Comparative Technical Process and Product Assessment of Catalytic and Thermal Pyrolysis of Lignocellulosic Biomass
Abstract
:1. Introduction
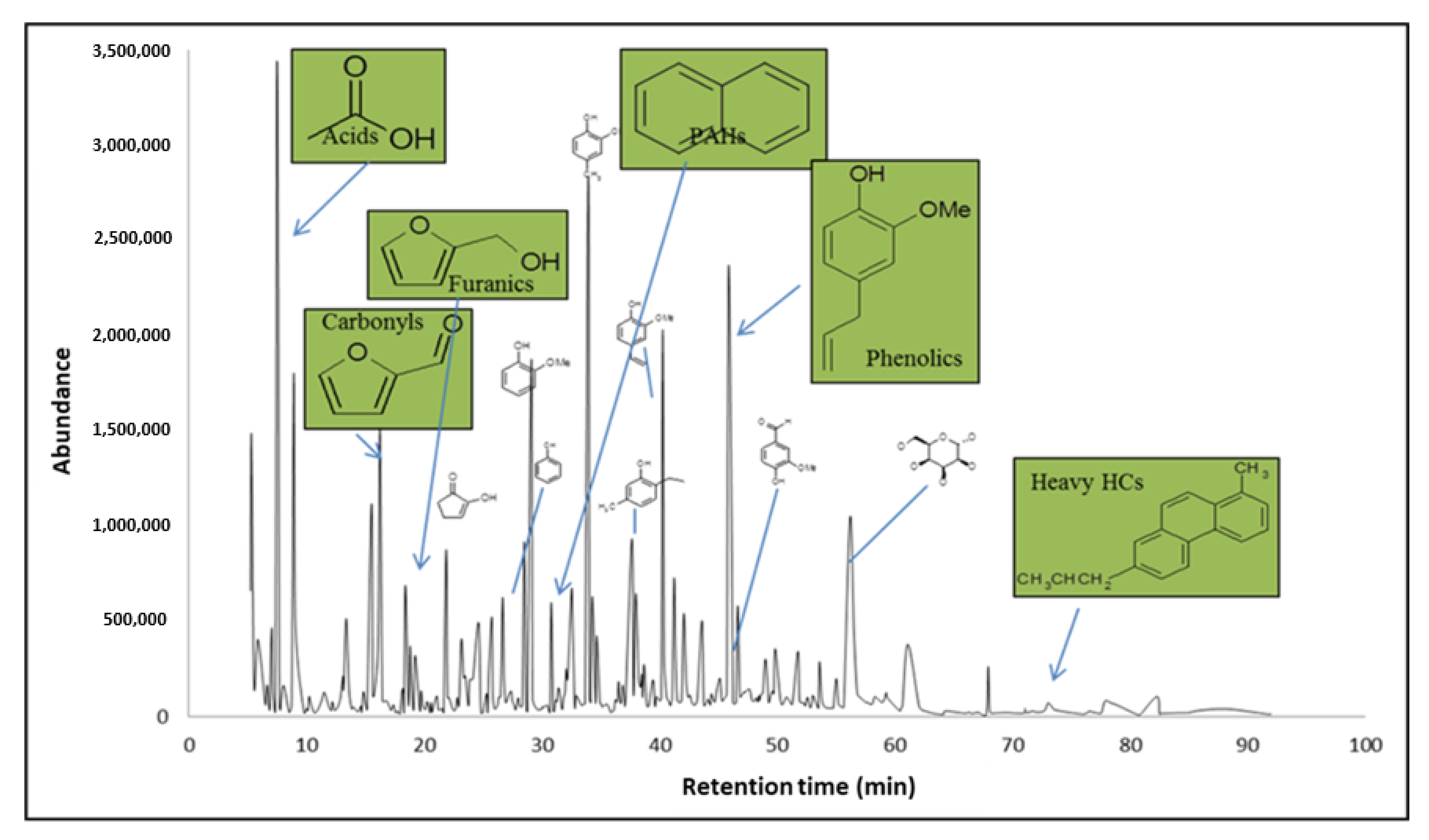
2. Laboratory Experiments
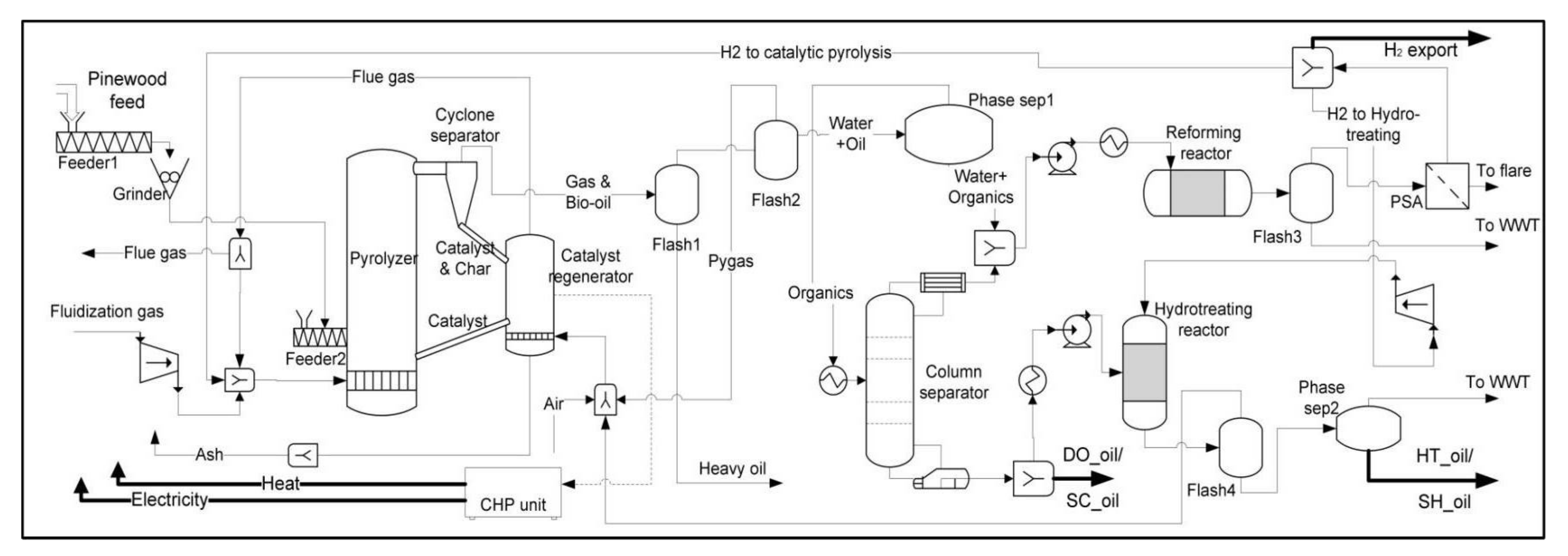
3. Process Model
3.1. Feedstock and Pretreatment
3.2. Pyrolysis
