How Do Indirect Effects of Contaminants Inform Ecotoxicology? A Review
Abstract
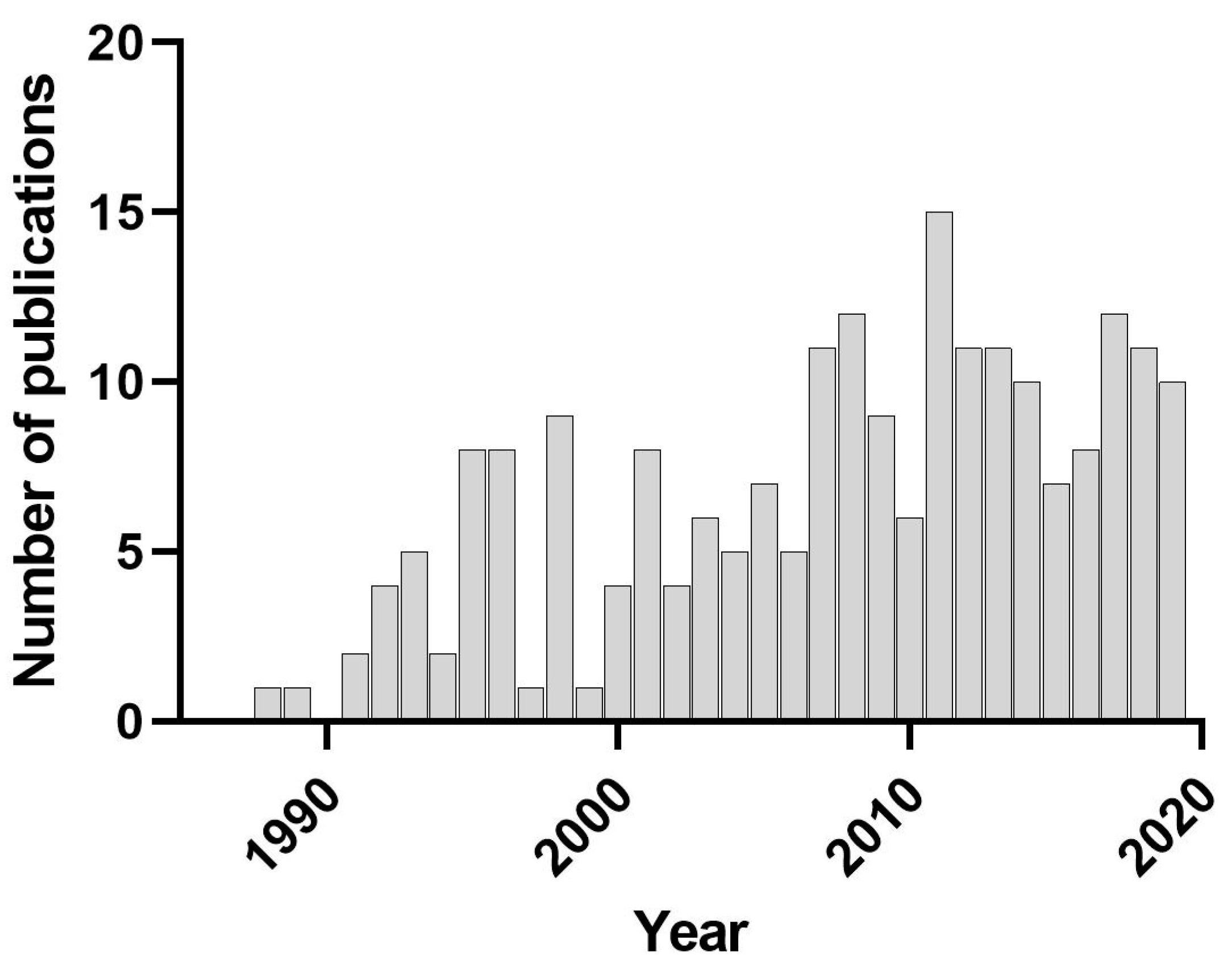
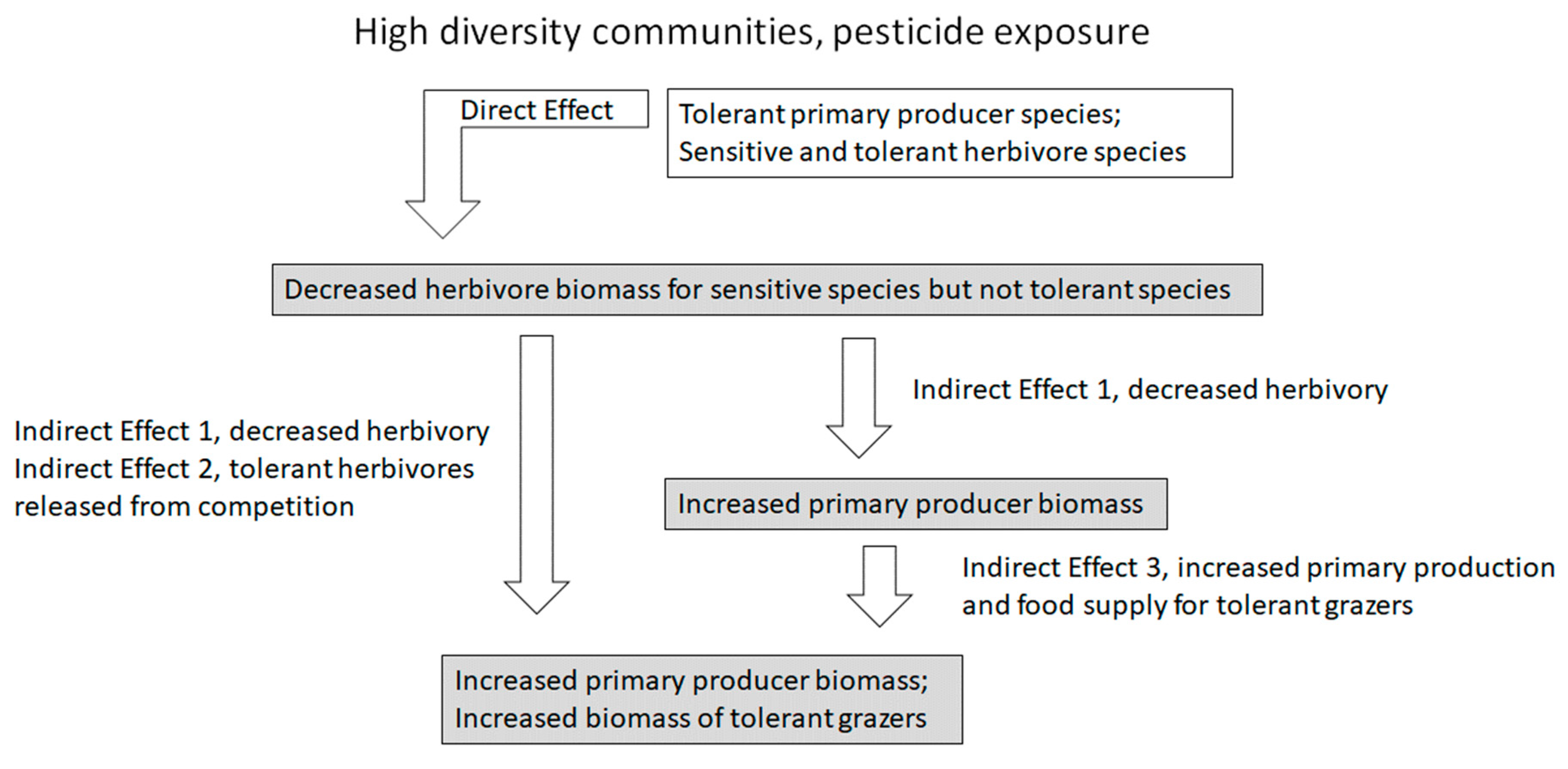
:1. Introduction to Indirect Effects
2. Indirect Effects and Behavior
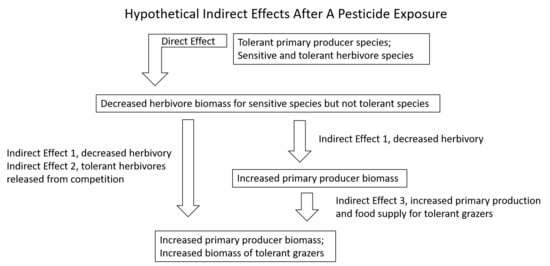
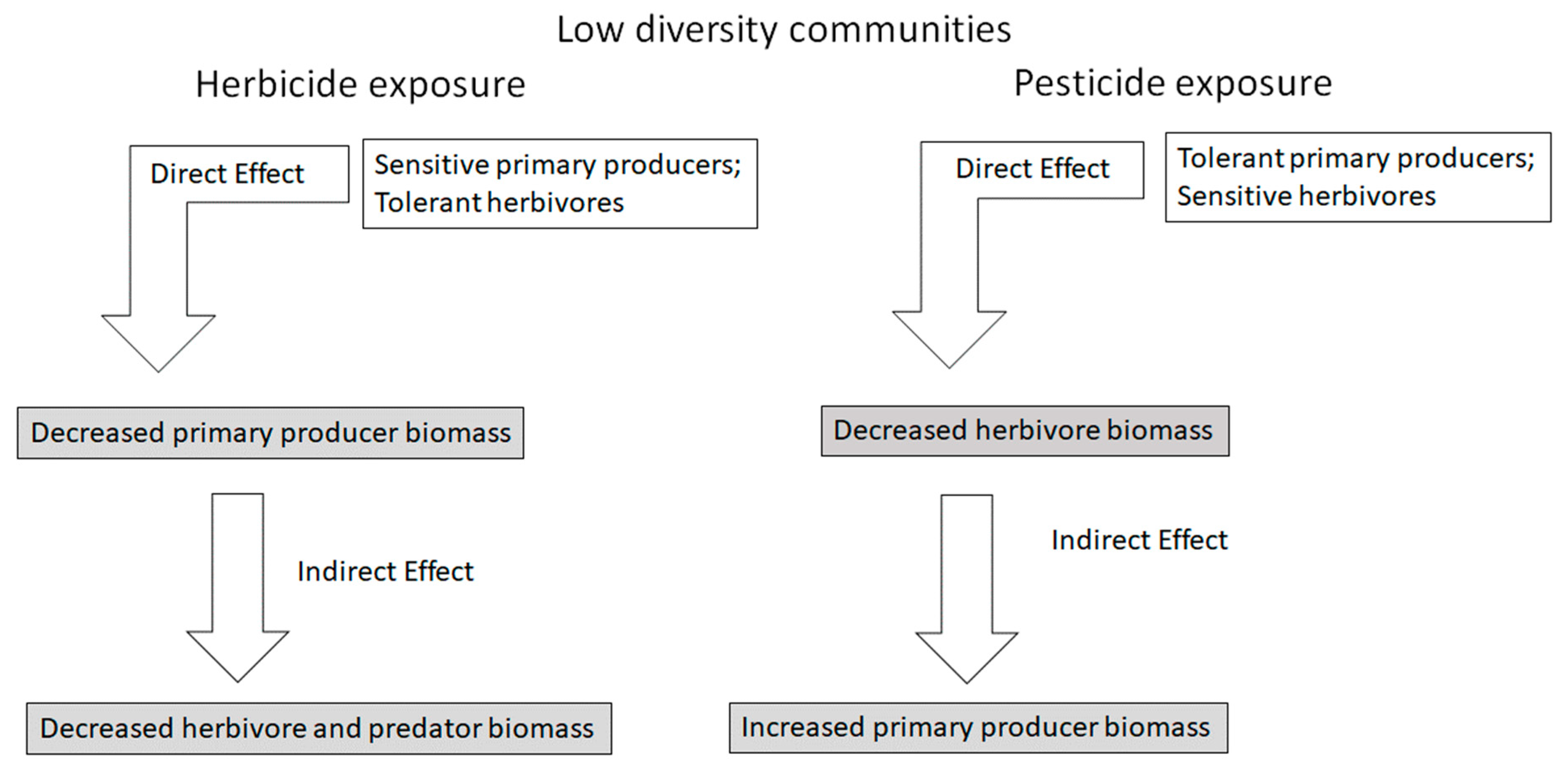
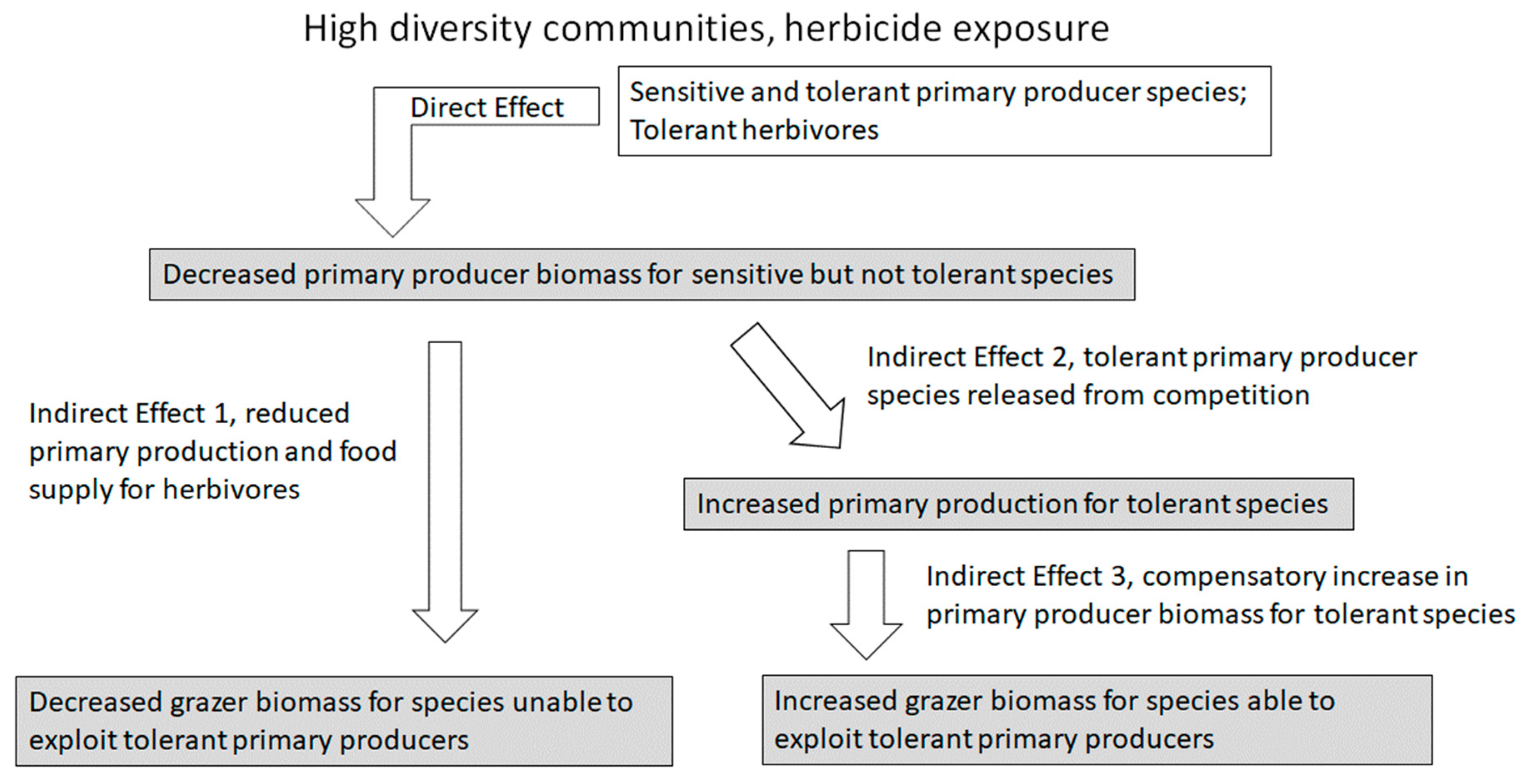
3. Indirect Effects and Community Ecology
4. Ecosystem Function/Services and Indirect Effects
5. Indirect Effects and Ecological Risk Assessment
6. Indirect Effects and Multiple Contaminants/Stressors
7. Indirect Effects and Experimental Studies
8. Indirect Effects and Models
9. Final Comments on Indirect Effects
Funding
Conflicts of Interest
References
- Fleeger, J.W.; Carman, K.R.; Nisbet, R.M. Indirect effects of contaminants on aquatic ecosystems. Sci. Total Environ. 2003, 317, 207–233. [Google Scholar] [CrossRef]
- Clements, W.H.; Rohr, J.R. Community responses to contaminants: Using basic ecological principles to predict ecotoxicological effects. Environ. Toxicol. Chem. 2009, 28, 1789–1800. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Kerby, J.L.; Sih, A. Community ecology as a framework for predicting contaminant effects. Trends Ecol. Evol. 2006, 21, 606–613. [Google Scholar] [CrossRef]
- Halstead, N.T.; McMahon, T.A.; Johnson, S.A.; Raffel, T.R.; Romansic, J.M.; Crumrine, P.W.; Rohr, J.R. Community ecology theory predicts the effects of agrochemical mixtures on aquatic biodiversity and ecosystem properties. Ecol. Lett. 2014, 17, 932–941. [Google Scholar] [CrossRef]
- Relyea, R.; Hoverman, J. Assessing the ecology in ecotoxicology: A review and synthesis in freshwater systems. Ecol. Lett. 2006, 9, 1157–1171. [Google Scholar] [CrossRef]
- Schmitt-Jansen, M.; Veit, U.; Dudel, G.; Altenburger, R. An ecological perspective in aquatic ecotoxicology: Approaches and challenges. Basic Appl. Ecol. 2008, 9, 337–345. [Google Scholar] [CrossRef]
- Preston, B.L. Indirect effects in aquatic ecotoxicology: Implications for ecological risk assessment. Environ. Manag. 2002, 29, 311–323. [Google Scholar] [CrossRef]
- Saaristo, M.; Brodin, T.; Balshine, S.; Bertram, M.G.; Brooks, B.W.; Ehlman, S.M.; McCallum, E.S.; Sih, A.; Sundin, J.; Wong, B.B.M.; et al. Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife. Proc. R. Soc. B-Biol. Sci. 2018, 285. [Google Scholar] [CrossRef]
- Kidd, K.A.; Paterson, M.J.; Rennie, M.D.; Podemski, C.L.; Findlay, D.L.; Blanchfield, P.J.; Liber, K. Direct and indirect responses of a freshwater food web to a potent synthetic oestrogen. Philos. Trans. R. Soc. B-Biol. Sci. 2014, 369. [Google Scholar] [CrossRef] [Green Version]
- Baert, J.M.; De Laender, F.; Janssen, C.R. The Consequences of nonrandomness in species-sensitivity in relation to functional traits for ecosystem-level effects of chemicals. Environ. Sci. Technol. 2017, 51, 7228–7235. [Google Scholar] [CrossRef]
- Rico, A.; Van den Brink, P.J.; Gylstra, R.; Focks, A.; Brock, T.C.M. Developing ecological scenarios for the prospective aquatic risk assessment of pesticides. Integr. Environ. Assess. Manag. 2016, 12, 510–521. [Google Scholar] [CrossRef]
- De Laender, F.; Morselli, M.; Baveco, H.; Van den Brink, P.J.; Di Guardo, A. Theoretically exploring direct and indirect chemical effects across ecological and exposure scenarios using mechanistic fate and effects modelling. Environ. Int. 2015, 74, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zang, W.; Yan, Z.; Hong, Y.; Liu, Z.; Yi, X.; Wang, X.; Liu, T.; Zhou, L. Species sensitivity analysis of heavy metals to freshwater organisms. Ecotoxicology 2015, 24, 1621–1631. [Google Scholar] [CrossRef]
- Long, E.R. Ranges in chemical concentrations in sediments associated with adverse biological effects. Mar. Poll. Bull. 1992, 24, 38–45. [Google Scholar] [CrossRef]
