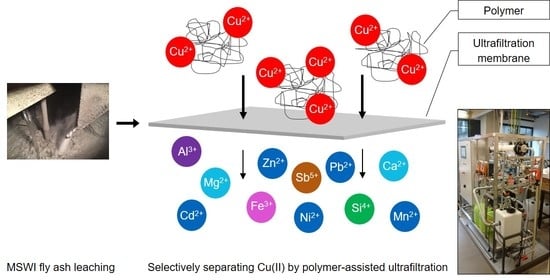
3.1. Metal Retention of Different PEIs in MSWI Fly Ash Extracts
Three different polyethyleneimines were investigated regarding their retention behavior towards all metal species contained in the fly ash extracts from MVA Ingolstadt and KEBAG Zuchwil. Composition of the fly ash extracts was previously analyzed; the resulting main metal components and chloride concentrations are shown in
Table S1.
Figure 2 shows the retention of Cd(II), Cu(II), Ni(II), Pb(II), Zn(II), Fe(III), and Sb(V) using HB-PEI (
Figure 2a,b), PE-PEI (
Figure 2c,d), and MOD-PEI (
Figure 2e,f). HB-PEI contains primary, secondary, and tertiary amino groups. Each PEI was investigated using the fly ash extracts from both MVA Ingolstadt (
Figure 2b,d,f) and KEBAG Zuchwil (
Figure 2a,c,e).
As illustrated in
Figure 2a–f, Cu(II) is the only metal ion retained at approximately 100% from pH 3.0 upwards. At this pH, Cd(II) and Pb(II) are retained at about 10% using PE-PEI (
Figure 2c,d), and 15–30% using HB-PEI (
Figure 2a,b). The fly ash extract from MVA Ingolstadt additionally contains Fe(III) and Sb(V), which are increasingly retained by the membrane starting from pH 3.0 on (
Figure 2b,d,f).
As shown in
Table 2, both fly ash extracts contained many more metal ions than depicted in
Figure 2. The retention of Li, Na, K, Rb, Mg, Ca, Sr, Si, Al, and Mn was also investigated in the same PAUF experiments described in
Figure 2. All were barely retained by the membrane, as can be seen in
Table 2. Regarding the monovalent alkali metal ions that was to be expected, however, the nonbinding especially of divalent alkaline earth metal ions is a crucial result regarding the target Cu(II) selectivity in the treatment of highly saline fly ash extracts.
The high Cu(II) retention observed is based on the interaction of Cu(II) with the respective PEI. Cu(II) forms tetraamminecopper(II) complexes with amine groups of the three PEIs. Ni(II) and Zn(II) are retained by forming ammine complexes as well but only at pH > 3.0.
To clarify the observed retention of additional metal ions (
Figure 2a–f), ultrafiltration experiments were carried out using fly ash extract without the addition of PEI. The results obtained are shown in
Figure S2.
To some extent, Pb, Fe, and Sb were retained in the PAUF experiments described above, but they also showed similar retention in the experiments without the addition of PEI (
Figure S2). During these experiments, visible turbidity of the feed solutions was observed; the feed samples were centrifuged and the supernatants were analyzed. A continuously decreasing Fe concentration above pH 3.0 was observed: Between pH 3.0 and 5.0 Fe(II) and Fe(III) may both exist, but Fe(II)-hydroxide requires a pH > 7.0 for precipitation [
3]. Therefore, Fe(III) was precipitated as solid iron(III)oxide hydrate and retained by the ultrafiltration membrane in the PAUF experiments (
Figure 2b,d,f).
The Sb concentration of the centrifuged feed samples also decreased above pH 3.0. This could occur due to the precipitation of Na[Sb(OH)
6], formed with sodium ions contained in the fly ash extract and also resulting from adjusting the pH with sodium hydroxide. Furthermore, Sb(V) and Sb(III) may coexist [
31], enabling coprecipitation of Sb(OH)
3 with Fe(III) oxide hydrate. Similar retention progression of antimony and iron, shown in
Figure 2b,f, indicates this.