3.3. Combined Heat and Power (CHP) Section
3.4. Separation
3.5. Hydrogen Production
3.6. Hydrotreating Section
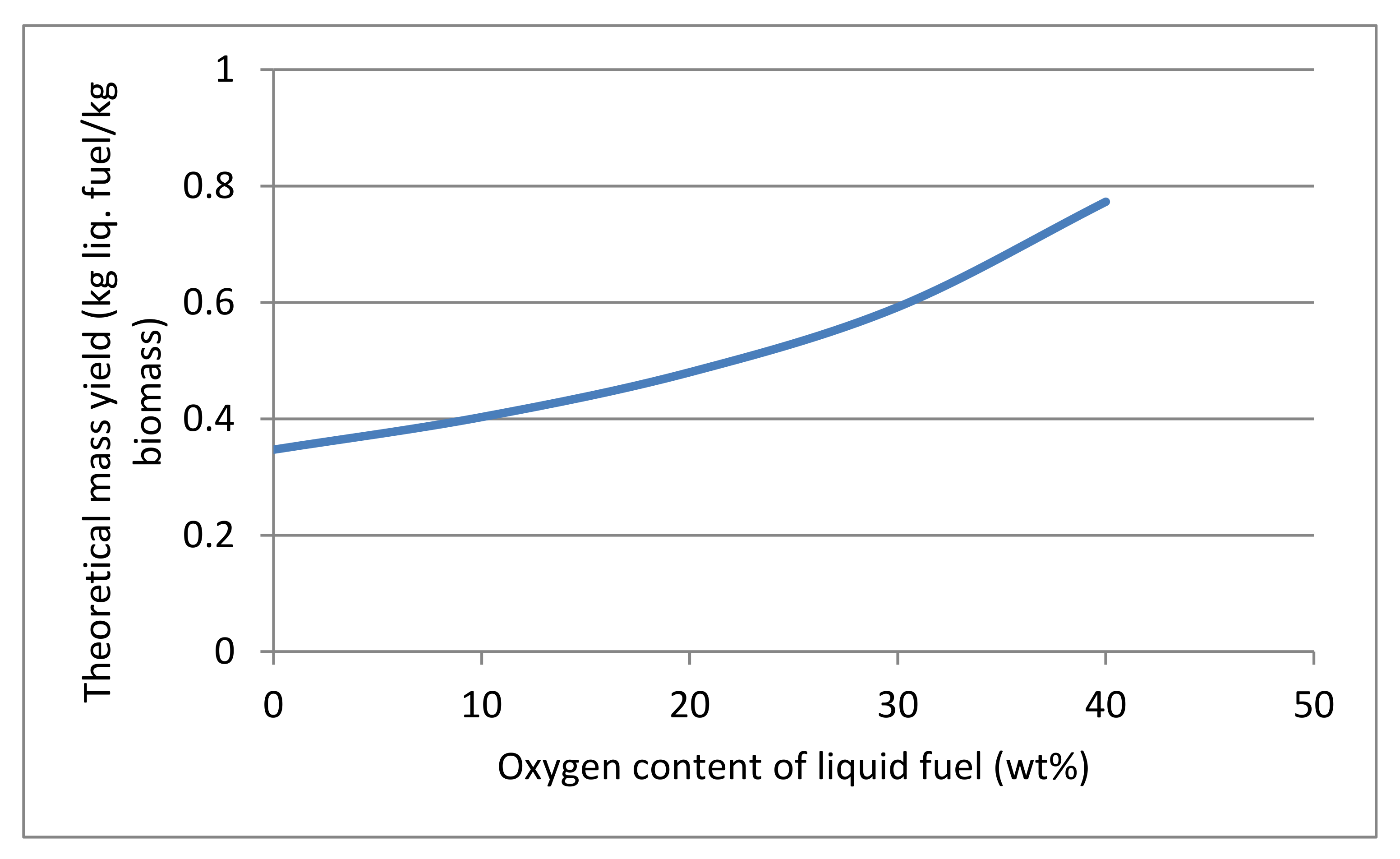
3.7. Fuel Specifications and Properties
3.8. Product Scenarios
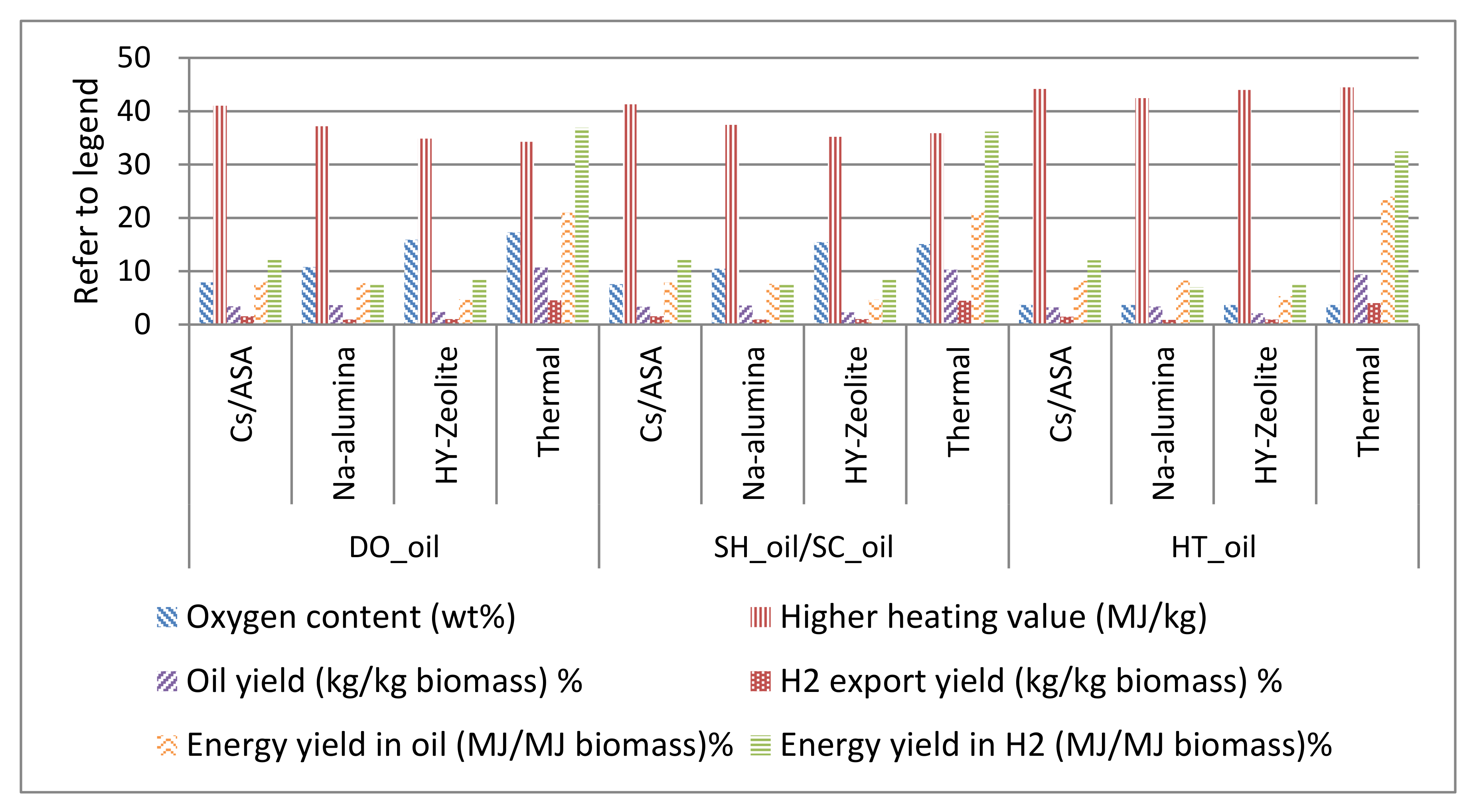
3.8.1. Direct Oil (DO_Oil)
3.8.2. Hydrotreated Oil (HT_Oil)
3.8.3. Selectively Hydrotreated Oil (SH_Oil)
3.8.4. Selectively Catalyzed Oil (SC_Oil)
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- International Energy Agency. World Energy Outlook 2012. Available online: https://www.iea.org/reports/world-energy-outlook-2012 (accessed on 3 December 2020).
