Why Is Batch Processing Still Dominating the Biologics Landscape? Towards an Integrated Continuous Bioprocessing Alternative
Abstract
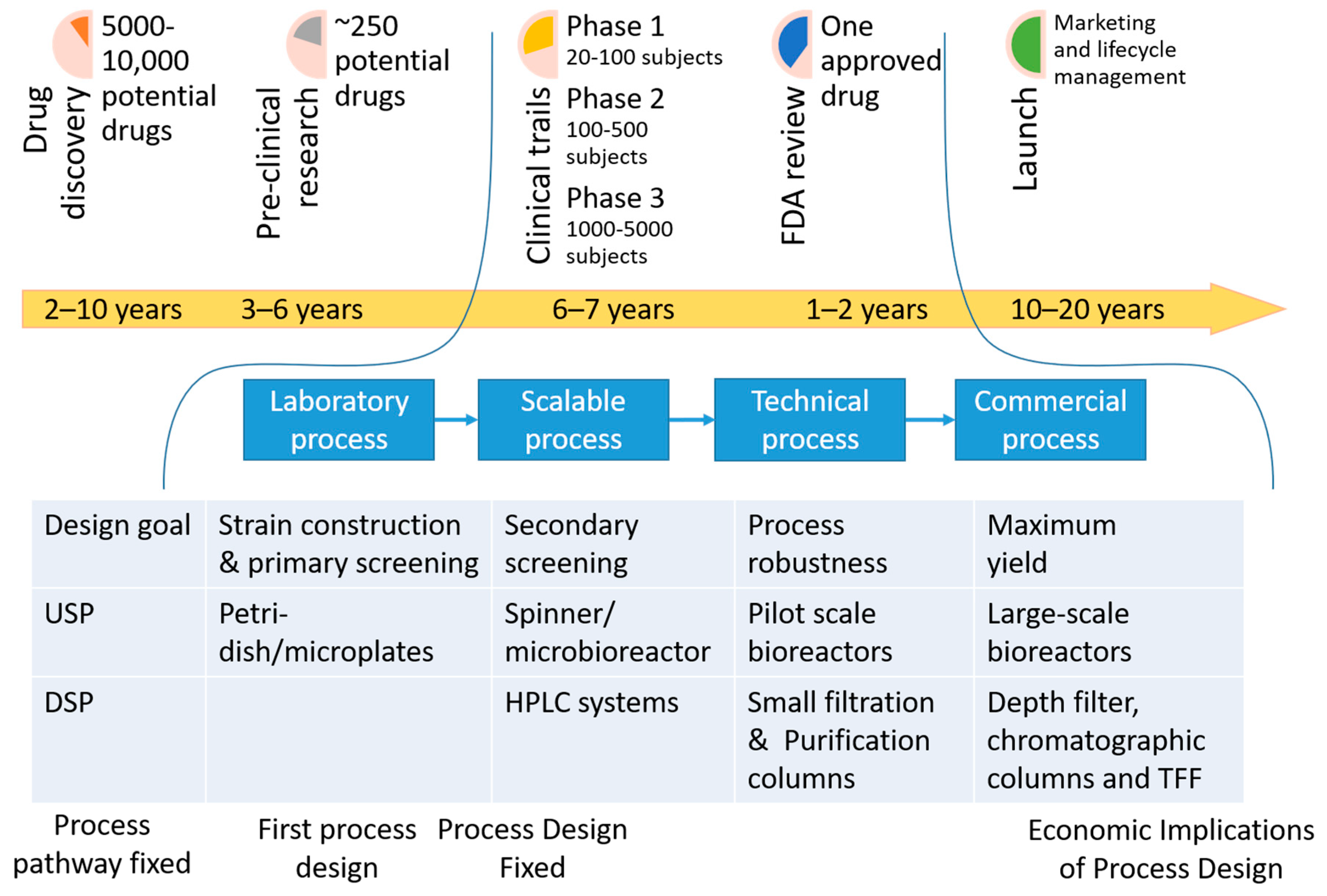
:1. Introduction: Where Are We Now
1.1. Critical Views on Current Practices
1.2. Academic Engagements Supporting the Advancement
2. What Is Creating This Disjoint
2.1. Are Costs or Regulators to Blame
2.2. Realization of Flexible and Intensified Manufacturing
2.3. The Dilemma of Technology Evolution—Continuous Stainless Steel vs. Single-Use
2.4. Organisational Readiness
3. What Can Be Done to Improve the Situation
3.1. Need for More PSE Case Studies to Build Confidence
3.2. PAT Solutions and Robotics for Better Control
3.3. Modeling and Simulation
3.4. The Clarity in Regulation for Continuous Biologics Manufacturing
4. Sustainability Considerations of Continuous Manufacturing of Biopharmaceuticals: Process Integration and Automation
5. Digitalization
5.1. From Smart Sensors to Big Data
5.2. Digital Twins
5.3. Application of Modeling in Regulatory Decision
5.4. Leveraging Process Data
5.5. Hybrid Facilities: Acknowledging the Best of Both Worlds
6. Summary and Outlook
- Technical:
- Improvement in automation to allow flexibility in the design and control of continuous processes;
- Adoption of a “digital twin” of processes to reduce the costs and risks linked with a decision made based on a limited set of experimental results;
- Application of detailed modeling and expert systems to support the development and regulatory requirements, such as scale-down modeling;
- Working on hybrid approaches such as single-use and multi-use to obtain best-of-all outcomes, thus enabling continuous manufacturing;
- Application of “big data” to support process development, control, and regulatory filing of a project.