- Zhao, Q.H.; De Laender, F.; Van den Brink, P.J. Community composition modifies direct and indirect effects of pesticides in freshwater food webs. Sci. Total Environ. 2020, 739. [Google Scholar] [CrossRef]
- Delmas, E.; Besson, M.; Brice, M.H.; Burkle, L.A.; Dalla Riva, G.V.; Fortin, M.J.; Gravel, D.; Guimaraes, P.R.; Hembry, D.H.; Newman, E.A.; et al. Analysing ecological networks of species interactions. Biol. Rev. 2019, 94, 16–36. [Google Scholar] [CrossRef] [Green Version]
- Baert, J.M.; De Laender, F.; Sabbe, K.; Janssen, C.R. Biodiversity increases functional and compositional resistance, but decreases resilience in phytoplankton communities. Ecology 2016, 97, 3433–3440. [Google Scholar] [CrossRef]
- Carman, K.R.; Fleeger, J.W.; Pomarico, S. Response of a benthic food web to hydrocarbon contamination. Limnol. Oceanogr. 1997, 42, 561–571. [Google Scholar] [CrossRef]
- Van den Brink, P.J.; Bracewell, S.A.; Bush, A.; Chariton, A.; Choung, C.B.; Compson, Z.G.; Dafforn, K.A.; Korbel, K.; Lapen, D.R.; Mayer-Pinto, M.; et al. Towards a general framework for the assessment of interactive effects of multiple stressors on aquatic ecosystems: Results from the Making Aquatic Ecosystems Great Again (MAEGA) workshop. Sci. Total Environ. 2019, 684, 722–726. [Google Scholar] [CrossRef]
- Orr, J.A.; Vinebrooke, R.D.; Jackson, M.C.; Kroeker, K.J.; Kordas, R.L.; Mantyka-Pringle, C.; Van den Brink, P.J.; De Laender, F.; Stoks, R.; Holmstrup, M.; et al. Towards a unified study of multiple stressors: Divisions and common goals across research disciplines. Proc. R. Soc. B-Biol. Sci. 2020, 287. [Google Scholar] [CrossRef]
- Rohr, J.R.; Schotthoefer, A.M.; Raffel, T.R.; Carrick, H.J.; Halstead, N.; Hoverman, J.T.; Johnson, C.M.; Johnson, L.B.; Lieske, C.; Piwoni, M.D.; et al. Agrochemicals increase trematode infections in a declining amphibian species. Nature 2008, 455, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Man, Y.B.; Mo, W.Y.; Man, K.Y.; Wong, M.H. Direct and indirect effects of microplastics on bivalves, with a focus on edible species: A mini-review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2109–2143. [Google Scholar] [CrossRef]
- Zhai, Y.; Brun, N.R.; Bundschuh, M.; Schrama, M.; Hin, E.; Vijver, M.G.; Hunting, E.R. Microbially-mediated indirect effects of silver nanoparticles on aquatic invertebrates. Aquat. Sci. 2018, 80. [Google Scholar] [CrossRef] [Green Version]
- Gredelj, A.; Barausse, A.; Grechi, L.; Palmeri, L. Deriving predicted no-effect concentrations (PNECs) for emerging contaminants in the river Po, Italy, using three approaches: Assessment factor, species sensitivity distribution and AQUATOX ecosystem modelling. Environ. Int. 2018, 119, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Relyea, R. Chemical cocktails in aquatic systems: Pesticide effects on the response and recovery of >20 animal taxa. Environ. Poll. 2014, 189, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Galic, N.; Hommen, U.; Baveco, J.; van den Brink, P.J. Potential application of population models in the European ecological risk assessment of chemicals II: Review of models and their potential to address environmental protection aims. Integr. Environ. Assess. Manag. 2010, 6, 338–360. [Google Scholar] [CrossRef] [PubMed]
- Agerstrand, M.; Arnold, K.; Balshine, S.; Brodin, T.; Brooks, B.W.; Maack, G.; McCallum, E.S.; Pyle, G.; Saaristo, M.; Ford, A.T. Emerging investigator series: Use of behavioural endpoints in the regulation of chemicals. Environ. Sci. Process. Impacts 2020, 22, 49–65. [Google Scholar] [CrossRef] [Green Version]
- Sievers, M.; Hale, R.; Parris, K.M.; Melvin, S.D.; Lanctot, C.M.; Swearer, S.E. Contaminant-induced behavioural changes in amphibians: A meta-analysis. Sci. Total Environ. 2019, 693. [Google Scholar] [CrossRef]
- Brodin, T.; Piovano, S.; Fick, J.; Klaminder, J.; Heynen, M.; Jonsson, M. Ecological effects of pharmaceuticals in aquatic systems-impacts through behavioural alterations. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130580. [Google Scholar] [CrossRef] [Green Version]
