Methanogenic Microorganisms in Industrial Wastewater Anaerobic Treatment
Abstract
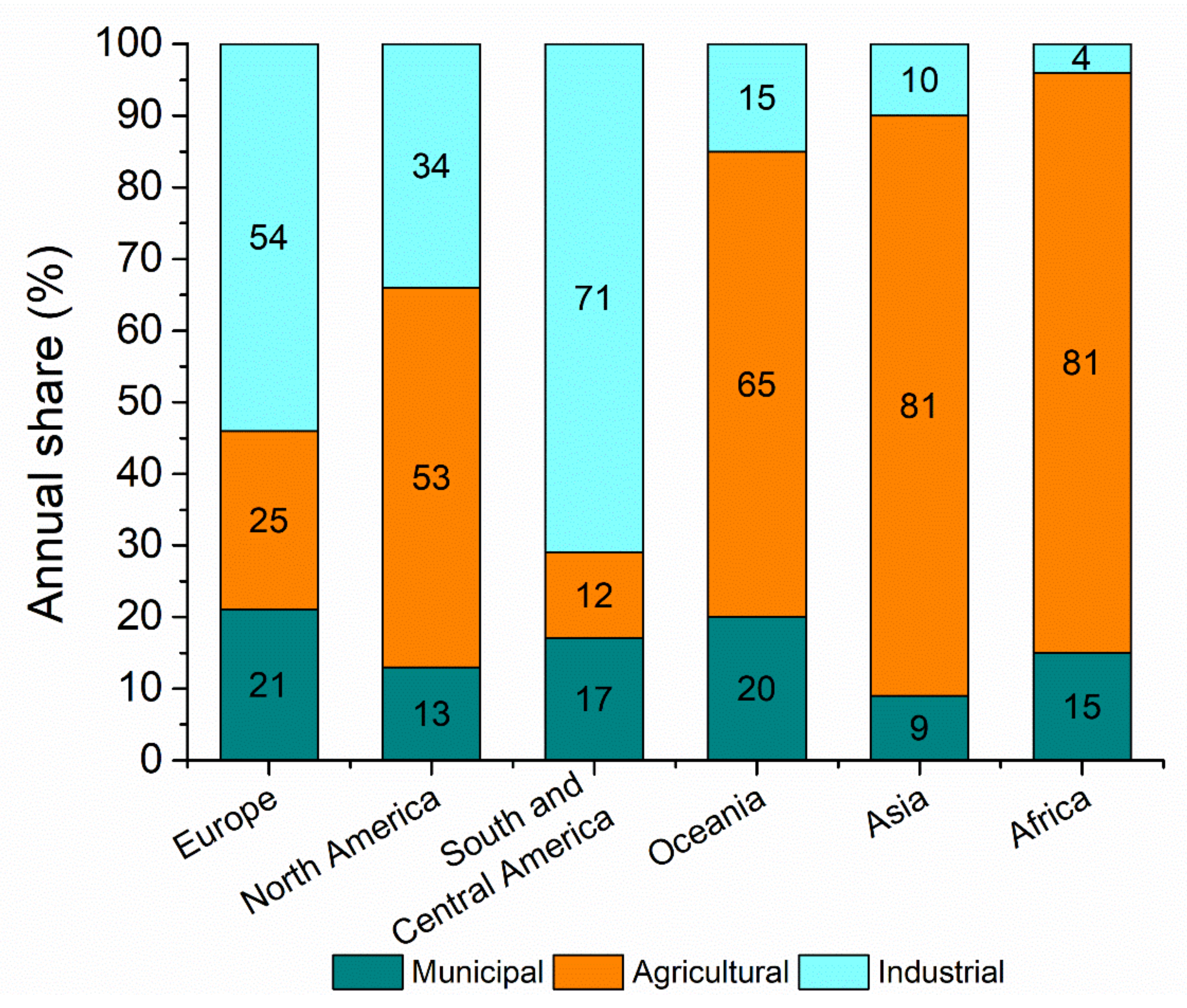
:1. Introduction
2. Industrial Wastewater
2.1. Industrial Wastewater Types
2.2. Industrial Water Suitable for Anaerobic Treatment and Methane Production
- Meat and poultry processing (slaughterhouses)
- Alcohol, beer, starch production
- Pulp and paper manufacture
- Chemical industry waste
- Other food and drink processing (dairy products, vegetable oil, fruits and vegetables, canneries, juice making, etc.)
2.3. Composition of Selected Industrial Wastewater
3. Anaerobic Wastewater Treatment
3.1. Aerobic Versus Anaerobic Wastewater Treatment
3.2. Anaerobic Digestion
3.3. Anaerobic Bioreactors
4. Methanogenic Microorganisms in Industrial Wastewaters
4.1. Inhibitors and Methanogenic Activity
4.2. Slaughterhouse Wastewater
4.3. Brewery Wastewater
4.4. Distilleries Wastewater
4.5. Pulp and Paper Industry Wastewater
4.6. Food and Drink Processing (Dairy Products, Vegetable Oil, Fruits and Vegetables, Canneries, Juice Making, etc.) Wastewater
4.6.1. Dairy Industry Wastewater
4.6.2. Vegetable Oil Industry Wastewater
4.6.3. Fruit and Vegetable Processing Industry Wastewater
4.7. Chemical Industry Wastewater
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mourelatou, A.; European Environment Agency. Environmental Indicator Report 2018: In Support to the Monitoring of the Seventh Environment Action Programme; European Environment Agency: Copenhagen, Denmark, 2018; ISBN 978-92-9480-017-6. [Google Scholar]
- Ritchie, H.; Roser, M. Water Use and Stress. Available online: https://ourworldindata.org/water-use-stress (accessed on 8 September 2020).
- The State of the World’s Land and Water Resources for Food and Agriculture: Managing Systems at Risk, 1st ed.; Earthscan: Abingdon, UK; New York, NY, USA, 2011; ISBN 978-1-84971-326-9.