- Management:
- Training on realistic situations highlighting risks and benefits of continuous manufacturing will allow removing the barrier caused by preexisting perceptions;
- Acquisition of trained staff who can support the adoption of new technology;
- Identifying key stakeholders from across the organization and getting them involved in the migration processes;
- Early alignment of R&D and commercial manufacturing business drivers to realize the extensive benefits of continuous biologics manufacturing.
- Regulatory:
- Clarity on the regulation of continuous vs. batch definitions under newer integrated and hybrid biomanufacturing process designs will be very useful;
- Increase in acceptability of digital twins as evidence for regulatory clearance;
- A joint effort by regulatory agencies and industries to develop a possible roadmap for the integrated continuous manufacturing will be highly beneficial for the biologics sector;
- Harmonization of continuous manufacturing standards and regulations.
Author Contributions
Funding
Conflicts of Interest
References
- Chatterjee, S. FDA Perspective on Continuous Manufacturing. In Proceedings of the IFPAC Annual Meeting, Baltimore, MD, USA, 22–25 January 2012. [Google Scholar]
- ICH Expert Working Group. Q13 Continuous Manufacturing of Drug Substances and Drug Products. Available online: https://database.ich.org/sites/default/files/Q13_EWG_Concept_Paper.pdf (accessed on 11 December 2020).
- Lee, S.L. Current FDA Perspective for Continuous Manufacturing. In Proceedings of the MIT-CMAC 2nd International Symposium on Continuous Manufacturing of Pharmaceuticals, Cambridge, MA, USA, 26–27 September 2016. [Google Scholar]
- Nasr, M.; Krumme, M.; Matsuda, Y.; Trout, B.L.; Badman, C.; Mascia, S.; Cooney, C.; Jensen, K.D.; Florence, A.; Johnston, C.; et al. Regulatory Perspectives on Continuous Pharmaceutical Manufacturing: Moving From Theory to Practice. J. Pharm. Sci. 2017, 106, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Croughan, M.S.; Konstantinov, K.B.; Cooney, C. The future of industrial bioprocessing: Batch or continuous? Biotechnol. Bioeng. 2015, 112, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Konstantinov, K.B.; Cooney, C. White Paper on Continuous Bioprocessing 20–21 May 2014 Continuous Manufacturing Symposium. J. Pharm. Sci. 2015, 104, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Gernaey, K.V.; Gani, R. A model-based systems approach to pharmaceutical product-process design and analysis. Chem. Eng. Sci. 2010, 65, 5757–5769. [Google Scholar] [CrossRef]
- Schaber, S.D.; Gerogiorgis, D.I.; Ramachandran, R.; Evans, J.M.B.; Barton, P.I.; Trout, B.L. Economic Analysis of Integrated Continuous and Batch Pharmaceutical Manufacturing: A Case Study. Ind. Eng. Chem. Res. 2011, 50, 10083–10092. [Google Scholar] [CrossRef] [Green Version]
- Department of Health and Human Services, Food and Drug Administration. PAT Guidance for Industry-Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance. Available online: https://www.fda.gov/media/71012/download (accessed on 11 December 2020).
- FDA. Quality Considerations for Continuous Manufacturing Guidance for Industry. Food Drug Adm. Available online: https://www.fda.gov/media/121314/download (accessed on 11 December 2020).