- Lamers, P.; Hamelinck, C.; Junginger, M.; Faaij, A. International bioenergy trade—A review of past developments in the liquid biofuel market. Renew. Sustain. Energy Rev. 2011, 15, 2655–2676. [Google Scholar] [CrossRef]
- Perlack, R.D.; Stokes, B.J. US Billion-Ton Update: Biomass Supply for a Bioenergy and Bioproducts Industry; ORNL/TM-2011/224; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2011. [Google Scholar]
- Eisentraut, A. Sustainable Production of Second Generation Biofuels Potential and Perspectives in Major Economies and Developing Countries; IEA Energy Papers 2010/1; OECD Publishing: Paris, France, 2010. [Google Scholar]
- Anex, R.P.; Aden, A.; Kazi, F.K.; Fortman, J.; Swanson, R.M.; Wright, M.M.; Satrio, J.A.; Brown, R.C.; Daugaard, D.E.; Platon, A.; et al. Techno-economic comparison of biomass-to-transportation fuels via pyrolysis, gasification, and biochemical pathways. Fuel 2010, 89 (Suppl. 1), S29–S35. [Google Scholar] [CrossRef]
- Meerman, J.C.; Ramírez, A.; Turkenburg, W.C.; Faaij, A.P.C. Performance of simulated flexible integrated gasification polygeneration facilities. Part A: A technical-energetic assessment. Renew. Sustain. Energy Rev. 2011, 15, 2563–2587. [Google Scholar] [CrossRef]
- Humbrid, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol; NREL/TP-5100-47764; NREL: Golden, CO, USA, 2011. [Google Scholar]
- Wright, M.M.; Daugaard, D.E.; Satrio, J.A.; Brown, R.C. Techno-economic analysis of biomass fast pyrolysis to transportation fuels. Fuel 2010, 89 (Suppl. 1), S2–S10. [Google Scholar] [CrossRef] [Green Version]
- Lane, J. Biofuels Digest. Available online: https://www.biofuelsdigest.com/bdigest/author/jmldigest/ (accessed on 3 December 2020).
- Meerman, J.C.; Ramírez, A.; Turkenburg, W.C.; Faaij, A.P.C. Performance of simulated flexible integrated gasification polygeneration facilities, Part B: Economic evaluation. Renew. Sustain. Energy Rev. 2012, 16, 6083–6102. [Google Scholar] [CrossRef]
- Ringer, M.; Putsche, V.; Scahill, J. Large-Scale Pyrolysis Oil Production: A Technology Assessment and Economic Analysis; NREL/TP-510-37779; National Renewable Energy Laboratory: Golden, CO, USA, 2006. [Google Scholar]
- Kersten, S.R.A.; van Swaaij, W.P.M.; Lefferts, L.; Seshan, K. Options for Catalysis in the Thermochemical Conversion of Biomass into Fuels. In Catalysis for Renewables; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 119–145. [Google Scholar]
- Bridgwater, A.V. Biomass fast pyrolysis. Therm. Sci. 2004, 8, 21. [Google Scholar] [CrossRef]
- Zabeti, M.; Nguyen, T.S.; Lefferts, L.; Heeres, H.J.; Seshan, K. In situ catalytic pyrolysis of lignocellulose using alkali-modified amorphous silica alumina. Bioresour. Technol. 2012, 118, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.S.; Zabeti, M.; Lefferts, L.; Brem, G.; Seshan, K. Catalytic upgrading of biomass pyrolysis vapours using faujasite zeolite catalysts. Biomass Bioenergy 2013, 48, 100. [Google Scholar] [CrossRef]
- Nguyen, T.S.; Zabeti, M.; Lefferts, L.; Brem, G.; Seshan, K. Conversion of lignocellulosic biomass to green fuel oil over sodium based catalysts. Bioresour. Technol. 2013, 142, 353–360. [Google Scholar] [CrossRef]
- KiOR. KiOR Ships First Cellulosic Diesel: World’s First Renewable Diesel En Route to American Vehicles; KiOR: Pasadena, TX, USA, 2013. [Google Scholar]
- Bulushev, D.A.; Ross, J.R.H. Catalysis for conversion of biomass to fuels via pyrolysis and gasification: A review. Catal. Today 2011, 171, 1–13. [Google Scholar] [CrossRef]
- Carlson, T.R.; Tompsett, G.A.; Conner, W.C.; Huber, G.W. Aromatic production from catalytic fast pyrolysis of biomass-derived feedstocks. Top. Catal. 2009, 52, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Ateay, F.; Miskolczi, N.; Borsodi, N. Comparision of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part I: Product yields, gas and pyrolysis oil properties. Bioresour. Technol. 2013, 133, 443–454. [Google Scholar]
- Pattiya, A.; Titiloye, J.O.; Bridgwater, A.V. Fast pyrolysis of cassava rhizome in the presence of catalysts. J. Anal. Appl. Pyrolysis 2008, 81, 72–79. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babich, I.V.; van der Hulst, M.; Lefferts, L.; Moulijn, J.A.; OConnor, P.; Seshan, K. Catalytic pyrolysis of microalgae to high-quality liquid bio-fuels. Biomass Bioenergy 2011, 35, 3199–3207. [Google Scholar] [CrossRef]
- Jones, S.B.; Valkenburg, C.; Walton, C.W.; Elliot, D.; Holladay, J.E.; Stevens, D.J.; Kinchin, C.; Czernik, S. Production of Gasoline and Diesel from Biomass Via Fast Pyrolysis, Hydrotreating and Hydrocracking: A Design Case; PNNL-18284; Pacific Northwest National Lab.(PNNL): Richland, WA, USA, 2009. [Google Scholar]
- Li, B.; Ou, L.; Dang, Q.; Meyer, P.; Jones, S.; Brown, R.; Wright, M. Techno-economic and uncertainty analysis of in situ and ex situ fast pyrolysis for biofuel production. Bioresour. Technol. 2015, 196, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Collard, F.; Blin, J.; Bensakhria, A.; Valette, J. Influence of impregnated metal on the pyrolysis conversion of biomass constituents. J. Anal. Appl. Pyrolysis 2012, 95, 213–226. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Liang, T.; Zhou, Y.; Luo, Z. Mechanism research on cellulose pyrolysis by Py-GC/MS and subsequent density functional theory studies. Bioresour. Technol. 2012, 104, 722–728. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, X.; Dong, C.; Zhang, Z.; Zhang, X.; Zhu, X. Influence of pyrolysis temperature and time on the cellulose fast pyrolysis products: Analytical Py-GC/MS study. J. Anal. Appl. Pyrolysis 2011, 92, 430–438. [Google Scholar] [CrossRef]
- Phyllis2: Database for Biomass and Waste; Energy Research Centre of the Netherlands: Patten, The Netherlands, 5 February 2013. Available online: https://phyllis.nl/ (accessed on 3 December 2020).