Pb(II) was retained to different degrees using HB-PEI, PE-PEI, and MOD-PEI (
Figure 2) and also without the addition of PEI (
Figure S2). Dissolved Pb
2+ forms lead chloro complexes such as [PbCl]
+, [PbCl
3]
−, [PbCl
4]
2− and/or hardly soluble PbCl
2 in the presence of 60 g L
−1 chloride [
32], as contained in the fly ash extracts. Weibel et al., thoroughly investigated Pb-chloro complex formation in fly ash extract and identified [PbCl
3]
− and [PbCl
4]
2− as the mainly present species [
3,
6]. Presumably, a certain percentage of the Pb(II) binds to protonated amino groups of the PEIs via these negatively charged chloro complexes. Especially tertiary amino groups of polyethyleneimine may act as anion exchangers and therefore bind negatively charged chloro complexes. The lead retention observed in the experiments without PEI, however, may arise from lead precipitated as PbCl
2.
As shown in
Figure 2a–d, Cd(II) was also retained to a small extent. This may be due to the formation of stable negatively charged Cd(II) chloro complexes binding to polyethyleneimine analogous to Pb(II) [
33]. Weibel et al., found [CdCl
4]
2− and [CdCl
3]
− to be the dominant Cd-chloro complexes formed in fly ash extracts [
6]. Cadmium, however, did not show any retention without PEI (
Figure S2), because CdCl
2 is water-soluble, in contrast to PbCl
2.
Conclusively, at pH 3.0, Cu(II) was the only metal ion being bound to the investigated PEIs via metal complex formation with amine groups.
After enrichment, Cu(II) has to be released from the polyethyleneimine in order to achieve selective Cu(II) separation. At the same time, a complete metal release means regeneration of the polymer, which can then be reused in further enrichment cycles.
We investigated the Cu(II) release by decreasing the pH of the feed solution at the end of each PAUF experiment. For all six feed solutions shown in
Figure 2, Cu(II) retention at pH 1.0 was 0% using fly ash extract from KEBAG Zuchwil (
Figure 2a,c,e) and 4–5% using fly ash extract from MVA Ingolstadt (
Figure 2b,d,f). Therefore, Cu(II) was successfully released from all investigated PEIs at pH ≤ 1.
Summarizing the sorption behavior of MOD-PEI, Cu(II) was selectively separated at pH 3.0 from 16 different metal ions, including additional heavy metals, alkaline and alkaline earth metals. Moreover, the very high chloride concentration of 60 g L
−1 in the highly saline fly ash extracts did not influence the pH-dependent Cu(II) chelating behavior. This also applies for HB-PEI [
19] and PE-PEI [
20]. The selective Cu(II) separation from real fly ash extract is even more exceptional, as the Cu(II) concentration in fly ash extracts is up to 380 times lower compared to interfering ions, such as alkaline (earth) metals or zinc. The highest selectivity regarding Cu(II) separation was achieved with MOD-PEI (
Figure 2e,f). Compared to HB-PEI and PE-PEI MOD-PEI provides a lower number of amino groups. This leads to an early displacement of other competing metal ions by Cu(II) because Cu(II) forms the most stable transition-metal complex compounds among all metal ions present in the investigated fly ash extracts [
34].
3.4. Operating Data of PAUF Pilot Plant Using HB-PEI
Operating data of the PAUF pilot plant were investigated by treating a fly ash extract from MVA Ingolstadt containing different concentrations of HB-PEI by tangential flow ultrafiltration. The pilot plant was operated at a transmembrane pressure (TMP) of 5 bar, and the temperature in the filtration circuit was 40 °C. The water-soluble polymer was trapped inside the filtration circuit and the polymer-free permeate was led back into the feed reservoir of the pilot plant.
The influence of polymer concentration and tangential velocity on the permeate flux and specific power uptake of the pilot plant are summarized in
Figure 4. Power uptake included the power consumption of the pressure and the tangential flow pump. Performance of the tangential flow filtration clearly increased with increasing tangential velocity and decreased with increasing polymer concentration in the filtration circuit.