- Jacquin, L.; Petitjean, Q.; Cote, J.; Laffaille, P.; Jean, S. Effects of pollution on fish behavior, personality, and cognition: Some research perspectives. Front. Ecol. Evol. 2020, 8. [Google Scholar] [CrossRef] [Green Version]
- Sousa, B.; Nunes, B. Reliability of behavioral test with fish: How neurotransmitters may exert neuromodulatory effects and alter the biological responses to neuroactive agents. Sci. Total Environ. 2020, 734, 139372. [Google Scholar] [CrossRef] [PubMed]
- Tierney, K.B.; Baldwin, D.H.; Hara, T.J.; Ross, P.S.; Scholz, N.L.; Kennedy, C.J. Olfactory toxicity in fishes. Aquat. Toxicol. 2010, 96, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Dabrowski, J.M. Combined effects of predatory fish and sublethal pesticide contamination on the behavior and mortality of mayfly nymphs. Environ. Toxicol. Chem. 2001, 20, 2537–2543. [Google Scholar] [CrossRef] [PubMed]
- Neuman-Lee, L.A.; Hopkins, G.R.; Brodie, E.D.; French, S.S. Sublethal contaminant exposure alters behavior in a common insect: Important implications for trophic transfer. J. Environ. Sci. Health Part B 2013, 48, 442–448. [Google Scholar] [CrossRef]
- Brodin, T.; Fick, J.; Jonsson, M.; Klaminder, J. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science 2013, 339, 814–815. [Google Scholar] [CrossRef] [PubMed]
- Jacquin, L.; Gandar, A.; Aguirre-Smith, M.; Perrault, A.; Le Henaff, M.; De Jong, L.; Paris-Palacios, S.; Laffaille, P.; Jean, S. High temperature aggravates the effects of pesticides in goldfish. Ecotoxicol. Environ. Saf. 2019, 172, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Lagesson, A.; Saaristo, M.; Brodin, T.; Fick, J.; Kiaminder, J.; Martin, J.M.; Wong, B.B.M. Fish on steroids: Temperature-dependent effects of 17 beta-trenbolone on predator escape, boldness, and exploratory behaviors. Environ. Poll. 2019, 245, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.V.M.; Pereira, K.C.; Sparaventi, E.; Gonzalez-Ortegon, E.; Blasco, J. Contamination may induce behavioural plasticity in the habitat selection by shrimps: A cost-benefits balance involving contamination, shelter and predation. Environ. Poll. 2020, 263. [Google Scholar] [CrossRef]
- Gessner, M.O.; Tlili, A. Fostering integration of freshwater ecology with ecotoxicology. Freshwat. Biol. 2016, 61, 1991–2001. [Google Scholar] [CrossRef] [Green Version]
- Evans, A.N.; Llanos, J.E.M.; Kunin, W.E.; Evison, S.E.F. Indirect effects of agricultural pesticide use on parasite prevalence in wild pollinators. Agric. Ecosyst. Environ. 2018, 258, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Bontje, D.; Kooi, B.W.; van Hattum, B. Sublethal toxic effects in a generic aquatic ecosystem. Ecotoxicol. Environ. Saf. 2011, 74, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Schuler, M.S.; Hintz, W.D.; Stoler, A.B.; Jones, D.K.; Mattes, B.M.; Relyea, R.A. Salty fertile lakes: How salinization and eutrophication alter the structure of freshwater communities. Ecosphere 2018, 9, 19. [Google Scholar] [CrossRef]
- Thrupp, T.J.; Runnalls, T.J.; Scholze, M.; Kugathas, S.; Kortenkamp, A.; Sumpter, J.P. The consequences of exposure to mixtures of chemicals: Something from ‘nothing’ and ‘a lot from a little’ when fish are exposed to steroid hormones. Sci. Total Environ. 2018, 619-620, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Graymore, M.; Stagnitti, F.; Allinson, G. Impacts of atrazine in aquatic ecosystems. Environ. Int. 2001, 26, 483–495. [Google Scholar] [CrossRef]
- Groner, M.L.; Relyea, R.A. A tale of two pesticides: How common insecticides affect aquatic communities. Freshwat. Biol. 2011, 56, 2391–2404. [Google Scholar] [CrossRef]