- O’Connor, P.; Cerqueira, H.S.; Bartek, R.; Yanik, S. Biomass Catalytic Conversion Process and Apparatus for Use Therein. U.S. Patent 8524959, 3 September 2013. [Google Scholar]
- Patel, M.K.; Crank, M.; Dornburg, V.; Hermann, B.; Roes, L.; Hüsing, B.; Overbeek, L.; Terragni, F.; Recchia, E. Medium and Long-Term Opportunities and Risks of the Biotechnological Production of Bulk Chemicals from Renewable Resources; UU CHEM NW&S (Copernicus): Uthrecht, The Netherlands, 2006. [Google Scholar]
- Oasmaa, A.; Czernik, S. Fuel oil quality of biomass pyrolysis oils: State of the art for the end users. Energy Fuels 1999, 13, 914–921. [Google Scholar] [CrossRef]
- Vispute, T.P.; Huber, G.W. Production of hydrogen, alkanes and polyols by aqueous phase processing of wood-derived pyrolysis oils. Green Chem. 2009, 11, 1433–1445. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Nie, J.; Ren, M.; Guo, Q. Effective Phase Separation of Biomass Pyrolysis Oils by Adding Aqueous Salt Solutions. Energy Fuels 2009, 23, 3307–3312. [Google Scholar] [CrossRef]
- Miller, M.M.; Wasik, S.P.; Huang, G.L.; Shiu, W.Y.; Mackay, D. Relationships between octanol-water partition coefficient and aqueous solubility. Environ. Sci. Technol. 1985, 19, 522. [Google Scholar] [CrossRef] [PubMed]
- Meylan, W.M.; Howard, P.H.; Boethling, R.S. Improved method for estimating water solubility from octanol/water partition coefficient. Environ. Toxicol. Chem. 1996, 15, 100. [Google Scholar] [CrossRef]
- De Vlieger, D.J.M.; Thakur, D.B.; Lefferts, L.; Seshan, K. Carbon nanotubes: A promising catalyst support material for supercritical water gasification of biomass waste. ChemCatChem 2012, 4, 2068–2074. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Neuenschwander, G.G.; Rotness, L.J.; Zacher, A.H. Catalytic hydroprocessing of biomass fast pyrolysis bio-oil to produce hydrocarbon products. Environ. Prog. Sustain. Energy 2009, 28, 441. [Google Scholar] [CrossRef]
- Zacher, A.H.; Olarte, M.V.; Santosa, D.M.; Elliott, D.C.; Jones, S.B. A review and perspective of recent bio-oil hydrotreating research. Green Chem. 2013, 16, 885–896. [Google Scholar] [CrossRef]
- European Union. Directive 2009/30/EC of the European Parliament and of the Councilof 23 April 2009, amending Directives 98/70/EC and 1999/32/EC and repealing Directive 93/12/EEC 2009, Brussels, Belgium. 5 June 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32009L0030 (accessed on 3 December 2020).