- Bal, A.S.; Dhagat, N.N. Upflow anaerobic sludge blanket reactor—A review. Indian J. Environ. Health 2001, 43, 1–82. [Google Scholar] [PubMed]
- Macarie, H. Overview of the application of anaerobic treatment to chemical and petrochemical wastewaters. Water Sci. Technol. 2000, 42, 201–214. [Google Scholar] [CrossRef]
- Cisneros, B.J. Safe Sanitation in Low Economic Development Areas. In Treatise on Water Science; Elsevier: Amsterdam, The Netherlands, 2011; pp. 147–200. ISBN 978-0-444-53199-5. [Google Scholar]
- Singh, K.S.; Harada, H.; Viraraghavan, T. Low-strength wastewater treatment by a UASB reactor. Bioresour. Technol. 1996, 55, 187–194. [Google Scholar] [CrossRef]
- Ma, B.; Peng, Y.; Zhang, S.; Wang, J.; Gan, Y.; Chang, J.; Wang, S.; Wang, S.; Zhu, G. Performance of anammox UASB reactor treating low strength wastewater under moderate and low temperatures. Bioresour. Technol. 2013, 129, 606–611. [Google Scholar] [CrossRef]
- Lay, C.-H.; Vo, T.-P.; Lin, P.-Y.; Abdul, P.M.; Liu, C.-M.; Lin, C.-Y. Anaerobic hydrogen and methane production from low-strength beverage wastewater. Int. J. Hydrog. Energy 2019, 44, 14351–14361. [Google Scholar] [CrossRef]
- Muralikrishna, I.V.; Manickam, V. Industrial Wastewater Treatment Technologies, Recycling, and Reuse. In Environmental Management; Elsevier: Amsterdam, The Netherlands, 2017; pp. 295–336. ISBN 978-0-12-811989-1. [Google Scholar]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; Wastewater Microbiology Series; Wiley-Interscience: Hoboken, NJ, USA, 2003; ISBN 978-0-471-20693-4. [Google Scholar]
- Speece, R.E. Anaerobic biotechnology for industrial wastewater treatment. Environ. Sci. Technol. 1983, 17, 416A–427A. [Google Scholar] [CrossRef]
- Wu, P.F.; Mittal, G. Characterization of provincially inspected slaughterhouse wastewater in Ontario, Canada. Can. Biosyst. Eng. 2012, 54, 6.9–6.18. [Google Scholar] [CrossRef]
- Caixeta, C.E.T.; Cammarota, M.C.; Xavier, A.M.F. Slaughterhouse wastewater treatment: Evaluation of a new three-phase separation system in a UASB reactor. Bioresour. Technol. 2002, 81, 61–69. [Google Scholar] [CrossRef]
- Del Nery, V.; Damianovic, M.H.Z.; Moura, R.B.; Pozzi, E.; Pires, E.C.; Foresti, E. Poultry slaughterhouse wastewater treatment plant for high quality effluent. Water Sci. Technol. 2016, 73, 309–316. [Google Scholar] [CrossRef]
- Gomes, A.J.G.; Atambo, D.O.; Das, K.K.; Cocke, D.L.; Das, K.P. Electrochemical remediation of chicken processing plant wastewater. J. Environ. Chem. Eng. 2018, 6, 6028–6036. [Google Scholar] [CrossRef]
- Erden, G.; Buyukkamaci, N.; Filibeli, A. Effect of low frequency ultrasound on anaerobic biodegradability of meat processing effluent. Desalination 2010, 259, 223–227. [Google Scholar] [CrossRef]
- Choi, Y.S.; Hong, S.W.; Kim, S.J.; Chung, I.H. Development of a biological process for livestock wastewater treatment using a technique for predominant outgrowth of Bacillus species. Water Sci. Technol. 2002, 45, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Monroy, O.H.F.; Vázquez, M.; Derramadero, J.C.; Guyot, J.P. Anaerobic-aerobic treatment of cheese wastewater with national technology in Mexico: The case of “El Sauz”. Water Sci. Technol. 1995, 32, 149–156. [Google Scholar] [CrossRef]
- Kasapgil, B.; Anderson, G.K.; Ince, O. An Investigation into the Pre-Treatment of Dairy Wastewater Prior to Aerobic Biological Treatment. Water Sci. Technol. 1994, 29, 205–212. [Google Scholar] [CrossRef]
- Ozturk, I.; Eroglu, V.; Ubay, G.; Demir, I. Hybrid upflow anaerobic sludge blanket reactor (HUASBR) treatment of dairy effluents. Water Sci. Technol. 1993, 28, 77–85. [Google Scholar] [CrossRef]
- Rao, A.G.; Reddy, T.S.K.; Prakash, S.S.; Vanajakshi, J.; Joseph, J.; Sarma, P.N. pH regulation of alkaline wastewater with carbon dioxide: A case study of treatment of brewery wastewater in UASB reactor coupled with absorber. Bioresour. Technol. 2007, 98, 2131–2136. [Google Scholar] [CrossRef]
- Sunil Kumar, G.; Gupta, S.K.; Singh, G. Biodegradation of distillery spent wash in anaerobic hybrid reactor. Water Res. 2007, 41, 721–730. [Google Scholar] [CrossRef]
- Kharayat, Y. Distillery wastewater: Bioremediation approaches. J. Integr. Environ. Sci. 2012, 9, 69–91. [Google Scholar] [CrossRef]
- Won, S.G.; Baldwin, S.A.; Lau, A.K.; Rezadehbashi, M. Optimal operational conditions for biohydrogen production from sugar refinery wastewater in an ASBR. Int. J. Hydrog. Energy 2013, 38, 13895–13906. [Google Scholar] [CrossRef]
- Velasquez-Orta, S.B.; Head, I.M.; Curtis, T.P.; Scott, K. Factors affecting current production in microbial fuel cells using different industrial wastewaters. Bioresour. Technol. 2011, 102, 5105–5112. [Google Scholar] [CrossRef]
- Eskelinen, K.; Särkkä, H.; Kurniawan, T.A.; Sillanpää, M.E.T. Removal of recalcitrant contaminants from bleaching effluents in pulp and paper mills using ultrasonic irradiation and Fenton-like oxidation, electrochemical treatment, and/or chemical precipitation: A comparative study. Desalination 2010, 255, 179–187. [Google Scholar] [CrossRef]
- Habets, L.H.A.; De Vegt, A.L. Anaerobic treatment of bleached TMP and CTMP effluent in the biopaq UASB system. Water Sci. Technol. 1991, 24, 331–345. [Google Scholar] [CrossRef]
- Valta, K.; Damala, P.; Panaretou, V.; Orli, E.; Moustakas, K.; Loizidou, M. Review and Assessment of Waste and Wastewater Treatment from Fruits and Vegetables Processing Industries in Greece. Waste Biomass Valorization 2017, 8, 1629–1648. [Google Scholar] [CrossRef]
- Ma, Z.; Lei, T.; Ji, X.; Gao, X.; Gao, C. Submerged membrane bioreactor for vegetable oil wastewater treatment. Chem. Eng. Technol. 2015, 38, 101–109. [Google Scholar] [CrossRef]
- Galliou, F.; Markakis, N.; Fountoulakis, M.S.; Nikolaidis, N.; Manios, T. Production of organic fertilizer from olive mill wastewater by combining solar greenhouse drying and composting. Waste Manag. 2018, 75, 305–311. [Google Scholar] [CrossRef]
- Kalat, D.G.; Yüceer, A. Anaerobic mesophilic and thermophilic treatability of vegetable oil refining wastewater. Process Saf. Environ. Prot. 2017, 109, 151–157. [Google Scholar] [CrossRef]
- Mohamed, A.A.H.; Al Shariff, S.M.; Ouf, S.A.; Benghanem, M. Atmospheric pressure plasma jet for bacterial decontamination and property improvement of fruit and vegetable processing wastewater. J. Phys. D Appl. Phys. 2016, 49. [Google Scholar] [CrossRef]
