northwestern Saudi Arabia. Chemosphere 2016, 144, 1928–1936. [Google Scholar] [CrossRef]
- Almasoud, F.; Ababneh, Z.Q.; Alanazi, Y.J.; Khandaker, M.U.; Sayyed, M. Assessment of radioactivity contents in bedrock groundwater samples from the northern region of Saudi Arabia. Chemosphere 2020, 242, 125181. [Google Scholar] [CrossRef]
- Bonotto, D.M.; Bueno, T.O.; Tessari, B.W.; Silva, A. The natural radioactivity in water by gross alpha and beta measurements. Radiat. Meas. 2009, 44, 92–101. [Google Scholar] [CrossRef]
- Kumar, A.; Karpe, R.; Rout, S.; Gautam, Y.P.; Mishra, M.; Ravi, P.; Tripathi, R. Activity ratios of 234 U/ 238 U and 226 Ra/ 228 Ra for transport mechanisms of elevated uranium in alluvial aquifers of groundwater in south-western (SW) Punjab, India. J. Environ. Radioact. 2016, 151, 311–320. [Google Scholar] [CrossRef]
- Shabana, E.; Kinsara, A. Radioactivity in the groundwater of a high background radiation area. J. Environ. Radioact. 2014, 137, 181–189. [Google Scholar] [CrossRef]
- Turhan, Ş.; Özçıtak, E.; Taşkın, H.; Varinlioğlu, A. Determination of natural radioactivity by gross alpha and beta measurements in ground water samples. Water Res. 2013, 47, 3103–3108. [Google Scholar] [CrossRef] [PubMed]
- AlEissa, K.A.; Alghamdi, A.S.; Almasoud, F.; Islam, S. Measurement of radon levels in groundwater supplies of Riyadh with liquid scintillation counter and the associated radiation dose. Radiat. Prot. Dosim. 2013, 154, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Al-Zubari, W. Sustainable Water Consumption in Arab Countries. Arab Environ. 8-Sustain. Consum. Better Resour. Manag. 2015, 108–113. [Google Scholar]
- Al-Hamarneh, I.F.; Alkhomashi, N.; Almasoud, F. Study on the radioactivity and soil-to-plant transfer factor of 226Ra, 234U and 238U radionuclides in irrigated farms from the northwestern Saudi Arabia. J. Environ. Radioact. 2016, 160, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Viglasova, E.; Krajnak, A.; Galambos, M.; Rosskopfova, O. Removal of Uranium from Water Media by Bentonite and Zeolite; CHÉMIA: Bratislava, Slovak Republic, 2014. [Google Scholar]
- Chalupnik, S.; Franus, W.; Wysocka, M.; Gzyl, G. Application of zeolites for radium removal from mine water. Environ. Sci. Pollut. Res. 2013, 20, 7900–7906. [Google Scholar] [CrossRef] [PubMed]
- Lumiste, L.; Münter, R.; Sutt, J.; Kivimäe, T.; Eensalu, T. Removal of radionuclides from Estonian groundwater using aeration, oxidation, and filtration. Proc. Estonian Acad. Sci. 2012, 61, 58–64. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Erenturk, S.; Kaygun, A.K. Removal of 226Ra from aqueous media and its thermodynamics and kinetics. J. Radioanal. Nucl. Chem. 2017, 311, 1227–1233. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.-Y.; Shao, H.-B.; Shao, M. The remediation of the lead-polluted garden soil by natural zeolite. J. Hazard. Mater. 2009, 169, 1106–1111. [Google Scholar] [CrossRef]
- Osmanlioglu, A.E. Treatment of radioactive liquid waste by sorption on natural zeolite in Turkey. J. Hazard. Mater. 2006, 137, 332–335. [Google Scholar] [CrossRef]
- Ovhal, S.; Butler, I.S.; Xu, S. The Potential of Zeolites to Block the Uptake of Radioactive Strontium-90 in Organisms. Contemp. Chem. 2018, 1, 1–13. [Google Scholar]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamarneh, I.F.; Almasoud, F. A comparative study of different radiometric methodologies for the determination of 226 Ra in water. Nucl. Eng. Technol. 2018, 50, 159–164. [Google Scholar] [CrossRef]
- Cooper, E.; Brown, R.; Milton, G. Determination of 222Rn and 226Ra in environmental waters by liquid scintillation counting. Environ. Int. 1988, 14, 335–340. [Google Scholar] [CrossRef]