- Mensens, C.; De Laender, F.; Janssen, C.R.; Rivera, F.C.; Sabbe, K.; De Troch, M. Selective and context-dependent effects of chemical stress across trophic levels at the basis of marine food webs. Ecol. Appl. 2018, 28, 1342–1353. [Google Scholar] [CrossRef] [Green Version]
- Becker, J.M.; Ganatra, A.A.; Kandie, F.; Muhlbauer, L.; Ahlheim, J.; Brack, W.; Torto, B.; Agola, E.L.; McOdimba, F.; Hollert, H.; et al. Pesticide pollution in freshwater paves the way for schistosomiasis transmission. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and ecosystem functioning. Ann. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Jordan, F.; Gjata, N.; Mei, S.; Yule, C.M. Simulating food web dynamics along a gradient: Quantifying human influence. PLoS ONE 2012, 7, e40280. [Google Scholar] [CrossRef] [Green Version]
- Rasher, D.B.; Steneck, R.S.; Halfar, J.; Kroeker, K.J.; Ries, J.B.; Tinker, M.T.; Chan, P.T.W.; Fietzke, J.; Kamenos, N.A.; Konar, B.H.; et al. Keystone predators govern the pathway and pace of climate impacts in a subarctic marine ecosystem. Science 2020, 369, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.B.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, D.L.; Petchey, O.L.; et al. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, T.C.; Burkepile, D.E.; Holbrook, S.J.; Carpenter, R.C.; Claudet, J.; Loiseau, C.; Thiault, L.; Brooks, A.J.; Washburn, L.; Schmitt, R.J. Landscape-scale patterns of nutrient enrichment in a coral reef ecosystem: Implications for coral to algae phase shifts. Ecol. Appl. 2020, e2227. [Google Scholar] [CrossRef]
- Pimentao, A.R.; Pascoal, C.; Castro, B.B.; Cassio, F. Fungistatic effect of agrochemical and pharmaceutical fungicides on non-target aquatic decomposers does not translate into decreased fungi- or invertebrate-mediated decomposition. Sci. Total Environ. 2020, 712, 10. [Google Scholar] [CrossRef] [PubMed]
- Pereda, O.; Solagaistua, L.; Atristain, M.; de Guzman, L.; Larranaga, A.; von Schiller, D.; Elosegi, A. Impact of wastewater effluent pollution on stream functioning: A whole-ecosystem manipulation experiment. Environ. Poll. 2020, 258. [Google Scholar] [CrossRef] [PubMed]
- Deegan, L.A.; Johnson, D.S.; Warren, R.S.; Peterson, B.J.; Fleeger, J.W.; Fagherazzi, S.; Wollheim, W.M. Coastal eutrophication as a driver of salt marsh loss. Nature 2012, 490, 388–394. [Google Scholar] [CrossRef]
- Johnston, E.L.; Mayer-Pinto, M.; Crowe, T.P. Chemical contaminant effects on marine ecosystem functioning. J. Appl. Ecol. 2015, 52, 140–149. [Google Scholar] [CrossRef] [Green Version]
- De Laender, F.; Janssen, C.R. Brief communication: The ecosystem perspective in ecotoxicology as a way forward for the ecological risk assessment of chemicals. Integr. Environ. Assess. Manag. 2013, 9, E34–E38. [Google Scholar] [CrossRef]
- Lenihan, H.S.; Peterson, C.H.; Miller, R.J.; Kayal, M.; Potoski, M. Biotic disturbance mitigates effects of multiple stressors in a marine benthic community. Ecosphere 2018, 9. [Google Scholar] [CrossRef]
- Beketov, M.A.; Liess, M. Ecotoxicology and macroecology—Time for integration. Environ. Poll. 2012, 162, 247–254. [Google Scholar] [CrossRef]
- Chagnon, M.; Kreutzweiser, D.; Mitchell, E.A.; Mitchell, E.A.; Morrissey, C.A.; Noome, D.A.; Noome, D.A.; Van der Sluijs, J.P.; Van der Sluijs, J.P. Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ. Sci. Pollut. Res. 2015, 22, 119–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, K.; Bundschuh, M.; Schäfer, R.B. Review on the effects of toxicants on freshwater ecosystem functions. Environ. Poll. 2013, 180, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Markandya, A.; Taylor, T.; Longo, A.; Murty, M.N.; Murty, S.; Dhavala, K. Counting the cost of vulture decline - An appraisal of the human health and other benefits of vultures in India. Ecol. Econ. 2008, 67, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Grechi, L.; Franco, A.; Palmeri, L.; Pivato, A.; Barausse, A. An ecosystem model of the lower Po river for use in ecological risk assessment of xenobiotics. Ecol. Modell. 2016, 332, 42–58. [Google Scholar] [CrossRef]