- Martinez, J.D.; Lapuerta, M.; Garcia-Contreras, R.; Murillo, R.; Garcia, T. Fuel Properties of Tire Pyrolysis Liquid and its Blends with Diesel Fuel. Energy Fuel. 2013, 27, 3296–3305. [Google Scholar] [CrossRef]
- Marker, T.; Felix, L.; Linck, M.; Roberts, M.; Ortiz-Toral, P. Integrated hydropyrolysis and hydroconversion (IH2®) for the direct production of gasoline and diesel fuels or blending components from biomass, Part 2: Continuous testing. Environ. Prog. Sustain. Energy 2013, 33, 762–768. [Google Scholar] [CrossRef]
- Eschenbacher, A.; Saraeian, A.; Shanks, B.; Jensen, P.; Li, C.; Duus, J.; Hansen, A.B.; Mentzel, U.V.; Henriksen, U.B.; Ahrenfeldt, J.; et al. Enhancing bio-oil quality and energy recovery by atmospheric hydrodeoxygenation of wheat straw pyrolysis vapors using Pt and Mo-based catalysts. Sustain. Energy Fuels 2020, 4, 1991–2008. [Google Scholar] [CrossRef]
- Triantafyllidis, K.S.; Iliopoulou, E.F.; Antonakou, E.V.; Lappas, A.A.; Wang, H.; Pinnavaia, T.J. Hydrothermally stable mesoporous aluminosilicates (MSU-S) assembled from zeolite seeds as catalysts for biomass pyrolysis. Microporous Mesoporous Mater. 2007, 99, 132–139. [Google Scholar] [CrossRef]
- Ebbesen, S.D.; Mojet, B.L.; Lefferts, L. Effect of pH on the nitrite hydrogenation mechanism over Pd/Al2O3 and Pt/Al2O3: Details obtained with ATR-IR spectroscopy. J. Phys. Chem. C 2010, 115, 1186–1194. [Google Scholar] [CrossRef]
- Hong, X.; Li, B.; Wang, Y.; Lu, J.; Hu, G.; Luo, M. Stable Ir/SiO2 catalyst for selective hydrogenation of crotonaldehyde. Appl. Surf. Sci. 2013, 270, 388–394. [Google Scholar] [CrossRef]
| Technology | Fuel Type | Feasible Processing Scale and Infrastructure | Feedstock Logistics | Fuel Logistics | Energy Yield in Fuel |
|---|---|---|---|---|---|
| Biochemical | Ethanol, Butanol— Blendable with gasoline to a certain extent | Medium- to large-scale process and new infrastructure | Transportation of solid biomass over long distances | Blendable fuel—Needs partly new transport infrastructure | Low |
| Gasification | Alkane chains produced via Fischer Tropsch— Direct use in existing engines. | Very large-scale process and new infrastructure | Transportation of solid biomass over long distances | Drop-in fuel—Existing transport infrastructure can be used | High |