- Langer, E. Biomanufacturing: Demand for Continuous Bioprocessing Increasing. BioPharm Int. 2020, 33, 5. [Google Scholar]
- Godawat, R.; Konstantinov, K.; Rohani, M.; Warikoo, V. End-to-end integrated fully continuous production of recombinant monoclonal antibodies. J. Biotechnol. 2015, 213, 13–19. [Google Scholar] [CrossRef]
- Aakesson, M.; Heitmann, M.; Tiainen, P. Integrated Continuous Biomanufacturing Process. U.S. Patent Application No. 15/306,938, 2 March 2017. [Google Scholar]
- Desai, S.G. Continuous and semi-continuous cell culture for production of blood clotting factors. J. Biotechnol. 2015, 213, 20–27. [Google Scholar] [CrossRef]
- Farid, S.S.; Thompson, B.; Davidson, A. Continuous bioprocessing: The real thing this time? In Proceedings of the10th Annual bioProcessUK Conference, London, UK, 3–4 December 2013. [Google Scholar]
- Jones, S.; Castillo, F.; Levine, H. Advances in the development of therapeutic monoclonal antibodies. BioPharm. Int. 2007, 20, 96–114. [Google Scholar]
- Agrawal, V.; Bal, M. Strategies for rapid production of therapeutic proteins in mammalian cells. BioProcess Int. 2012, 10, 32–48. [Google Scholar]
- Ebeler, M.; Lind, O.; Norrman, N.; Palmgren, R.; Franzreb, M. One-step integrated clarification and purification of a monoclonal antibody using Protein A Mag Sepharose beads and a cGMP-compliant high-gradient magnetic separator. New Biotechnol. 2018, 42, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Käppler, T.; Cerff, M.; Ottow, K.E.; Hobley, T.J.; Posten, C. In situ magnetic separation for extracellular protein production. Biotechnol. Bioeng. 2009, 102, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, X.; Huang, C.; Angelo, J.; Oliveira, C.L.; Xu, M.; Xu, X.; Temel, D.; Ding, J.; Ghose, S.; et al. Biomanufacturing evolution from conventional to intensified processes for productivity improvement: A case study. mAbs 2020, 12, 1770669. [Google Scholar] [CrossRef] [PubMed]
- Haringa, C.; Mudde, R.F.; Noorman, H.J. From industrial fermentor to CFD-guided downscaling: What have we learned? Biochem. Eng. J. 2018, 140, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Tajsoleiman, T.; Spann, R.; Bach, C.; Gernaey, K.V.; Huusom, J.K.; Krühne, U. A CFD based automatic method for compartment model development. Comput. Chem. Eng. 2019, 123, 236–245. [Google Scholar] [CrossRef]
- Gargalo, C.L.; Heras, S.C.; de Las Jones, M.N.; Mansouri, S.S.; Krühne, U.; Gernaey, K.V. Towards the Development of Digital Twins for the Bio-Manufacturing Industry. Available online: https://doi.org/10.1007/10_2020_142 (accessed on 11 December 2020).
- Busse, C.; Biechele, P.; de Vries, I.; Reardon, K.F.; Solle, D.; Scheper, T. Sensors for disposable bioreactors. Eng. Life Sci. 2017, 17, 940–952. [Google Scholar] [CrossRef]
- Kornecki, M.; Schmidt, A.; Lohmann, L.; Huter, M.; Mestmäcker, F.; Klepzig, L.S.; Mouellef, M.; Zobel-Roos, S.; Strube, J. Accelerating Biomanufacturing by Modeling of Continuous Bioprocessing—Piloting Case Study of Monoclonal Antibody Manufacturing. Processes 2019, 7, 495. [Google Scholar] [CrossRef] [Green Version]
- Huter, M.; Strube, J. Model-Based Design and Process Optimization of Continuous Single Pass Tangential Flow Filtration Focusing on Continuous Bioprocessing. Processes 2019, 7, 317. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, R.J.; Castilho, L.R.; Subramanian, G. Tools Enabling Continuous and Integrated Upstream and Downstream Processes in the Manufacturing of Biologicals. In Continuous Biomanufacturing-Innovative Technologies and Methods; Wiley: Hoboken, NJ, USA, 2017; pp. 31–68. [Google Scholar]
- Castilho, L.; Anspach, F. CFD-aided design of a dynamic filter for mammalian cell separation. Biotechnol. Bioeng. 2003, 83, 514–524. [Google Scholar] [CrossRef]
- Biomanufacturing Consortium (BioMAN)|MIT Center for Biomedical Innovation. Available online: http://cbi.mit.edu/research-overview/bioman/ (accessed on 31 August 2020).