- Lombardo, A.; Franco, A.; Pivato, A.; Barausse, A. Food web modeling of a river ecosystem for risk assessment of down-the-drain chemicals: A case study with AQUATOX. Sci. Total Environ. 2015, 508, 214–227. [Google Scholar] [CrossRef]
- Zhang, L.L.; Liu, J.L. AQUATOX coupled foodweb model for ecosystem risk assessment of Polybrominated diphenyl ethers (PBDEs) in lake ecosystems. Environ. Poll. 2014, 191, 80–92. [Google Scholar] [CrossRef]
- Rodrigues, A.C.M.; Machado, A.L.; Bordalo, M.D.; Saro, L.; Simao, F.C.P.; Rocha, R.J.M.; Golovko, O.; Zlabek, V.; Barata, C.; Soares, A.; et al. Invasive species mediate insecticide effects on community and ecosystem functioning. Environ. Sci. Technol. 2018, 52, 4889–4900. [Google Scholar] [CrossRef]
- Chagaris, D.D.; Patterson, W.F.; Allen, M.S. Relative effects of multiple stressors on reef food webs in the northern Gulf of Mexico revealed via ecosystem modeling. Front. Mar. Sci. 2020, 7, 17. [Google Scholar] [CrossRef]
- Macneale, K.H.; Kiffney, P.M.; Scholz, N.L. Pesticides, aquatic food webs, and the conservation of Pacific salmon. Front. Ecol. Environ. 2010, 8, 475–482. [Google Scholar] [CrossRef]
- Aschehoug, E.T.; Sivakoff, F.S.; Cayton, H.L.; Morris, W.F.; Haddad, N.M. Habitat restoration affects immature stages of a wetland butterfly through indirect effects on predation. Ecology 2015, 96, 1761–1767. [Google Scholar] [CrossRef]
- Watts, C.; Thornburrow, D.; Cave, V. Responses of invertebrates to herbicide in Salix cinerea invaded wetlands: Restoration implications. Ecol. Manag. Restor. 2016, 17, 243–249. [Google Scholar] [CrossRef]
- Peterson, C.H.; Rice, S.D.; Short, J.W.; Esler, D.; Bodkin, J.L.; Ballachey, B.E.; Irons, D.B. Long-term ecosystem response to the Exxon Valdez oil spill. Science 2003, 302, 2082–2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hook, S.E. Beyond thresholds: A holistic approach to impact assessment is needed to enable accurate predictions of environmental risk from oil spills. Integr. Environ. Assess. Manag. 2020, 16, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, H.; Yuan, Y.; Zhao, Y.; Li, G.; Zhang, F. Indirect effect of nutrient accumulation intensified toxicity risk of metals in sediments from urban river network. Environ. Sci. Pollut. Res. 2020, 27, 6193–6204. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.J.; De Schamphelaere, K.; Clements, W.H.; Sorensen, M.T.; Van den Brink, P.J.; Liess, M. Combined and interactive effects of global climate change and toxicants on populations and communities. Environ. Toxicol. Chem. 2013, 32, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sievers, M.; Hale, R.; Parris, K.M.; Swearer, S.E. Impacts of human-induced environmental change in wetlands on aquatic animals. Biol. Rev. Camb. Philos. Soc. 2018, 93, 529–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, S.P.; Grabowski, J.H.; Roman, H.; Geggel, A.; Rouhani, S.; Oehrig, J.; Baker, M. Consequences of large-scale salinity alteration during the Deepwater Horizon oil spill on subtidal oyster populations. Mar. Ecol. Prog. Ser. 2017, 576, 175–187. [Google Scholar] [CrossRef]
- Deis, D.R.; Fleeger, J.W.; Johnson, D.S.; Mendelssohn, I.A.; Lin, Q.; Graham, S.A.; Zengel, S.; Hou, A. Recovery of the salt marsh periwinkle (Littoraria irrorata) 9 years after the Deepwater Horizon oil spill: Size matters. Mar. Poll. Bull. 2020, 160, 111581. [Google Scholar] [CrossRef]
- Fleeger, J.W.; Johnson, D.S.; Zengel, S.; Mendelssohn, I.A.; Deis, D.R.; Graham, S.A.; Lin, Q.; Christman, M.C.; Riggio, M.R.; Pant, M. Macroinfauna responses and recovery trajectories after an oil spill differ from those following saltmarsh restoration. Mar. Environ. Res. 2020, 155. [Google Scholar] [CrossRef]