- Escobar, V.G.; Tomé, F.V.; Lozano, J.; Sánchez, A.M. Determination of 222Rn and 226Ra in aqueous samples using a low-level liquid scintillation counter. Appl. Radiat. Isot. 1996, 47, 861–867. [Google Scholar] [CrossRef]
- Prichard, H.M.; Gesell, T.F. Rapid Measurements of 222Rn Concentrations in Water with a Commercial Liquid Scintillation Counter. Health Phys. 1977, 33, 577–581. [Google Scholar] [CrossRef]
- Zouridakis, N.; Ochsenkühn, K.; Savidou, A. Determination of uranium and radon in potable water samples. J. Environ. Radioact. 2002, 61, 225–232. [Google Scholar] [CrossRef]
- Giles, C.H.; D’Silva, A.P.; Easton, I.A. A general treatment and classification of the solute adsorption isotherm part. II. Experimental interpretation. J. Colloid Interface Sci. 1974, 47, 766–778. [Google Scholar] [CrossRef]
- Lee, S.-Z.; Allen, H.E.; Huang, C.P.; Sparks, N.L.; Sanders, P.F.; Peijnenburg, W.J.G.M. Predicting Soil−Water Partition Coefficients for Cadmium. Environ. Sci. Technol. 1996, 30, 3418–3424. [Google Scholar] [CrossRef]
- Rafique, M.I.; Usman, A.R.A.; Ahmad, M.; Sallam, A.; Al-Wabel, M.I. In situ immobilization of Cr and its availability to maize plants in tannery waste–contaminated soil: Effects of biochar feedstock and pyrolysis temperature. J. Soils Sediments 2020, 20, 330–339. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Ma, F.; Tankpa, V.; Bai, S.; Guo, X.; Wang, X. Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: A review. Sci. Total. Environ. 2019, 668, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Houhoune, F.; Khemaissia, S.; Nibou, D.; Chegrouche, S.; Menacer, S. Kinetic study and sorption mechanism of uranium (VI) onto NaY zeolite. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 1994, p. 070008. [Google Scholar]
- WHO. Chapter 9 Radiological Aspects. In Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Misaelides, P. Application of natural zeolites in environmental remediation: A short review. Microporous Mesoporous Mater. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- El-Naggar, M.; El-Kamash, A.; El-Dessouky, M.; Ghonaim, A. Two-step method for preparation of NaA-X zeolite blend from fly ash for removal of cesium ions. J. Hazard. Mater. 2008, 154, 963–972. [Google Scholar] [CrossRef] [PubMed]
- El-Rahman, K.A.; El-Kamash, A.M.; El-Sourougy, M.R.; Abdel-Moniem, N.M. Thermodynamic modeling for the removal of Cs+, Sr2+, Ca2+ and Mg2+ ions from aqueous waste solutions using zeolite A. J. Radioanal. Nucl. Chem. 2006, 268, 221–230. [Google Scholar] [CrossRef]
- Bujdák, J. Adsorption kinetics models in clay systems. The critical analysis of pseudo-second order mechanism. Appl. Clay Sci. 2020, 191, 105630. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Saffar-Dastgerdi, M.H. Zeolite nanoparticle as a superior adsorbent with high capacity: Synthesis, surface modification and pollutant adsorption ability from wastewater. Microchem. J. 2019, 145, 74–83. [Google Scholar] [CrossRef]

| Sorbent | BET Surface Area (m2g−1) | Pore Radius (nm) | Total Pore Volume (cc g−1) |
|---|---|---|---|
| Zeolite | 44.3868 | 1.460 | 0.066 |
| montmorillonite | 38.1534 | 1.330 | 0.045 |
| Biochar | 54.3115 | 1.510 | 0.059 |
| Model | Parameters | Sorbents | ||
|---|---|---|---|---|
| Zeolite | Montmorillonite | Biochar | ||
| Linear | r2 | 0.9875 | 0.9998 | 0.9898 |
| kd | 4.44 | 0.086 | 0.022 | |
| X2 | 0.00722 | 3.39 × 10−5 | 0.00070 | |
| SSE | 0.00061 | 4.79 × 10−6 | 4.75 × 10−5 | |
| RSME | 0.025 | 0.002 | 0.0069 | |
| Langmuir | r2 | 0.6118 | 0.8155 | 0.6371 |
| qm | −0.124 | 0.765 | 0.498 | |
| kl | 7.27 | 0.16 | 0.056 | |
| X2 | 0.0962 | 0.0011 | 0.00096 | |
| SSE | 0.0278 | 0.0001 | 8.33 × 10−5 | |
| RSME | 0.1669 | 0.0114 | 0.0091 | |