PAUF is influenced by gel layer formation [
21,
22,
23], which occurs in the boundary layer on the membrane surface due to the retention of the water-soluble polymer. This results in additional flow resistance, decreasing the achievable permeate flux and increasing the specific power uptake of the process [
35]. Gel layer formation is reduced by increased tangential velocity in tangential flow filtration, as turbulent flow conditions on the membrane surface reduce the thickness of the boundary layer [
36]. Consequently, the permeate flux of the pilot plant increased with increasing tangential velocity.
The transition from laminar to turbulent flow regime in the channels of the tubular ceramic membranes appeared at tangential velocities between 0.3 and 0.8 m s−1 and was observable in a strong decrease of specific power uptake. Depending on the HB-PEI concentration, a minimum specific power uptake was observed at a tangential velocity of about 0.8 m s−1 (1–10 g L−1 HB-PEI) and 1.0 m s−1 (20 and 30 g L−1 HB-PEI). With further increase of tangential velocity, the specific power uptake rose moderately. This results from the logarithmic rise of the permeate flux, but a disproportional rise of pressure loss in the filtration circuit with increasing tangential velocity occurs.
The permeate flux achieved by the pilot plant is comparable to other work [
15,
21,
22,
23] but rather low. Reasons for this are the very high salt content of the treated fly ash extract, the high water-soluble polymer concentration used in the filtration circuit, and a progressive fouling of the ceramic tubular membranes that were used for the treatment of PEI containing fly ash extract. Irreversible fouling (i.e., by irreversible pore blocking), which cannot be reversed by counter-flushing of the membranes, was significant, but remained in a steady state. So, the pilot plant data given in this work represent realistic conditions using ceramic tubular membranes.
3.5. Multistage Process for Technical Scale Separation of Cu(II) from Fly Ash Extract
A PAUF-based process for the selective separation and purification of Cu(II) was investigated, as shown in
Figure 5. In the retention stage, fly ash extract and HB-PEI solution were fed to an ultrafiltration plant. Cu(II) was bound by the polymer and retained in the filtration circuit. A preconcentrate containing Cu(II) loaded HB-PEI in fly ash extract was (continuously) ejected from the filtration circuit, and the Cu(II) depleted permeate was discharged as wastewater. In order to improve the selectivity of Cu(II) toward interfering ions in the fly ash extract, the preconcentrate was purified in another ultrafiltration stage. This can be done either by thickening the preconcentrate, where the concentration of Cu(II) loaded polymer and therefore Cu(II) in the filtration circuit is enriched or by rinsing the filtration circuit with water in order to displace fly ash extract from the polymer solution. Depending on the technical realization, the steps may be done in a different order or at the same time. For Cu(II) recovery and regeneration of HB-PEI, the pH is decreased and Cu(II) is released from the polymer. Cu(II) is rinsed from the filtration circuit, producing a polymer-free Cu(II) concentrate. The regenerated HB-PEI solution is recirculated into the retention stage.
The technical implementation of the different steps (retention, enrichment/purification, regeneration) is investigated in the following sections. The cumulative volume of solutions fed into the pilot plant is given in ѳ (multiples of the filtration circuit volume). The concentration of elements in the filtration circuit is referenced to their concentration in the feed solution (in continuous operation) respective to their concentration in the filtration circuit at the beginning of the rinsing experiments (in batch operation).
3.5.1. Retention of Cu(II) in Continuous Operation
The retention of Cu(II) was investigated with two fly ash extracts containing 0.13 g L
−1 and 0.7 g L
−1 Cu(II). The pilot plant was filled with 4 g L
−1 pretreated HB-PEI in water. After startup, fly ash extract was fed to the plant and treated at pH 3.0.
Figure 6 shows the concentrations of Cu(II), Pb(II), Zn(II), and Ca(II) in the filtration circuit during both experiments.
At the beginning, the concentration of elements in the filtration circuit was lower than in the fly ash extract, resulting in an arithmetically negative enrichment factor. After the feed was switched to fly ash extract, the water in the filtration circuit was continuously displaced and the concentrations of Cu(II), Zn(II), Pb(II), and Ca(II) increased. After ѳ = 3, Ca(II) concentration in the filtration circuit was equal to the Ca(II) concentration in the feed solution and therefore the enrichment was zero. This implies that the displacement of water was completed and the filtration circuit from then on was only filled with fly ash extract and HB-PEI.