- Nadal, M.; Marques, M.; Mari, M.; Domingo, J.L. Climate change and environmental concentrations of POPs: A review. Environ. Res. 2015, 143, 177–185. [Google Scholar] [CrossRef]
- Pincebourde, S.; van Baaren, J.; Rasmann, S.; Rasmont, P.; Rodet, G.; Martinet, B.; Calatayud, P.A. Plant-insect interactions in a changing world. In Insect-Plant Interactions in a Crop Protection Perspective; Sauvion, N., Thiery, D., Calatayud, P.A., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 81, pp. 289–332. [Google Scholar]
- Sumner, A.W.; Johnston, T.A.; Lescord, G.L.; Branfireun, B.A.; Gunn, J.M. Mercury Bioaccumulation in lacustrine fish populations along a climatic gradient in northern Ontario, Canada. Ecosystems 2019. [Google Scholar] [CrossRef]
- Bates, M.L.; Nash, S.M.B.; Hawker, D.W.; Shaw, E.C.; Cropp, R.A. The distribution of persistent organic pollutants in a trophically complex Antarctic ecosystem model. J. Mar. Syst. 2017, 170, 103–114. [Google Scholar] [CrossRef]
- Cambronero, M.C.; Marshall, H.; De Meester, L.; Davidson, T.A.; Beckerman, A.P.; Orsini, L. Predictability of the impact of multiple stressors on the keystone species Daphnia. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Van den Brink, P.J. Ecological risk assessment: From book-keeping to chemical stress ecology. Environ. Sci. Technol. 2008, 42, 8999–9004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, A.; Price, O.R.; Marshall, S.; Jolliet, O.; Van den Brink, P.J.; Rico, A.; Focks, A.; De Laender, F.; Ashauer, R. Toward refined environmental scenarios for ecological risk assessment of down-the-drain chemicals in freshwater environments. Integr. Environ. Assess. Manag. 2017, 13, 233–248. [Google Scholar] [CrossRef] [Green Version]
- Fodrie, F.J.; Able, K.W.; Galvez, F.; Heck, K.L.; Jensen, O.P.; Lopez-Duarte, P.C.; Martin, C.W.; Turner, R.E.; Whitehead, A. Integrating organismal and population responses of estuarine fishes in Macondo spill research. Bioscience 2014, 64, 778–788. [Google Scholar] [CrossRef]
- Whitehead, A.; Dubansky, B.; Bodinier, C.; Garcia, T.I.; Miles, S.; Pilley, C.; Raghunathan, V.; Roach, J.L.; Walker, N.; Walter, R.B.; et al. Genomic and physiological footprint of the Deepwater Horizon oil spill on resident marsh fishes. Proc. Nat. Acad. Sci. USA 2012, 109, 20298–20302. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.W.; Lewis, K.A.; McDonald, A.M.; Spearman, T.P.; Alford, S.B.; Christian, R.C.; Valentine, J.F. Disturbance-driven changes to northern Gulf of Mexico nekton communities following the Deepwater Horizon oil spill. Mar. Poll. Bull. 2020, 155, 111098. [Google Scholar] [CrossRef]
- De Laender, F. Community- and ecosystem-level effects of multiple environmental change drivers: Beyond null model testing. Glob. Chang. Biol. 2018, 24, 5021–5030. [Google Scholar] [CrossRef] [Green Version]
- Liess, M.; Foit, K.; Becker, A.; Hassold, E.; Dolciotti, I.; Kattwinkel, M.; Duquesne, S. Culmination of low-dose pesticide effects. Environ. Sci. Technol. 2013, 47, 8862–8868. [Google Scholar] [CrossRef]
- Kattwinkel, M.; Liess, M. Competition matters: Species interactions prolong the long-term effects of pulsed toxicant stress on populations. Environ. Toxicol. Chem. 2014, 33, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Van den Brink, P.J.; Choung, C.B.; Landis, W.; Mayer-Pinto, M.; Pettigrove, V.; Scanes, P.; Smith, R.; Stauber, J. New approaches to the ecological risk assessment of multiple stressors. Mar. Freshwat. Res. 2016, 67, 429–439. [Google Scholar] [CrossRef]
- Galic, N.; Sullivan, L.L.; Grimm, V.; Forbes, V.E. When things don’t add up: Quantifying impacts of multiple stressors from individual metabolism to ecosystem processing. Ecol. Lett. 2018, 21, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Gobel, P.; Dierkes, C.; Coldewey, W.C. Storm water runoff concentration matrix for urban areas. J. Contam. Hydrol. 2007, 91, 26–42. [Google Scholar] [CrossRef]