- Jørgensen, S.E. (Ed.) Chapter 21 Manufacture of Organic Chemicals. In Industrial Waste Water Management; Studies in Environmental Science; Elsevier: Amsterdam, The Netherlands, 1979; Volume 5, pp. 273–282. [Google Scholar]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D.; Metcalf, E. (Eds.) Wastewater Engineering: Treatment and Reuse, 4th ed.; McGraw-Hill Series in Civil and Environmental Engineering; McGraw-Hill: Boston, MA, USA, 2003; ISBN 978-0-07-041878-3. [Google Scholar]
- van Lier, J.B.; Mahmoud, N.A.; Zeeman, G. Anaerobic Wastewater Treatment. In Biological Wastewater Treatment: Principles, Modelling and Design; IWA Publishing: London, UK, 2008; pp. 415–457. ISBN 978-1-84339-188-3. [Google Scholar]
- Riffat, R.; Sajjad, M.W.; Dararat, S. Anaerobic processes. Water Environ. Res. 1998, 70, 518–540. [Google Scholar] [CrossRef]
- Dionisi, D. Biological Wastewater Treatment Processes: Mass and Heat Balances; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2017; ISBN 978-1-4822-2926-4. [Google Scholar]
- Tchobanoglous, G.; Burton, F.L.; Metcalf, L.; Eddy, H.P. (Eds.) Wastewater Engineering: Treatment, Disposal, and Reuse, 3rd ed.; McGraw-Hill Series in Water Resources and Environmental Engineering; McGraw-Hill: New York, NY, USA, 1991; ISBN 978-0-07-041690-1. [Google Scholar]
- Young, J.C.; McCarty, P.L. The anaerobic filter for waste treatment. J. Water Pollut. Control Fed. 1969, 41, R160–R173. [Google Scholar]
- Khanal, S.K.; Giri, B.; Nitayavardhana, S.; Gadhamshetty, V. 10—Anaerobic Bioreactors/Digesters: Design and Development. In Current Developments in Biotechnology and Bioengineering; Lee, D.-J., Jegatheesan, V., Ngo, H.H., Hallenbeck, P.C., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 261–279. ISBN 978-0-444-63665-2. [Google Scholar]
- Cabezas, A.; de Araujo, J.C.; Callejas, C.; Galès, A.; Hamelin, J.; Marone, A.; Sousa, D.Z.; Trably, E.; Etchebehere, C. How to use molecular biology tools for the study of the anaerobic digestion process? Rev. Environ. Sci. Biotechnol. 2015, 14, 555–593. [Google Scholar] [CrossRef]
- Senés-Guerrero, C.; Colón-Contreras, F.A.; Reynoso-Lobo, J.F.; Tinoco-Pérez, B.; Siller-Cepeda, J.H.; Pacheco, A. Biogas-producing microbial composition of an anaerobic digester and associated bovine residues. Microbiol. Open 2019, 8, e00854. [Google Scholar] [CrossRef] [PubMed]
- Batstone, D.J.; Virdis, B. The role of anaerobic digestion in the emerging energy economy. Curr. Opin. Biotechnol. 2014, 27, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Holland, K.T.; Knapp, J.G.; Shoesmith, J.G. Anaerobic Bacteria. J. Basic Microbiol. 1990, 30, 379. [Google Scholar] [CrossRef]
- Hungate, R.E. Hydrogen as an intermediate in the rumen fermentation. Arch. Mikrobiol. 1967, 59, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.P. Microbial Methane Production—Theoretical Aspects2. J. Anim. Sci. 1979, 48, 193–201. [Google Scholar] [CrossRef]
- Merlin, G.; Boileau, H. Anaerobic Digestion of Agricultural Waste: State of the Art and Future Trends; Nova Science Publishers, Inc.: New York, NY, USA, 2013. [Google Scholar]
- Kushkevych, I.; Kováč, J.; Vítězová, M.; Vítěz, T.; Bartoš, M. The diversity of sulfate-reducing bacteria in the seven bioreactors. Arch. Microbiol. 2018, 200, 945–950. [Google Scholar] [CrossRef] [PubMed]
- McCarty, P.L. The development of anaerobic treatment and its future. Water Sci. Technol. 2001, 44, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Van Lier, J.B.; van der Zee, F.P.; Frijters, C.T.M.J.; Ersahin, M.E. Celebrating 40 years anaerobic sludge bed reactors for industrial wastewater treatment. Rev. Environ. Sci. Biotechnol. 2015, 14, 681–702. [Google Scholar] [CrossRef] [Green Version]
- Lettinga, G.; van der Geest, A.T.; Hobma, S.; Laan, J.V.D. Anaerobic treatment of methanolic wastes. Water Res. 1979, 13, 725–737. [Google Scholar] [CrossRef]
- Van Lier, J.B. High-rate anaerobic wastewater treatment: Diversifying from end-of-the-pipe treatment to resource-oriented conversion techniques. Water Sci. Technol. 2008, 57, 1137–1148. [Google Scholar] [CrossRef]
- Driessen, W.; Yspeert, P. Anaerobic treatment of low, medium and high strength effluent in the agro-industry. Water Sci. Technol. 1999, 40, 221–228. [Google Scholar] [CrossRef]
- Díaz, E.E.; Stams, A.J.M.; Amils, R.; Sanz, J.L. Phenotypic Properties and Microbial Diversity of Methanogenic Granules from a Full-Scale Upflow Anaerobic Sludge Bed Reactor Treating Brewery Wastewater. Appl. Environ. Microbiol. 2006, 72, 4942–4949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godon, J.J.; Zumstein, E.; Dabert, P.; Habouzit, F.; Moletta, R. Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl. Environ. Microbiol. 1997, 63, 2802–2813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekiguchi, Y.; Kamagata, Y.; Syutsubo, K.; Ohashi, A.; Harada, H.; Nakamura, K. Phylogenetic diversity of mesophilic and thermophilic granular sludges determined by 16S rRNA gene analysis. Microbiology 1998, 144, 2655–2665. [Google Scholar] [CrossRef] [Green Version]
- Keyser, M.; Britz, T.J.; Witthuhn, R.C. Fingerprinting and Identification of Bacteria Present in UASB Granules Used to Treat Winery, Brewery, Distillery or Peach-lye Canning Wastewater. S. Afr. J. Enol. Vitic. 2007, 28, 69–79. [Google Scholar] [CrossRef]
- Fernández, N.; Díaz, E.E.; Amils, R.; Sanz, J.L. Analysis of Microbial Community during Biofilm Development in an Anaerobic Wastewater Treatment Reactor. Microb. Ecol. 2008, 56, 121–132. [Google Scholar] [CrossRef]
- Narihiro, T.; Terada, T.; Kikuchi, K.; Iguchi, A.; Ikeda, M.; Yamauchi, T.; Shiraishi, K.; Kamagata, Y.; Nakamura, K.; Sekiguchi, Y. Comparative analysis of bacterial and archaeal communities in methanogenic sludge granules from upflow anaerobic sludge blanket reactors treating various food-processing, high-strength organic wastewaters. Microbes Environ. 2009, 24, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Peptidolytic Microbial Community of Methanogenic Reactors from Two Modified UASBs of Brewery Industries. Available online: https://www.scielo.br/scielo.php?script=sci_arttext&pid=S1517-83822010000300022&lng=en&nrm=iso&tlng=en (accessed on 22 October 2020).