| Pyrolysis | Mix of oxygen containing organics— Can be blended after treatment | Medium-scale process Existing oil processing infrastructure can be used | Transportation of solid biomass over shorter distances due to smaller processing capacity | Blendable or drop in after treatment—Existing infrastructure can be partially used | Medium |
| Characteristics. | Thermal Pyrolysis Bio-Oil | Fossil Fuel Oil |
|---|---|---|
| Water content (wt.%) | 15–35 | 0.1 |
| H (wt.%) | 5.2–7.0 | 85.0 |
| C (wt.%) | 50–64 | 11.1 |
| O (wt.%) | 35–40 | 1.0 |
| N (wt.%) | 0.05–0.40 | 0.3 |
| S (wt.%) | 0.05–0.30 | 2.3 |
| Heating value (MJ·kg−1) | 16–19 | 40.0 |
| Viscosity (cP at 50 °C) | 40–150 | 180.0 |
| pH | 2.4 | - |
| Waste Pinewood Properties | |
|---|---|
| Moisture content (wt.% as received) | 3.00 |
| Volatile matter (wt.% dry) | 79.38 |
| Fixed carbon (wt.% dry) | 11.34 |
| Ash (wt.% dry) | 9.28 |
| C (wt.% dry ash free) | 50.15 |
| H (wt.% dry ash free) | 5.41 |
| O (wt.% dry ash free) | 44.37 |
| N (wt.% dry ash free) | 0.06 |
| S (wt.% dry ash free) | 0.01 |
| Cl (wt.% dry ash free) | 0.00 |
| Heating value (HHV) (MJ/kg as received) | 17.50 |
| Average diameter (mm) | 10.00 |
| CHP Parameters | Value |
|---|---|
| Overall efficiency | 72.0% |
| Electricity factor | 0.613 |
| Losses for maintenance (heat/steam prod.) | 7.5% |
| Upper Limit | |
|---|---|
| Oxygen content | 3.70 wt.% |
| Benzene | 1.10 wt.% |
| Aromatics | 35.00 v.% |
| Olefins | 18.00 v.% |
| Gasoline | Diesel | Fuel Oil | |
|---|---|---|---|
| Carbon content (wt.%) | 87 | 86 | 85.3 |
| Hydrogen content (wt.%) | 13 | 14 | 11.5 |
| Oxygen content (wt.%) | 0 | 0 | 1 |
| H/C molar ratio | 1.79 | 1.95 | 1.62 |
| Higher heating value (MJ/kg) | 47.30 | 44.80 | 40 |
| Average molecular weight (g) | 114.23 (Octane) | 169.83 (Hexadecane) | 422 (based on C30 alkane) (C7 to >C50) |
| Average boiling point (°C) | 38–204 | >150 | 121–600 |
| Acid-sugar-carbonyl content (wt.%) | 0 | 0 | 0 |
| Pyrolysis Yields (without Further Treatment) | Cs/ASA | Na/γAl2O3 | HY-Zeolite | Thermal |
|---|---|---|---|---|
| Organics (wt.%) | 15.79 | 12.48 | 10.30 | 44.09 |
| Water (H2O) (wt.%) | 32.06 | 29.12 | 33.04 | 21.51 |
| Char + Coke (wt.%) | 18.55 | 25.13 | 28.44 | 11.43 |
| Gases (wt.%) | 24.61 | 24.27 | 19.22 | 13.98 |
| Ash (wt.%) | 9.00 | 9.00 | 9.00 | 9.00 |
| Organic fraction properties | ||||
| Carbon content (wt.%) | 65.45 | 69.65 | 57.75 | 52.03 |
| Hydrogen content (wt.%) | 5.35 | 5.27 | 5.06 | 5.69 |
| Oxygen content (wt.%) | 29.20 | 25.08 | 37.19 | 42.28 |
| Heating value (MJ/kg) | 24.92 | 26.98 | 20.44 | 18.49 |