| Freundlich | r2 | 0.9289 | 0.9988 | 0.9955 |
| kf | 43.33 | 0.102 | 0.0263 | |
| 1/n | 2.13 | 0.848 | 0.892 | |
| X2 | 0.0216 | 0.00026 | 0.0007 | |
| SSE | 0.0046 | 3.74 × 10−5 | 5.9 × 10−5 | |
| RSME | 0.0677 | 0.0061 | 0.0077 | |
| Temkin | r2 | 0.9970 | 0.9195 | 0.9130 |
| AT | 25.78 | 4.61 | 2.05 | |
| BT | 0.3255 | 0.0826 | 0.0427 | |
| X2 | 0.00063 | 0.0158 | 0.0067 | |
| SSE | 0.0001 | 0.0016 | 0.0004 | |
| RSME | 0.0120 | 0.0402 | 0.0201 | |
| Sorbent Type | Initial Concentrations, (Bq L−1) | Equilibrium Activity Concentrations, (Bq L−1) | Removal Efficiency (%) | Partition Coefficient (PC, L kg−1) |
|---|---|---|---|---|
| Zeolite | 1 | 0.045 | 95.50 | 1061 |
| 3 | 0.060 | 98.00 | 2450 | |
| 5 | 0.085 | 98.30 | 2891 | |
| 7 | 0.110 | 98.43 | 3132 | |
| Montmorillonite | 1 | 0.285 | 71.50 | 125.0 |
| 3 | 1.015 | 66.17 | 97.80 | |
| 5 | 1.735 | 65.30 | 94.10 | |
| 7 | 2.490 | 64.43 | 90.60 | |
| Biochar | 1 | 0.65 | 35.50 | 27.50 |
| 3 | 2.00 | 33.50 | 25.20 | |
| 5 | 3.51 | 29.90 | 21.30 | |
| 7 | 4.78 | 31.71 | 23.20 |
| Model | Parameters | Sorbents | ||
|---|---|---|---|---|
| Zeolite | Montmorillonite | Biochar | ||
| Pseudo-second-order | H | 0.228 | 0.007 | 0.002 |
| k2 | 3.665 | 0.121 | 0.039 | |
| qe | 0.250 | 0.242 | 0.233 | |
| r2 | 1.000 | 0.9999 | 0.9996 | |
| Initial first linear portion (Intraparticle diffusion) | kid1 | 0.0003 | 0.0042 | 0.0118 |
| a1 | 0.243 | 0.1337 | −0.0137 | |
| r2 | 0.9364 | 0.9407 | 0.9921 | |
| Initial second linear portion (Intraparticle diffusion) | Kid2 | 5.0 × 10−5 | 0.0007 | 0.0016 |
| a2 | 0.248 | 0.2099 | 0.1549 | |
| r2 | 0.8940 | 0.9976 | 0.9500 | |
| Order of Groundwater Wells | Sorbent Type | Initial Concentrations, (Bq L−1) | Equilibrium Activity Concentrations, (Bq L−1) (±1σ) | Removal Efficiency (%) (±1σ) |
|---|---|---|---|---|
| Well-1 | Zeolite | 1.12 | 0.04 ± 0.004 | 96.30 ± 0.01 |
| Montmorillonite | 0.32 ± 0.03 | 71.73 ± 2.27 | ||
| Biochar | 0.74 ± 0.09 | 34.56 ± 1.94 | ||
| Well-2 | Zeolite | 2.76 | 0.06 ± 0.01 | 97.71 ± 0.10 |
| Montmorillonite | 0.85 ± 0.04 | 69.04 ± 0.85 | ||
| Biochar | 1.84 ± 0.06 | 33.24 ± 1.18 | ||
| Well-3 | Zeolite | 3.65 | 0.12 ± 0.02 | 96.81 ± 0.28 |
| Montmorillonite | 1.19 ± 0.08 | 67.54 ± 1.25 | ||
| Biochar | 2.29 ± 0.10 | 37.33 ± 0.87 | ||
| Well-4 | Zeolite | 7.68 | 0.23 ± 0.02 | 97.07 ± 0.17 |
| Montmorillonite | 2.68 ± 0.07 | 68.08 ± 0.31 | ||
| Biochar | 5.63 ± 0.05 | 26.71 ± 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almasoud, F.I.; Al-Farraj, A.S.; Al-Wabel, M.I.; Usman, A.R.A.; Alanazi, Y.J.; Ababneh, Z.Q. The Potential Use of Zeolite, Montmorillonite, and Biochar for the Removal of Radium-226 from Aqueous Solutions and Contaminated Groundwater. Processes 2020, 8, 1537. https://doi.org/10.3390/pr8121537
Almasoud FI, Al-Farraj AS, Al-Wabel MI, Usman ARA, Alanazi YJ, Ababneh ZQ. The Potential Use of Zeolite, Montmorillonite, and Biochar for the Removal of Radium-226 from Aqueous Solutions and Contaminated Groundwater. Processes. 2020; 8(12):1537. https://doi.org/10.3390/pr8121537
Chicago/Turabian StyleAlmasoud, Fahad I., Abdullah S. Al-Farraj, Mohammad I. Al-Wabel, Adel R.A. Usman, Yousef J. Alanazi, and Zaid Q. Ababneh. 2020. "The Potential Use of Zeolite, Montmorillonite, and Biochar for the Removal of Radium-226 from Aqueous Solutions and Contaminated Groundwater" Processes 8, no. 12: 1537. https://doi.org/10.3390/pr8121537
APA StyleAlmasoud, F. I., Al-Farraj, A. S., Al-Wabel, M. I., Usman, A. R. A., Alanazi, Y. J., & Ababneh, Z. Q. (2020). The Potential Use of Zeolite, Montmorillonite, and Biochar for the Removal of Radium-226 from Aqueous Solutions and Contaminated Groundwater. Processes, 8(12), 1537. https://doi.org/10.3390/pr8121537