- Zhang, L.; Ban, Q.; Li, J.; Wan, C. Functional bacterial and archaeal dynamics dictated by pH stress during sugar refinery wastewater in a UASB. Bioresour. Technol. 2019, 288, 121464. [Google Scholar] [CrossRef]
- Leclerc, M.; Delgènes, J.P.; Godon, J.J. Diversity of the archaeal community in 44 anaerobic digesters as determined by single strand conformation polymorphism analysis and 16S rDNA sequencing. Environ. Microb. 2004, 6, 809–819. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Y.; Ni, B.-J.; Han, X.; Fan, L.; Yuan, Z. Dissecting microbial community structure and methane-producing pathways of a full-scale anaerobic reactor digesting activated sludge from wastewater treatment by metagenomic sequencing. Microb. Cell Fact. 2015, 14, 33. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Liu, G.; Li, H.; Xu, L.; Du, L.; Yang, B. Predictive functional profiling using marker gene sequences and community diversity analyses of microbes in full-scale anaerobic sludge digesters. Bioprocess Biosyst. Eng. 2016, 39, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, W.; Lee, C. Absolute dominance of hydrogenotrophic methanogens in full-scale anaerobic sewage sludge digesters. J. Environ. Sci. (China) 2013, 25, 2272–2280. [Google Scholar] [CrossRef]
- Vítěz, T.; Novák, D.; Lochman, J.; Vítězová, M. Methanogens Diversity during Anaerobic Sewage Sludge Stabilization and the Effect of Temperature. Processes 2020, 8, 822. [Google Scholar] [CrossRef]
- Struk, M.; Vítězová, M.; Vítěz, T.; Bartoš, M.; Kushkevych, I. Modřice Plant Anaerobic Digester: Microbial Distribution and Biogas Production. Water Air Soil Pollut. 2019, 230, 240. [Google Scholar] [CrossRef]
- Ferry, J.G.; Smith, P.H.; Wolfe, R.S. Methanospirillum, a New Genus of Methanogenic Bacteria, and Characterization of Methanospirillum hungatii sp.nov. Int. J. Syst. Bacteriol. 1974, 24, 465–469. [Google Scholar] [CrossRef]
- Maus, I.; Wibberg, D.; Stantscheff, R.; Eikmeyer, F.-G.; Seffner, A.; Boelter, J.; Szczepanowski, R.; Blom, J.; Jaenicke, S.; Konig, H.; et al. Complete Genome Sequence of the Hydrogenotrophic, Methanogenic Archaeon Methanoculleus bourgensis Strain MS2T, Isolated from a Sewage Sludge Digester. J. Bacteriol. 2012, 194, 5487–5488. [Google Scholar] [CrossRef] [Green Version]
- Imachi, H.; Sakai, S.; Sekiguchi, Y.; Hanada, S.; Kamagata, Y.; Ohashi, A.; Harada, H. Methanolinea tarda gen. nov., sp. nov., a methane-producing archaeon isolated from a methanogenic digester sludge. Int. J. Syst. Evol. Microbiol. 2008, 58, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Yu, G.; Louie, T.; Liu, T.; Zhu, C.; Xue, G.; Gao, P. From mesophilic to thermophilic digestion: The transitions of anaerobic bacterial, archaeal, and fungal community structures in sludge and manure samples. Appl. Microbiol. Biotechnol. 2015, 99, 10271–10282. [Google Scholar] [CrossRef]
- Kotelnikova, S.; Macario, A.J.L.; Pedersen, K. Methanobacterium subterraneum sp. nov., a new alkaliphilic, eurythermic and halotolerant methanogen isolated from deep granitic groundwater. Int. J. Syst. Bacteriol. 1998, 48, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Blotevogel, K.-H.; Fischer, U.; Mocha, M.; Jannsen, S. Methanobacterium thermoalcaliphilum spec. nov., a new moderately alkaliphilic and thermophilic autotrophic methanogen. Arch. Microbiol. 1985, 142, 211–217. [Google Scholar] [CrossRef]
- Winter, J.; Lerp, C.; Zabel, H.-P.; Wildenauer, F.X.; König, H.; Schindler, F. Methanobacterium wolfei, sp. nov., a New Tungsten-Requiring, Thermophilic, Autotrophic Methanogen. Syst. Appl. Microbiol. 1984, 5, 457–466. [Google Scholar] [CrossRef]
- Zeikus, J.G.; Wolfe, R.S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J. Bacteriol. 1972, 109, 707–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levén, L.; Eriksson, A.R.B.; Schnürer, A. Effect of process temperature on bacterial and archaeal communities in two methanogenic bioreactors treating organic household waste: Temperature effects on microbial communities in bioreactors. FEMS Microbiol. Ecol. 2007, 59, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Jérôme, V.; Freitag, R.; Mayer, H.K. Diversity of the resident microbiota in a thermophilic municipal biogas plant. Appl. Microbiol. Biotechnol. 2008, 81, 163. [Google Scholar] [CrossRef]
- Lettinga, G.; van Velsen, A.F.M.; Hobma, S.W.; de Zeeuw, W.; Klapwijk, A. Use of the upflow sludge blanket (USB) reactor concept for biological wastewater treatment, especially for anaerobic treatment. Biotechnol. Bioeng. 1980, 22, 699–734. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, Q.; Lu, Y. Inhibitory effects of ammonia on methanogen mcrA transcripts in anaerobic digester sludge. FEMS Microbiol. Ecol. 2014, 87, 368–377. [Google Scholar] [CrossRef] [Green Version]
- Vallee, B.L.; Ulmer, D.D. Biochemical Effects of Mercury, Cadmium, and Lead. Annu. Rev. Biochem. 1972, 41, 91–128. [Google Scholar] [CrossRef]
- Hendriksen, H.V.; Ahring, B.K. Effects of ammonia on growth and morphology of thermophilic hydrogen-oxidizing methanogenic bacteria. FEMS Microbiol. Lett. 1991, 85, 241–246. [Google Scholar] [CrossRef]
- Donlon, B.A.; Razo-Flores, E.; Field, J.A.; Lettinga, G. Toxicity of N-substituted aromatics to acetoclastic methanogenic activity in granular sludge. Appl. Environ. Microbiol. 1995, 61, 3889–3893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belay, N.; Daniels, L. Production of ethane, ethylene, and acetylene from halogenated hydrocarbons by methanogenic bacteria. Appl. Environ. Microbiol. 1987, 53, 1604–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinzema, A.; Boone, M.; van Knippenberg, K.; Lettinga, G. Bactericidal effect of long chain fatty acids in anaerobic digestion. Water Environ. Res. 1994, 66, 40–49. [Google Scholar] [CrossRef]
- Massé, D.I.; Masse, L. Characterization of wastewater from hog slaughterhouses in Eastern Canada and evaluation of their in-plant wastewater treatment systems. Can. Agric. Eng 2000, 42, 139–146. [Google Scholar]