- BioPhorum. Available online: https://www.biophorum.com/ (accessed on 31 August 2020).
- Udugama, I.A.; Feldman, H.; de las Heras, S.C.; Kizhedath, A.; Bryde-Jacobsen, J.; van den Berg, F.; Mansouri, S.S.; Gernaey, K.V. Biopro World Talent Campus: A week of real world challenge for biotechnology post-graduate students. Educ. Chem. Eng. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- DiCesare, C.; Yu, M.; Yin, J.; Zhou, W.; Hwang, C.; Tengtrakool, J.; Konstantinov, K. Development, qualification, and application of a bioreactor scale-down process: Modeling large-scale microcarrier perfusion cell culture. Bioprocess. Int. 2016, 14, 18–29. [Google Scholar]
- Tajsoleiman, T.; Mears, L.; Krühne, U.; Gernaey, K.V.; Cornelissen, S. An Industrial Perspective on Scale-Down Challenges Using Miniaturized Bioreactors. Trends Biotechnol. 2019, 37, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Ram, R.J. Tools for Continuous Bioprocess Development. BioPharm Int. 2016, 29, 18–25. [Google Scholar]
- Sartorius Ambr® 15 Fermentation-High throughput Automated System|Sartorius. Available online: https://www.sartorius.com/us-en/products/fermentation-bioreactors/ambr-multi-parallel-bioreactors/ambr-15-fermentation (accessed on 9 October 2020).
- Scott, C. Large-Scale Capacity Strategies: Single Use, Multiuse, or Both? Bioprocess. Int. 2019. Available online: https://bioprocessintl.com/manufacturing/facility-design-engineering/large-scale-capacity-strategies-single-use-multiuse-or-both/ (accessed on 11 December 2020).
- Goji, T.; Hayashi, Y.; Sakata, I. Evaluating “startup readiness” for researchers: Case studies of research-based startups with biopharmaceutical research topics. Heliyon 2020, 6, e04160. [Google Scholar] [CrossRef] [PubMed]
- Udugama, I.A.; Taube, M.A.; Mansouri, S.S.; Kirkpatrick, R.; Gernaey, K.V.; Yu, W.; Young, B.R. A Systematic Methodology for Comprehensive Economic Assessment of Process Control Structures. Ind. Eng. Chem. Res. 2018, 57, 13116–13130. [Google Scholar] [CrossRef] [Green Version]
- Thiess, H.; Gronemeyer, P.; Ditz, R.; Strube, J.; Zobel-Roos, S.; Subramanian, G. Engineering Challenges of Continuous Biomanufacturing Processes (CBP). In Continuous Biomanufacturing-Innovative Technologies and Methods; Wiley: Hoboken, NJ, USA, 2017; pp. 69–106. [Google Scholar]
- Montes, F.; Gernaey, K.V.; Sin, G. Implementation of a Radial Basis Function control strategy for the crystallization of Ibuprofen under uncertainty. Comput. Aided Chem. Eng. 2018, 44, 565–570. [Google Scholar] [CrossRef]
- O’Mahony, N.; Murphy, T.; Panduru, K.; Riordan, D.; Walsh, J. Adaptive process control and sensor fusion for process analytical technology. In Proceedings of the 2016 27th Irish Signals and Systems Conference (ISSC), Londonderry, UK, 21–22 June 2016; pp. 1–6. [Google Scholar]
- Allmendinger, R.; Simaria, A.S.; Turner, R.; Farid, S.S. Closed-loop optimization of chromatography column sizing strategies in biopharmaceutical manufacture. J. Chem. Technol. Biotechnol. 2013, 89, 1481–1490. [Google Scholar] [CrossRef] [Green Version]
- FDA. PAT Guidance for Industry—A Framework for Innovative Pharmaceutical Development; Manufacturing and Quality Assurance: Rockville, MD, USA, 2004. [Google Scholar]
- Randek, J.; Mandenius, C.-F. On-line soft sensing in upstream bioprocessing. Crit. Rev. Biotechnol. 2017, 38, 106–121. [Google Scholar] [CrossRef]
- Zimmermann, R.; Fiabane, L.; Gasteuil, Y.; Volk, R.; Pinton, J.-F. Measuring Lagrangian accelerations using an instrumented particle. Phys. Scr. 2013, 2013, 014063. [Google Scholar] [CrossRef]
- Freesense. Available online: https://www.freesense.dk (accessed on 11 December 2020).