- Laskowski, R.; Bednarska, A.J.; Kramarz, P.E.; Loureiro, S.; Scheil, V.; Kudłek, J.; Holmstrup, M. Interactions between toxic chemicals and natural environmental factors—A meta-analysis and case studies. Sci. Total Environ. 2010, 408, 3763–3774. [Google Scholar] [CrossRef]
- Sievers, M.; Hale, R.; Swearer, S.E.; Parris, K.M. Contaminant mixtures interact to impair predator-avoidance behaviours and survival in a larval amphibian. Ecotoxicol. Environ. Saf. 2018, 161, 482–488. [Google Scholar] [CrossRef]
- Kotalik, C.J.; Cadmus, P.; Clements, W.H. Indirect effects of iron oxide on stream benthic communities: Capturing ecological complexity with controlled mesocosm experiments. Environ. Sci. Technol. 2019, 53, 11532–11540. [Google Scholar] [CrossRef]
- Boyle, T.P.; Fairchild, J.F. The role of mesocosm studies in ecological risk analysis. Ecol. Appl. 1997, 7, 1099–1102. [Google Scholar] [CrossRef]
- Clemow, Y.H.; Manning, G.E.; Breton, R.L.; Winchell, M.F.; Padilla, L.; Rodney, S.I.; Hanzas, J.P.; Estes, T.L.; Budreski, K.; Toth, B.N.; et al. A Refined ecological risk assessment for California red-legged frog, delta smelt, and California tiger salamander exposed to malathion. Integr. Environ. Assess. Manag. 2018, 14, 224–239. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, C.A.; Crawley, M.J.; Wright, D.J.; Kuczynski, J.; Robinson, L.; Knight, R.; Abu Al-Soud, W.; Sorensen, S.J.; Deng, Y.; Zhou, J.Z.; et al. Identifying qualitative effects of different grazing types on below-ground communities and function in a long-term field experiment. Environ. Microbiol. 2015, 17, 841–854. [Google Scholar] [CrossRef]
- Gutierrez, Y.; Ott, D.; Scherber, C. Direct and indirect effects of plant diversity and phenoxy herbicide application on the development and reproduction of a polyphagous herbivore. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Riedl, V.; Agatz, A.; Benstead, R.; Ashauer, R. A standardized tritrophic small-scale system (tricosm) for the assessment of stressor-induced effects on aquatic community dynamics. Environ. Toxicol. Chem. 2018, 37, 1051–1060. [Google Scholar] [CrossRef]
- Duggan, S.B.; Kotalik, C.J.; Clements, W.H. Integrating results of field biomonitoring and mesocosm experiments to validate postspill impacts of petroleum hydrocarbons on stream benthic communities. Environ. Sci. Technol. 2018, 52, 13584–13590. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Schmidt, T.S.; Clements, W.H. Quantifying differences in responses of aquatic insects to trace metal exposure in field studies and short-term stream mesocosm experiments. Environ. Sci. Technol. 2018, 52, 4378–4384. [Google Scholar] [CrossRef] [PubMed]
- Fournier, B.; Dos Santos, S.P.; Gustavsen, J.A.; Imfeld, G.; Lamy, F.; Mitchell, E.A.D.; Mota, M.; Noll, D.; Planchamp, C.; Heger, T.J. Impact of a synthetic fungicide (fosetyl-Al and propamocarb-hydrochloride) and a biopesticide (Clonostachys rosea) on soil bacterial, fungal, and protist communities. Sci. Total Environ. 2020, 738. [Google Scholar] [CrossRef]
- Helander, M.; Pauna, A.; Saikkonen, K.; Saloniemi, I. Glyphosate residues in soil affect crop plant germination and growth. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Masion, A.; Mouneyrac, C.; de Garidel-Thoron, C.; Hendren, C.O.; Thiery, A.; Santaella, C.; Giamberini, L.; Bottero, J.-Y.; Wiesner, M.R.; et al. Contribution of mesocosm testing to a single-step and exposure-driven environmental risk assessment of engineered nanomaterials. Nanoimpact 2019, 13, 66–69. [Google Scholar] [CrossRef]
- Traas, T.P.; Janse, J.H.; van den Brink, P.J.; Brock, T.C.M.; Aldenberg, T. A freshwater food web model for the combined effects of nutrients and insecticide stress and subsequent recovery. Environ. Toxicol. Chem. 2004, 23, 521–529. [Google Scholar] [CrossRef]