- Del Pozo, R.; Diez, V. Integrated anaerobic–aerobic fixed-film reactor for slaughterhouse wastewater treatment. Water Res. 2005, 39, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Kayhanian, M. Performance of a high-solids anaerobic digestion process under various ammonia concentrations. J. Chem. Technol. Biotechnol. 1994, 59, 349–352. [Google Scholar] [CrossRef]
- Da Silva, M.L.B.; Cantão, M.E.; Mezzari, M.P.; Ma, J.; Nossa, C.W. Assessment of Bacterial and Archaeal Community Structure in Swine Wastewater Treatment Processes. Microb. Ecol. 2015, 70, 77–87. [Google Scholar] [CrossRef]
- Han, G.; Shin, S.G.; Cho, K.; Lee, J.; Kim, W.; Hwang, S. Temporal variation in bacterial and methanogenic communities of three full-scale anaerobic digesters treating swine wastewater. Environ. Sci. Pollut. Res. 2019, 26, 1217–1226. [Google Scholar] [CrossRef]
- Del Nery, V.; Pozzi, E.; Damianovic, M.H.R.Z.; Domingues, M.R.; Zaiat, M. Granules characteristics in the vertical profile of a full-scale upflow anaerobic sludge blanket reactor treating poultry slaughterhouse wastewater. Bioresour. Technol. 2008, 99, 2018–2024. [Google Scholar] [CrossRef]
- Shi, X.Y.; Jin, D.W.; Sun, Q.Y.; Li, W.W. Optimization of conditions for hydrogen production from brewery wastewater by anaerobic sludge using desirability function approach. Renew. Energy 2010, 35, 1493–1498. [Google Scholar] [CrossRef]
- Granada, C.E.; Hasan, C.; Marder, M.; Konrad, O.; Vargas, L.K.; Passaglia, L.M.P.; Giongo, A.; de Oliveira, R.R.; Pereira, L.D.M.; de Jesus Trindade, F.; et al. Biogas from slaughterhouse wastewater anaerobic digestion is driven by the archaeal family Methanobacteriaceae and bacterial families Porphyromonadaceae and Tissierellaceae. Renew. Energy 2018, 118, 840–846. [Google Scholar] [CrossRef]
- da Silva, A.M.E.V.; da Silva, R.J.N.B.; Camões, M.F.G.F.C. Optimization of the determination of chemical oxygen demand in wastewaters. Anal. Chim. Acta 2011, 699, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Maestrojuan, G.M.; Boone, D.R.; Xun, L.; Mah, R.A.; Zhang, L. Transfer of Methanogenium bourgense, Methanogenium marisnigri, Methanogenium olentangyi, and Methanogenium thermophilicum to the Genus Methanoculleus gen. nov., Emendation of Methanoculleus marisnigri and Methanogenium, and Description of New Strains of Methanoculleus bourgense and Methanoculleus marisnigri. Int. J. Syst. Bacteriol. 1990, 40, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, S.; Ueno, A.; Tamamura, S.; Naganuma, T.; Kaneko, K. Methanoculleus horonobensis sp. nov., a methanogenic archaeon isolated from a deep diatomaceous shale formation. Int. J. Syst. Evol. Microbiol. 2013, 63, 4320–4323. [Google Scholar] [CrossRef]
- Fillaudeau, L.; Blanpain-Avet, P.; Daufin, G. Water, wastewater and waste management in brewing industries. J. Clean. Prod. 2006, 14, 463–471. [Google Scholar] [CrossRef]
- Briggs, D.E. (Ed.) Brewing: Science and Practice; Woodhead Publishing Series in Food Science and Technology; CRC Press: Boca Raton, FL, USA; Woodhead Pub. Ltd: Cambridge, UK, 2004; ISBN 978-1-85573-490-6. [Google Scholar]
- Cronin, C.; Lo, K.V. Anaerobic treatment of brewery wastewater using UASB reactors seeded with activated sludge. Bioresour. Technol. 1998, 64, 33–38. [Google Scholar] [CrossRef]
- Baloch, M.I.; Akunna, J.C.; Collier, P.J. The performance of a phase separated granular bed bioreactor treating brewery wastewater. Bioresour. Technol. 2007, 98, 1849–1855. [Google Scholar] [CrossRef]
- Wu, W.M.; Hickey, R.F.; Zeikus, J.G. Characterization of metabolic performance of methanogenic granules treating brewery wastewater: Role of sulfate-reducing bacteria. Appl. Environ. Microbiol. 1991, 57, 3438–3449. [Google Scholar] [CrossRef] [Green Version]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Van Haandel, A.; De Vrieze, J.; Verstraete, W.; dos Santos, V.S. Methanosaeta dominate acetoclastic methanogenesis during high-rate methane production in anaerobic reactors treating distillery wastewaters: High-rate anaerobic digestion of vinasses. J. Chem. Technol. Biotechnol. 2014, 89, 1751–1759. [Google Scholar] [CrossRef]
- Arantes, M.K.; Alves, H.J.; Sequinel, R.; da Silva, E.A. Treatment of brewery wastewater and its use for biological production of methane and hydrogen. Int. J. Hydrog. Energy 2017, 42, 26243–26256. [Google Scholar] [CrossRef]
- Gomec, C.; Letsiou, I.; Ozturk, I.; Eroglu, V.; Wilderer, P. Identification of Archaeal population in the granular sludge of an UASB reactor treating sewage at low temperatures. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2008, 43, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.I.F.S.; Van Boekel, M.A.J.S. A kinetic model for the glucose/glycine Maillard reaction pathways. Food Chem. 2005, 90, 257–269. [Google Scholar] [CrossRef]
- Oleszkiewicz, J.A.; Sharma, V.K. Stimulation and inhibition of anaerobic processes by heavy metals—A review. Biol. Wastes 1990, 31, 45–67. [Google Scholar] [CrossRef]
- Agabo-García, C.; Pérez, M.; Solera, R. Adaptation of thermophilic sludge-inoculum to co-digestion with Sherry-wine distillery wastewater. Biomass Bioenergy 2020, 139, 105628. [Google Scholar] [CrossRef]
- Oosterkamp, M.J.; Bauer, S.; Ibáñez, A.B.; Méndez-García, C.; Hong, P.-Y.; Cann, I.; Mackie, R.I. Identification of methanogenesis and syntrophy as important microbial metabolic processes for optimal thermophilic anaerobic digestion of energy cane thin stillage. Bioresour. Technol. Rep. 2019, 7, 100254. [Google Scholar] [CrossRef]
- Town, J.R.; Links, M.G.; Fonstad, T.A.; Dumonceaux, T.J. Molecular characterization of anaerobic digester microbial communities identifies microorganisms that correlate to reactor performance. Bioresour. Technol. 2014, 151, 249–257. [Google Scholar] [CrossRef]