- Pontius, K.; Junicke, H.; Gernaey, K.V.; Bevilacqua, M. Monitoring yeast fermentations by nonlinear infrared technology and chemometrics—Understanding process correlations and indirect predictions. Appl. Microbiol. Biotechnol. 2020, 104, 5315–5335. [Google Scholar] [CrossRef] [PubMed]
- Landgrebe, D.; Haake, C.; Höpfner, T.; Beutel, S.; Hitzmann, B.; Scheper, T.; Rhiel, M.; Reardon, K.F. On-line infrared spectroscopy for bioprocess monitoring. Appl. Microbiol. Biotechnol. 2010, 88, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Gargalo, C.L.; Udugama, I.A.; Pontius, K.; Lopez, P.C.; Nielsen, R.F.; Hasanzadeh, A.; Mansouri, S.S.; Bayer, C.; Junicke, H.; Gernaey, K.V. Towards smart manufacturing: A perspective on recent developments in industrial measurement and monitoring technologies for bio-based production processes. J. Ind. Microbiol. Biotechnol. 2020, 47, 947–964. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-K.; Yoo, S.J.; Jeong, D.H.; Lee, J.M. Real-time estimation of glucose concentration in algae cultivation system using Raman spectroscopy. Bioresour. Technol. 2013, 142, 131–137. [Google Scholar] [CrossRef]
- De Assis, A.J.; Filho, R.M. Soft sensors development for on-line bioreactor state estimation. Comput. Chem. Eng. 2000, 24, 1099–1103. [Google Scholar] [CrossRef]
- Veloso, A.C.A.; Rocha, I.; Ferreira, E.C. Monitoring of fed-batch E. coli fermentations with software sensors. Bioprocess. Biosyst. Eng. 2009, 32, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Tambe, S.S. Soft-sensor development for biochemical systems using genetic programming. Biochem. Eng. J. 2014, 85, 89–100. [Google Scholar] [CrossRef]
- Krause, D.; Hussein, M.; Becker, T. Online monitoring of bioprocesses via multivariate sensor prediction within swarm intelligence decision making. Chemom. Intell. Lab. Syst. 2015, 145, 48–59. [Google Scholar] [CrossRef]
- Luttmann, R.; Bracewell, D.G.; Cornelissen, G.; Gernaey, K.V.; Glassey, J.; Hass, V.C.; Kaiser, C.; Preusse, C.; Striedner, G.; Mandenius, C.-F. Soft sensors in bioprocessing: A status report and recommendations. Biotechnol. J. 2012, 7, 1040–1048. [Google Scholar] [CrossRef]
- Ramin, P.; Mansouri, S.S.; Udugama, I.; Benyahia, B.; Gernaey, K.V. Modelling continuous pharmaceutical and bio-based processes at plant-wide level: A roadmap towards efficient decision-making. Chim. Oggi Chem. Today 2018, 36, 26–30. [Google Scholar]
- Jacquemart, R.; VanderSluis, M.; Zhao, M.; Sukhija, K.; Sidhu, N.; Stout, J. A Single-use Strategy to Enable Manufacturing of Affordable Biologics. Comput. Struct. Biotechnol. J. 2016, 14, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zürcher, P.; Shirahata, H.; Badr, S.; Sugiyama, H. Multi-stage and multi-objective decision-support tool for biopharmaceutical drug product manufacturing: Equipment technology evaluation. Chem. Eng. Res. Des. 2020, 161, 240–252. [Google Scholar] [CrossRef]
- Shirahata, H.; Hirao, M.; Sugiyama, H. Multiobjective decision-support tools for the choice between single-use and multi-use technologies in sterile filling of biopharmaceuticals. Comput. Chem. Eng. 2019, 122, 114–128. [Google Scholar] [CrossRef]
- Dallinger, D.; Kappe, C.O. Why flow means green–Evaluating the merits of continuous processing in the context of sustainability. Curr. Opin. Green Sustain. Chem. 2017, 7, 6–12. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Poechlauer, P.; Broxterman, Q.B.; Yang, B.-S.; Ende, D.A.; Baird, J.; Bertsch, C.; Hannah, R.E.; Dell’Orco, P.; Noorman, H.; et al. Key Green Engineering Research Areas for Sustainable Manufacturing: A Perspective from Pharmaceutical and Fine Chemicals Manufacturers. Org. Process. Res. Dev. 2011, 15, 900–911. [Google Scholar] [CrossRef]
- May, M. Modular Bioprocessing Makes Adaptability a Snap: By swapping out and adding bioprocessing modules, biomanufacturers can modify functionality and adjust capacity quickly and economically. Genet. Eng. Biotechnol. News 2019, 39, 38–40. [Google Scholar] [CrossRef]
- Scott, C. Sustainability in Bioprocessing. BioProcess Int. 2011, 9. Available online: https://bioprocessintl.com/manufacturing/monoclonal-antibodies/sustainability-in-bioprocessing-323438/ (accessed on 11 December 2020).