- Spromberg, J.A.; John, B.M.; Landis, W.G. Metapopulation dynamics: Indirect effects and multiple distinct outcomes in ecological risk assessment. Environ. Toxicol. Chem. 1998, 17, 1640–1649. [Google Scholar] [CrossRef]
- Howick, G.L.; Giddings, J.M.; Denoyelles, F.; Ferrington, L.C.; Kettle, W.D.; Baker, D. Rapid establishment of test conditions and trophic-level interactions in 0.04-hectare earthen pond mesocosms. Environ. Toxicol. Chem. 1992, 11, 107–114. [Google Scholar] [CrossRef]
- Forbes, V.E.; Salice, C.J.; Birnir, B.; Bruins, R.J.F.; Calow, P.; Ducrot, V.; Galic, N.; Garber, K.; Harvey, B.C.; Jager, H.; et al. A framework for predicting impacts on ecosystem services from (sub)organismal responses to chemicals. Environ. Toxicol. Chem. 2017, 36, 845–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galic, N.; Schmolke, A.; Forbes, V.; Baveco, H.; van den Brink, P.J. The role of ecological models in linking ecological risk assessment to ecosystem services in agroecosystems. Sci. Total Environ. 2012, 415, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Van den Brink, P.J.; Baird, D.J.; Baveco, H.; Focks, A. The use of traits-based approaches and eco(toxico)logical models to advance the ecological risk assessment framework for chemicals. Integr. Environ. Assess. Manag. 2013, 9, E47–E57. [Google Scholar] [CrossRef]
- Strona, G.; Fattorini, S.; Fiasca, B.; Di Lorenzo, T.; Di Cicco, M.; Lorenzetti, W.; Boccacci, F.; Galassi, D.M.P. AQUALIFE software: A new tool for a standardized ecological assessment of groundwater dependent ecosystems. Water 2019, 11, 2574. [Google Scholar] [CrossRef] [Green Version]
- Etterson, M.; Garber, K.; Odenkirchen, E. Mechanistic modeling of insecticide risks to breeding birds in North American agroecosystems. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.C.; Jin, X.; Nakada, N.; Sumpter, J.P. Learning from the past and considering the future of chemicals in the environment. Science 2020, 367, 384–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rico, A.; Van den Brink, P.J. Evaluating aquatic invertebrate vulnerability to insecticides based on intrinsic sensitivity, biological traits, and toxic mode of action. Environ. Toxicol. Chem. 2015, 34, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Xia, P.; Wang, P.P.; Yang, J.H.; Baird, D.J. Omics advances in ecotoxicology. Environ. Sci. Technol. 2018, 52, 3842–3851. [Google Scholar] [CrossRef]
- Ciallella, H.L.; Russo, D.P.; Aleksunes, L.M.; Grimm, F.A.; Zhu, H. Predictive modeling of estrogen receptor agonism, antagonism, and binding activities using machine- and deep-learning approaches. Lab. Investig. 2020. [Google Scholar] [CrossRef]
- Ollivier, M.; Lesieur, V.; Raghu, S.; Martin, J.F. Characterizing ecological interaction networks to support risk assessment in classical biological control of weeds. Curr. Opin. Insect Sci. 2020, 38, 40–47. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Kong, X.Z.; Qin, N.; He, Q.S.; Liu, W.X.; Bai, Z.L.; Wang, Y.; Xu, F.L. Combining species sensitivity distribution (SSD) model and thermodynamic index (exergy) for system-level ecological risk assessment of contaminates in aquatic ecosystems. Environ. Int. 2019, 133. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleeger, J.W. How Do Indirect Effects of Contaminants Inform Ecotoxicology? A Review. Processes 2020, 8, 1659. https://doi.org/10.3390/pr8121659
Fleeger JW. How Do Indirect Effects of Contaminants Inform Ecotoxicology? A Review. Processes. 2020; 8(12):1659. https://doi.org/10.3390/pr8121659
Chicago/Turabian StyleFleeger, John W. 2020. "How Do Indirect Effects of Contaminants Inform Ecotoxicology? A Review" Processes 8, no. 12: 1659. https://doi.org/10.3390/pr8121659
APA StyleFleeger, J. W. (2020). How Do Indirect Effects of Contaminants Inform Ecotoxicology? A Review. Processes, 8(12), 1659. https://doi.org/10.3390/pr8121659