- Wong, S.; Teng, T.; Ahmad, A.; Zuhairi, A.; Najafpour, G. Treatment of pulp and paper mill wastewater by polyacrylamide (PAM) in polymer induced flocculation. J. Hazard. Mater. 2006, 135, 378–388. [Google Scholar] [CrossRef]
- Thakur, I.S. Screening and identification of microbial strains for removal of colour and adsorbable organic halogens in pulp and paper mill effluent. Process Biochem. 2004, 39, 1693–1699. [Google Scholar] [CrossRef]
- Lacorte, S. Organic compounds in paper-mill process waters and effluents. TrAC Trends Anal. Chem. 2003, 22, 725–737. [Google Scholar] [CrossRef]
- Patt, R.; Kordsachia, O.; Süttinger, R.; Ohtani, Y.; Hoesch, J.F.; Ehrler, P.; Eichinger, R.; Holik, H.; Hamm, U.; Rohmann, M.E.; et al. Paper and Pulp. In Ullmann’s Encyclopedia of Industrial Chemistry; American Cancer Society: Atlanta, GA, USA, 2000; ISBN 978-3-527-30673-2. [Google Scholar]
- Jantharadej, K.; Mhuantong, W.; Limpiyakorn, T.; Mongkolsuk, S.; Sirikanchana, K.; Suwannasilp, B.B. Identification of sulfate-reducing and methanogenic microbial taxa in anaerobic bioreactors from industrial wastewater treatment plants using next-generation sequencing and gene clone library analyses. J. Environ. Sci. Health Part A 2020, 55, 1–11. [Google Scholar] [CrossRef]
- Roest, K.; Heilig, H.G.H.J.; Smidt, H.; de Vos, W.M.; Stams, A.J.M.; Akkermans, A.D.L. Community analysis of a full-scale anaerobic bioreactor treating paper mill wastewater. Syst. Appl. Microbiol. 2005, 28, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Nadais, M.H.G.A.G.; Capela, M.I.A.P.F.; Arroja, L.M.G.A.; Hung, Y.-T. Anaerobic Treatment of Milk Processing Wastewater. In Environmental Bioengineering; Wang, L.K., Tay, J.-H., Tay, S.T.L., Hung, Y.-T., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 555–627. ISBN 978-1-58829-493-7. [Google Scholar]
- Chartrain, M.; Zeikus, J.G. Microbial ecophysiology of whey biomethanation: Intermediary metabolism of lactose degradation in continuous culture. Appl. Environ. Microbiol. 1986, 51, 180–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsay, I.R.; Pullammanappallil, P.C. Protein degradation during anaerobic wastewater treatment: Derivation of stoichiometry. Biodegradation 2001, 12, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Suwannoppadol, S.; Ho, G.; Cord-Ruwisch, R. Overcoming sodium toxicity by utilizing grass leaves as co-substrate during the start-up of batch thermophilic anaerobic digestion. Bioresour. Technol. 2012, 125, 188–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAteer, P.G.; Christine Trego, A.; Thorn, C.; Mahony, T.; Abram, F.; O’Flaherty, V. Reactor configuration influences microbial community structure during high-rate, low-temperature anaerobic treatment of dairy wastewater. Bioresour. Technol. 2020, 307, 123221. [Google Scholar] [CrossRef]
- Singh, S.; Rinta-Kanto, J.M.; Kettunen, R.; Tolvanen, H.; Lens, P.; Collins, G.; Kokko, M.; Rintala, J. Anaerobic treatment of LCFA-containing synthetic dairy wastewater at 20 °C: Process performance and microbial community dynamics. Sci. Total Environ. 2019, 691, 960–968. [Google Scholar] [CrossRef]
- Shahbandeh, M. Global Vegetable Oil Consumption, 2019/20. Available online: https://www.statista.com/statistics/263937/vegetable-oils-global-consumption/ (accessed on 27 October 2020).
- Ahmed, Y.; Yaakob, Z.; Akhtar, P.; Sopian, K. Production of biogas and performance evaluation of existing treatment processes in palm oil mill effluent (POME). Renew. Sustain. Energy Rev. 2015, 42, 1260–1278. [Google Scholar] [CrossRef]
- Atallah, E.; Kwapinski, W.; Ahmad, M.N.; Leahy, J.J.; Zeaiter, J. Effect of water-sludge ratio and reaction time on the hydrothermal carbonization of olive oil mill wastewater treatment: Hydrochar characterization. J. Water Process Eng. 2019, 31, 100813. [Google Scholar] [CrossRef]
- Alves, M.M.; Pereira, M.A.; Sousa, D.Z.; Cavaleiro, A.J.; Picavet, M.; Smidt, H.; Stams, A.J.M. Waste lipids to energy: How to optimize methane production from long-chain fatty acids (LCFA). Microb. Biotechnol. 2009, 2, 538–550. [Google Scholar] [CrossRef] [Green Version]
- O-Thong, S.; Boe, K.; Angelidaki, I. Thermophilic anaerobic co-digestion of oil palm empty fruit bunches with palm oil mill effluent for efficient biogas production. Appl. Energy 2012, 93, 648–654. [Google Scholar] [CrossRef]
- Doula, M.K.; Moreno-Ortego, J.L.; Tinivella, F.; Inglezakis, V.J.; Sarris, A.; Komnitsas, K. Chapter 2—Olive mill waste: Recent advances for the sustainable development of olive oil industry. In Olive Mill Waste; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 29–56. ISBN 978-0-12-805314-0. [Google Scholar]
- Miller, T.L. Methanosphaera. In Bergey’s Manual of Systematics of Archaea and Bacteria; American Cancer Society: Atlanta, GA, USA, 2015; pp. 1–6. ISBN 978-1-118-96060-8. [Google Scholar]
- McIlroy, S.J.; Kirkegaard, R.H.; McIlroy, B.; Nierychlo, M.; Kristensen, J.M.; Karst, S.M.; Albertsen, M.; Nielsen, P.H. MiDAS 2.0: An ecosystem-specific taxonomy and online database for the organisms of wastewater treatment systems expanded for anaerobic digester groups. Database (Oxford) 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zuo, J.; Ji, R.; Chen, X.; Liu, F.; Wang, K.; Yang, Y. Methanogenic community dynamics in anaerobic co-digestion of fruit and vegetable waste and food waste. J. Environ. Sci. 2012, 24, 1288–1294. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.; Liu, X.; Ma, H.; Wu, J.; Zuo, J.; Wang, K. A new method of two-phase anaerobic digestion for fruit and vegetable waste treatment. Bioresour. Technol. 2016, 211, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Nakasaki, K.; Kwon, S.H.; Ikeda, H. Identification of microorganisms in the granules generated during methane fermentation of the syrup wastewater produced while canning fruit. Process Biochem. 2013, 48, 912–919. [Google Scholar] [CrossRef]
- USDA Citrus: World Markets and Trade. Available online: https://apps.fas.usda.gov/psdonline/circulars/citrus.pdf (accessed on 27 October 2020).