| DO_Oil Table | ||||
|---|---|---|---|---|
| Flow Rates | Cs/ASA | Na-Alumina | HY-Zeolite | Thermal |
| Oil production (kg/hr) | 685 | 731 | 474 | 2141 |
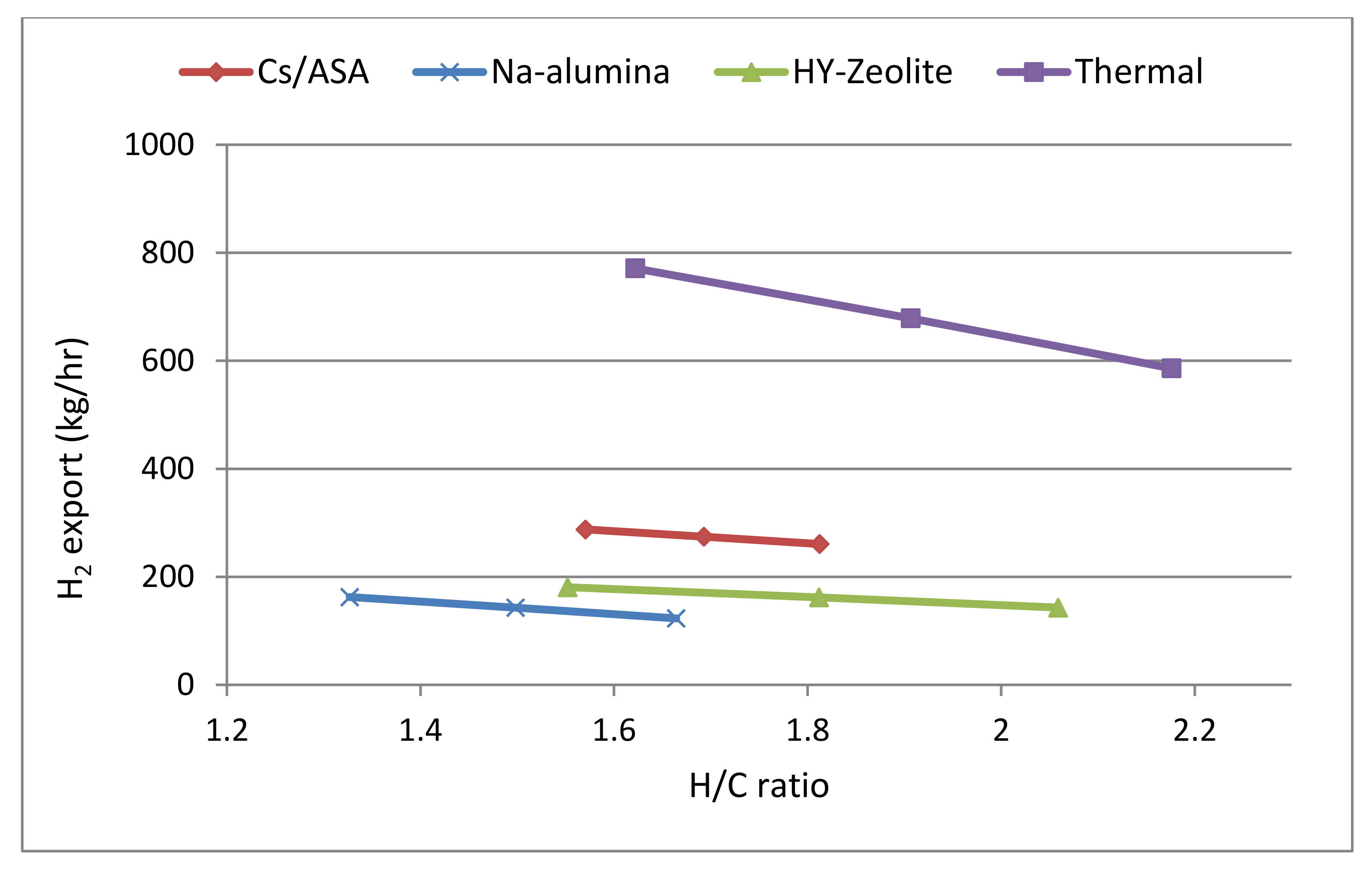
| H2 export (kg/hr) a | 308 | 192 | 209 | 910 |
| Combustion energy (Char+coke+gases) (MJ/h) | 187,660 | 212,923 | 223,145 | 133,078 |
| Electricity export (kW) b | 11,855 | 13,787 | 14,568 | 7682 |
| Steam export (MJ/hr) b | 68,582 | 80,820 | 85,962 | 28,888 |
| Overall energy yield (MJ/MJ biomass)% c | 52.5 | 53.1 | 53.0 | 74.3 |
| Oil Properties | ||||
| Carbon content (wt.%) | 82.2 | 81.3 | 75.9 | 74.5 |
| Hydrogen content (wt.%) | 9.9 | 7.8 | 8.1 | 8.2 |
| Oxygen content (wt.%) | 7.9 | 10.8 | 15.9 | 17.3 |
| Higher heating value (MJ/kg) | 41.05 | 37.19 | 34.86 | 34.28 |
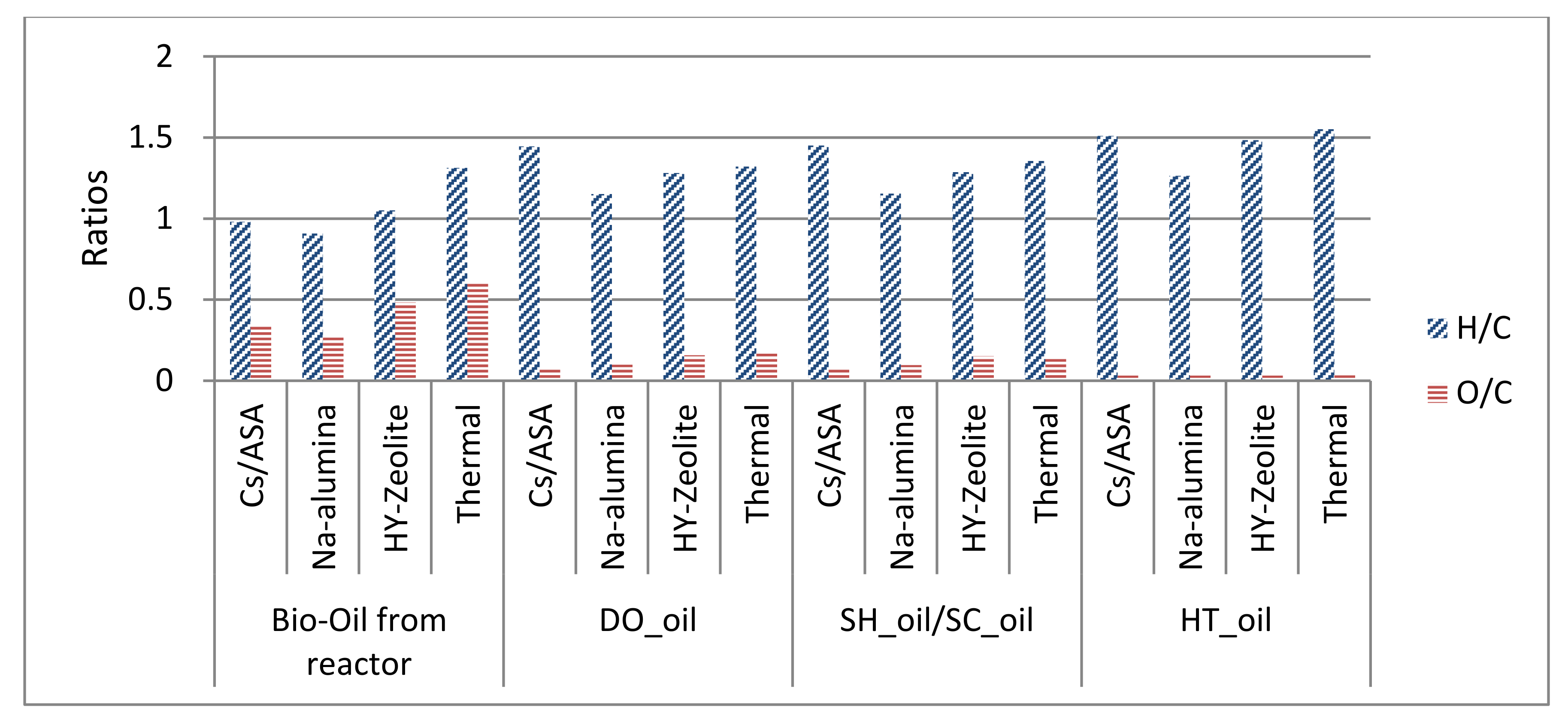
| H/C molar ratio | 1.45 | 1.15 | 1.28 | 1.32 |
| O/C molar ratio | 0.07 | 0.10 | 0.16 | 0.17 |
| O/H molar ratio | 0.05 | 0.09 | 0.12 | 0.13 |
| Average molecular weight (g) | 139.81 | 122.04 | 140.79 | 146.66 |
| Average boiling point (deg C) | 202.42 | 200.04 | 215.04 | 220.49 |
| Acids (wt.%) | 0.00 | 0.00 | 0.03 | 0.97 |
| Sugars (wt.%) | 0.00 | 0.00 | 0.00 | 2.16 |
| Carbonyls (wt.%) | 1.41 | 1.70 | 2.02 | 2.80 |
| Total problem components (wt.%) | 1.42 | 1.70 | 2.04 | 5.92 |
| Benzene (wt.%) | 0.00 | 0.00 | 0.00 | 0.00 |
| Aromatics (vol%) | 19.90 | 29.25 | 1.22 | 0.74 |
| Olefins (vol%) d | 11.56 | 4.01 | 1.38 | 2.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, A.D.; Zabeti, M.; Seshan, K.; Patel, M.K. Comparative Technical Process and Product Assessment of Catalytic and Thermal Pyrolysis of Lignocellulosic Biomass. Processes 2020, 8, 1600. https://doi.org/10.3390/pr8121600
Patel AD, Zabeti M, Seshan K, Patel MK. Comparative Technical Process and Product Assessment of Catalytic and Thermal Pyrolysis of Lignocellulosic Biomass. Processes. 2020; 8(12):1600. https://doi.org/10.3390/pr8121600
Chicago/Turabian StylePatel, Akshay D., Masoud Zabeti, K. Seshan, and Martin K. Patel. 2020. "Comparative Technical Process and Product Assessment of Catalytic and Thermal Pyrolysis of Lignocellulosic Biomass" Processes 8, no. 12: 1600. https://doi.org/10.3390/pr8121600
APA StylePatel, A. D., Zabeti, M., Seshan, K., & Patel, M. K. (2020). Comparative Technical Process and Product Assessment of Catalytic and Thermal Pyrolysis of Lignocellulosic Biomass. Processes, 8(12), 1600. https://doi.org/10.3390/pr8121600