- Lonza Innovations in Pharma Biotech & Nutrition. Available online: https://annualreport.lonza.com/2019/segments/pharma-biotech-nutrition/innovations.html (accessed on 11 December 2020).
- Udugama, I.A.; Gargalo, C.L.; Yamashita, Y.; Taube, M.A.; Palazoglu, A.; Young, B.R.; Gernaey, K.V.; Kulahci, M.; Bayer, C. The Role of Big Data in Industrial (Bio)chemical Process Operations. Ind. Eng. Chem. Res. 2020, 59, 15283–15297. [Google Scholar] [CrossRef]
- Lopez, P.C.; Udugama, I.A.; Thomsen, S.T.; Roslander, C.; Junicke, H.; Mauricio-Iglesias, M.; Gernaey, K.V. Towards a digital twin: A hybrid data-driven and mechanistic digital shadow to forecast the evolution of lignocellulosic fermentation. Biofuels, Bioprod. Biorefining 2020, 14, 1046–1060. [Google Scholar] [CrossRef]



| Collaborative Platforms | Kind/Lead | Purpose |
|---|---|---|
| Biomanufacturing consortium (BioMAN) | Industry–academia collaboration lead by Massachusetts Institute of Technology (MIT) | Several stakeholders work together to develop new knowledge, science, technologies, and strategies to improve biomanufacturing [29]. |
| BioPhorum consortium | Cross-industry collaboration | Connecting most big biopharmaceutical manufacturers and suppliers collaboratively to produce technical documents to explore, propose, and define industry best practices on the topics mentioned earlier [30]. |
| National Institute for Innovation in Manufacturing Biopharmaceuticals (NIIMBL) | Public–private partnership, manufacturing innovation institutes funded through a cooperative agreement with the National Institute of Standards and Technology (NIST) | To achieve a public–private partnership to enable more efficient and rapid manufacturing capabilities and biopharmaceutical manufacturing workforce to accelerate biopharmaceutical innovation. |
| BIOPRO cluster | Industry–academia collaboration lead by Technical University of Denmark (DTU) | Developing new ways of making bio-based production more efficient and sustainable by reducing the consumption of energy and raw materials while improving yields [31]. |
| Multi-Use Stainless Steel Systems | Single-Use Systems |
|---|---|
| Benefits | |
|
|
| Challenges | |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Udugama, I.A.; Gargalo, C.L.; Gernaey, K.V. Why Is Batch Processing Still Dominating the Biologics Landscape? Towards an Integrated Continuous Bioprocessing Alternative. Processes 2020, 8, 1641. https://doi.org/10.3390/pr8121641
Kumar A, Udugama IA, Gargalo CL, Gernaey KV. Why Is Batch Processing Still Dominating the Biologics Landscape? Towards an Integrated Continuous Bioprocessing Alternative. Processes. 2020; 8(12):1641. https://doi.org/10.3390/pr8121641
Chicago/Turabian StyleKumar, Ashish, Isuru A. Udugama, Carina L. Gargalo, and Krist V. Gernaey. 2020. "Why Is Batch Processing Still Dominating the Biologics Landscape? Towards an Integrated Continuous Bioprocessing Alternative" Processes 8, no. 12: 1641. https://doi.org/10.3390/pr8121641
APA StyleKumar, A., Udugama, I. A., Gargalo, C. L., & Gernaey, K. V. (2020). Why Is Batch Processing Still Dominating the Biologics Landscape? Towards an Integrated Continuous Bioprocessing Alternative. Processes, 8(12), 1641. https://doi.org/10.3390/pr8121641