- Corsino, S.F.; Di Trapani, D.; Torregrossa, M.; Viviani, G. Aerobic granular sludge treating high strength citrus wastewater: Analysis of pH and organic loading rate effect on kinetics, performance and stability. J. Environ. Manag. 2018, 214, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Mendoza, E.S.; Méndez-Contreras, J.M.; Martínez-Sibaja, A.; Vallejo-Cantú, N.A.; Alvarado-Lassman, A. Correction to: Anaerobic digestion of citrus industry effluents using an Anaerobic Hybrid Reactor. Clean Technol. Environ. Policy 2018, 20, 1399. [Google Scholar] [CrossRef] [Green Version]
- Nasr, F.A.; Doma, H.S.; Abdel-Halim, H.S.; El-Shafai, S.A. Chemical industry wastewater treatment. Environmentalist 2007, 27, 275–286. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Chen, T.; Li, Y.-Y.; Chui, H.-K. Degradation of phenol in wastewater in an upflow anaerobic sludge blanket reactor. Water Res. 1996, 30, 1353–1360. [Google Scholar] [CrossRef]
- Muñoz Sierra, J.D.; Oosterkamp, M.J.; Wang, W.; Spanjers, H.; van Lier, J.B. Comparative performance of upflow anaerobic sludge blanket reactor and anaerobic membrane bioreactor treating phenolic wastewater: Overcoming high salinity. Chem. Eng. J. 2019, 366, 480–490. [Google Scholar] [CrossRef]
- Yang, Y.; Tsukahara, K.; Sawayama, S. Biodegradation and methane production from glycerol-containing synthetic wastes with fixed-bed bioreactor under mesophilic and thermophilic anaerobic conditions. Process Biochem. 2008, 43, 362–367. [Google Scholar] [CrossRef]
- Vasconcelos, E.A.F.; Santaella, S.T.; Viana, M.B.; dos Santos, A.B.; Pinheiro, G.C.; Leitão, R.C. Composition and ecology of bacterial and archaeal communities in anaerobic reactor fed with residual glycerol. Anaerobe 2019, 59, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Niu, Q.; Li, L.; Hu, Y.; Mribet, C.; Hojo, T.; Li, Y.-Y. A gradual change between methanogenesis and sulfidogenesis during a long-term UASB treatment of sulfate-rich chemical wastewater. Sci. Total Environ. 2018, 636, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, Y.; Xiao, W.; Zhou, Z.; Yan, X. Performance and spatial community succession of an anaerobic baffled reactor treating acetone–butanol–ethanol fermentation wastewater. Bioresour. Technol. 2011, 102, 7407–7414. [Google Scholar] [CrossRef]
- Platen, H.; Schink, B. Methanogenic degradation of acetone by an enrichment culture. Arch. Microbiol. 1987, 149, 136–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enright, A.-M.; Collins, G.; O’Flaherty, V. Temporal microbial diversity changes in solvent-degrading anaerobic granular sludge from low-temperature (15 °C) wastewater treatment bioreactors. Syst. Appl. Microbiol. 2007, 30, 471–482. [Google Scholar] [CrossRef]
- Danshita, T.; Miyaoka, Y.; Sumino, H.; Iguchi, A.; Yamaguchi, T.; Syutsubo, K. Evaluation of process performance and retained sludge properties of a psychrophilic UASB reactor for treatment of iso-plophyl alcohol (2-propanol)-containing wastewater. J. Environ. Sci. Health Part A 2018, 53, 1177–1184. [Google Scholar] [CrossRef]





| Category | Common Features | Pollutants | Typical Industrial Sectors |
|---|---|---|---|
| Minimal contamination (can be land spread) | Wastewater contains no pollutants, nutrients can be useful for agricultural plants development, levels of toxic substances is very low | Nitrogen, phosphorus | Food and drink |
| Equivalent to domestic-type effluents | Organic pollutants similar content as in municipal wastewater | Degradable organic matter | Food and drink |
| Low flow and non-domestic type pollutants at low concentrations | Wastewater containing small concentrations of other pollutants not present in urban effluents | Pesticides, hormones, nano-plastics and endocrine disrupters | Chemicals |
| Metals | Wastewater containing metals or metalloids from industry | Metals | Metal processing and mineral industry |
| High nutrient loading | Wastewater containing high concentrations of nitrogen compounds, phosphates, with higher conductivity | Substances increasing eutrophication | Chemicals: fertilizers |
| Effluent streams requiring pH adjustment | Wastewater streams with very low or very high pH | Acids or alkalis | Chemicals and mineral industry |
| Persistent organics content | Wastewater containing not easily degradable organic pollutants (persistent hydrocarbons or bioaccumulative organic toxic substances | Persistent organics | Textiles and chemicals |
| Emerging substances | Wastewater contains new pollutants or has characteristics that are not currently monitored | New parameters or compounds not frequently measured | Pharmaceuticals |
| Acetaldehyde | Crotonic acid | Isobutyric acid | Isopropyl alcohol |
| Acetic anhydride | Diacetone gulusonic acid | Isopropanol | Propionate |
| Acetone | Dimethoxy benzoic acid | Lactic acid | Propylene glycol |
| Acrylic acid | Ethanol | Maleic acid | Protocatechuic acid |
| Adipic acid | Ethyl acetate | Methyl acetate | Resorcinol |
| Aniline | Ethyl acrylate | Methyl acrylate | Sec-butanol |
| 1-amino-2-propanol | Ferulic acid | Methyl ethyl ketone | Sec-butylamine |
| 4-amino butyric acid | Formaldehyde | Methyl formate | Sorbic acid |
| Benzoic acid | Formic acid | Nitrobenzene | Syringaldehyde |
| Butanol | Fumaric acid | Pentaerythritol | Syringic acid |
| Butyraldehyde | Glutamic acid | Pentanol | Succinic acid |
| Butylene glycerol | Glutaric acid | Phenol | Tert-butanol |
| Catechol | Glycerol | Phthalic acid | Vanillic acid |
| Cresol | Hexanoic acid | Propanal | Vinyl acetate |
| Crotonaldehyde | Hydroquinone | Propanol |
| Agriculture wastes | Corn processing wastes | Chemical industry wastes | Seafood and shellfish wastes |
| Alcohol stillage | Dairy wastes | Meat packing wastes | Slaughterhouse and meat packing |
| Animal wastes | Egg processing wastes | Pear wastes | Sugar processing wastes |
| Bagasse | Fruit Leachate | Peat wastes | Tannery wastes |
| Bean blanching water | Giant kelp wastes | Pectin wastes | Vegetable processing wastes |
| Beverage production wastes | Guar gum wastes | Petroleum wastes | Wheat and grain processing wastes |
| Brewery wastes | H2-CO pyrolysis wastes | Pharmaceutical | Wine processing wastes |
| Canning wastes | Heat-treated activated sludge | Potato processing wastes | Wood processing wastes |
| Coking mill wastes | Cheese processing wastes | Pulp and paper wastes | Wool scouring wastes |
| Wastewater Type | COD | BOD | TS | SS | VSS | TN | TP | N-NH4+ | pH | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Slaughterhouse | 2000–11,588 | 1300–4635 (BOD5) | 6394 | 850–6300 | 660–5250 | 850 | 15–48 | 20–66 | 6.3–6.98 | [13,14] |
| Poultry slaughterhouse | 2790–5520 | 1558–2988 | - | - | - | 62–313 (KN) | - | 16–95 | 6.8–7.8 | [15] |
| Poultry processing | 1140 | 570 (BOD5) | - | 264 | - | - | - | 2.7 | - | [16] |
| Meat processing | 5160 | - | 2028 | 1820 | 1380 | - | - | - | 7.5 | [17] |
| Livestock breeding | 6190–78,600 | 3940–34,600 | - | 1850–29,000 | - | 1530–6500 | 116–1770 | - | - | [18] |
| Dairy industry | - | 10,000–50,000 | - | 220–340 | 200–300 | 188 | 100 | 18 | 9–10.5 | [19] |
| Milk plant | 2000–6000 | 1200–4000 (BOD5) | - | 350–1000 | 330–940 | 50–60 (KN) | - | - | 8.0–11.0 | [20] |
| Butter production | 52,000 | - | - | 1500 | - | 1120 (KN) | - | - | 4.3–5.9 | [20,21] |
| Brewery | 2000–6000 | 1200–3600 | 5100–8750 | 2901–3000 | - | 25–80 (KN) | 10.0–50.0 | - | 3.0–12.0 | [22] |
| Raw distillery wastewater | 80,000–120,000 | 45,000–60,000 | 100,000 | 10,000 | 100–2800 | 100–64,000 | 240–65,000 | - | 3.5–5.2 | [23,24] |
| Sugar factory | 572–6612 | - | 3840–5780 | 30–170 | 560–6470 | - | 2.0–4.0 | 3.7–10.1 | 4.7–5.2 | [25] |
| Cellulose Processing | 600–10,400 | 221–3700 | - | 20–3200 | - | - | - | - | 6.3–9.0 | [26,27,28] |
| Fruits and Vegetables Processing | 1500–4300 | 500–2500 | 400–1200 | 6–10 | [29] | |||||
| Vegetable oil mills | 1355–1987 | 712–1136 (BOD5) | - | - | - | - | - | 3.6–14.4 | 0.9–2.3 | [30] |
| Olive oil mills | 57,200 | - | 49,100 | - | - | 1600 | 300 | - | 4.9 | [31] |
| Refining of vegetable oils | 17,688–24,787 | 4120–4560 (BOD5) | - | 791–3544 | - | <10 | - | <10 | 10.0–10.4 | [32] |
| Blackberry processing | 930 | - | 840 | - | - | 92 | - | - | 5.9 | [33] |
| Dates processing | 410 | - | 471 | - | - | 37 | - | - | 6.1 | [33] |
| Tomato processing | 294 | - | 322 | - | - | 21 | - | - | 5.3 | [33] |
| Beetroot processing | 501 | - | 630 | - | - | 43 | - | - | 6.2 | [33] |
| Butadiene and styrene | 800–1500 | 4000–8000 (BOD5) | - | 200–500 | - | - | - | - | - | [34] |
| Acrylates | 2000–3200 | 1000–2000 (BOD5) | - | 50–100 | - | - | - | - | - | [34] |
| Acetaldehyde | 40,000–60,000 | 15,000–25,000 (BOD5) | - | 150–300 | - | - | - | - | - | [34] |
| Ketones | 20,000–40,000 | 10,000–20,000 (BOD5) | - | 50–100 | - | - | - | - | - | [34] |
| Methyl acrylate acid | 7000–12,000 | - | - | 6000–12,000 | - | - | - | - | - | [34] |
| Organic acids | 5000–15,000 | 300–600 (BOD5) | - | 100–200 | - | - | - | - | - | [34] |
| Raw materials for the pigment industry | 1000–2000 | 200–400 | - | 80–200 | - | - | - | - | - | [34] |
| Aerobic Treatment | Anaerobic Treatment | |
|---|---|---|
| Transformation of input substrate | 50% microbial biomass 50% CO2 | 5% microbial biomass 95% biogas |
| Energy balance | 60% microbial biomass 40% reaction heat | 90% biogas 5–7% microbial biomass 3–5% reaction heat |
| Process | Inflow COD | Hydraulic Retention Time | Organic Loading Rate | COD Removal Efficiency |
|---|---|---|---|---|
| (mg/L) | (h) | (kgCOD/m3 per day) | (%) | |
| Anaerobic lagoons | N.A. | 24–1200 | 0.04–1 | 30–50 |
| Anaerobic contact process | 1500–5000 | 2–14 | 0.5–5.3 | 75–90 |
| Fixed Bed reactor | 10,000–70,000 | 24–48 | 1–15 | 75–85 |
| Upflow Anaerobic Sludge Blanket (UASB) reactor | 5000–90,000 | 4–12 | 4–12 | 75–85 |
| Expanded Granular Sludge Bed (EGSB) reactor | 1000–90,000 | 5–10 | 5–30 | 80–85 |
| Internal Circulation (IC) reactor | 5000–90,000 | 3–25 | 5–40 | 80–87 |
| Genera of Methanogens | Methanogenic Metabolic Pathway | Industry | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Slaughterhause | Brewery | Distillery | Paper | Dairy | Vegetable Oil | Fruit and Vegetable | Chemical | ||
| Methanothrix | acetoclastic | ● | ● | ● | ● | ● | ● | ● | ● |
| Methanosarcina | acetoclastic hydrogenotrophic methylotrophic | ● | ● | ● | ● | ● | ● | ● | |
| Methanomicrobium | hydrogenotrophic | ● | ● | ||||||
| Methanobrevibacter | hydrogenotrophic | ● | ● | ● | |||||
| Methanocalculus | hydrogenotrophic | ● | |||||||
| Methanoculleus | hydrogenotrophic | ● | ● | ● | ● | ● | |||
| Methanofollis | hydrogenotrophic | ● | ● | ||||||
| Methanobacterium | hydrogenotrophic | ● | ● | ● | ● | ● | |||
| Methanoregula | hydrogenotrophic | ● | ● | ||||||
| Methanococcus | hydrogenotrophic | ● | |||||||
| Methanospirillum | hydrogenotrophic | ● | ● | ● | ● | ||||
| Methanocorpuscullum | hydrogenotrophic | ● | ● | ||||||
| Methanogenium | hydrogenotrophic | ● | |||||||
| Methanimicrococcus | methylotrophic | ● | |||||||
| Methanosphaera | hydrogenotrophic | ● | ● | ||||||
| Methanothermobacter | hydrogenotrophic | ● | |||||||
| Methanolinea | hydrogenotrophic | ● | ● | ||||||
| Methanomethylovorans | methylotrophic | ● | |||||||
| Methanomassiliicoccus | methanol + H2 | ● | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vítězová, M.; Kohoutová, A.; Vítěz, T.; Hanišáková, N.; Kushkevych, I. Methanogenic Microorganisms in Industrial Wastewater Anaerobic Treatment. Processes 2020, 8, 1546. https://doi.org/10.3390/pr8121546
Vítězová M, Kohoutová A, Vítěz T, Hanišáková N, Kushkevych I. Methanogenic Microorganisms in Industrial Wastewater Anaerobic Treatment. Processes. 2020; 8(12):1546. https://doi.org/10.3390/pr8121546
Chicago/Turabian StyleVítězová, Monika, Anna Kohoutová, Tomáš Vítěz, Nikola Hanišáková, and Ivan Kushkevych. 2020. "Methanogenic Microorganisms in Industrial Wastewater Anaerobic Treatment" Processes 8, no. 12: 1546. https://doi.org/10.3390/pr8121546
APA StyleVítězová, M., Kohoutová, A., Vítěz, T., Hanišáková, N., & Kushkevych, I. (2020). Methanogenic Microorganisms in Industrial Wastewater Anaerobic Treatment. Processes, 8(12), 1546. https://doi.org/10.3390/pr8121546