Ca(II) enrichment remained zero in further operation of the pilot plant. In accordance with the laboratory experiments, Ca(II) was not retained in PAUF. It represents further elements that were not bound by HB-PEI at pH 3.0 which also showed zero enrichment (including chloride).
For Zn(II) and Pb(II), a slight enrichment in the filtration circuit was observed, reaching a steady state at ѳ = 4. This slight enrichment was limited to 10% for Zn(II) and 30% for Pb(II). The latter already showed a slight binding by HB-PEI in laboratory experiments. This originates from Pb-chloro complexes interacting electrostatically with HB-PEI (see
Section 3.1). At pH 3.0 Zn(II) can also form Zn-chloro complexes (weaker compared to Pb-chloro complexes) [
6]. Both metal ions do not bind to HB-PEI via metal complex formation at pH 3.0 and therefore showed no linear increase in the filtration circuit in continuous operation.
In contrast, the concentration of Cu(II) in the filtration circuit correlated linearly with the volume of fly ash extract treated, irrespective of whether a feed with 0.13 g L−1 or 0.7 g L−1 Cu(II) was used. With 4 g L−1 of HB-PEI, the effective Cu(II) concentration in the filtration circuit was limited to 0.8 g L−1 Cu(II) when loading of 200 mg Cu(II)/g polymer was accepted. Cu(II) exceeding this concentration was not sufficiently bound by HB-PEI and was lost in the following stages.
For Cu(II) retention in continuous operation, addition of sodium hydroxide solution to the filtration circuit is required, as otherwise the pH value drops and Cu(II) is no longer retained. The intense mixing due to the tangential flow and high buffer capacity of the polymer solution allowed perfect pH control in the filtration circuit. The process was able to selectively retain Cu(II) in the filtration circuit, even if strong fluctuation of the Cu(II) concentration occurred in the feed.
Though Cu(II) was enriched considerably, the achievable selectivity of Cu(II) toward Zn(II), Pb(II), and Ca(II) in a preconcentrate ejected from the filtration circuit was quite low (
Table 4). In steady state, the Cu(II) loaded polymer was in the fly ash extract the filtration circuit was filled with. To improve Cu(II) selectivity, the fly ash extract had to be rinsed out from the preconcentrate in a second step.
3.5.2. Cu(II) Enrichment and Purification
Rinsing of the preconcentrate with water in the batch process was investigated. The purpose of this procedure is to displace fly ash extract from the preconcentrate gained in the retention step. Due to its high concentration in the preconcentrate, Ca(II) concentration serves as reference for further unbound species in the filtration circuit. As can be seen in
Figure 7, the concentration of Ca(II) followed an exponential decrease during rinsing, which can be described by Equation (2). The concentration of an unbound species in the filtration circuit decreased from the initial concentration
according to
with ѳ giving the volume of rinsing water used (in multiples of filtration circuit volume). An evaluation of this equation is given in
Table 5. Though slightly bound by the polymer in laboratory experiments and in the retention step, Zn(II) and Pb(II) were successfully rinsed from the filtration circuit equally to unbound species.
An alternative way to increase Cu(II) selectivity is to thicken the preconcentrate. This is achieved by feeding Cu(II) loaded preconcentrate exceeding the volume of the filtration circuit to the pilot plant. The polymer is retained by the membrane and Cu(II) is enriched linearly with increasing polymer concentration in the filtration circuit, while the concentration of unbound species passing the membrane remains equal. Combining the thickening step with purification of the concentrate obtained by rinsing with water, a substantial enhancement of Cu(II) selectivity toward unbound species could be obtained (
Table 6).
When the preconcentrate was rinsed at an initial Cu(II) loading of 250 mg Cu(II)/g polymer, a significant loss of Cu(II) occurred. Therefore, rinsing was optimized by reducing the initial loading to 200 Cu(II)/g polymer
. As can be seen in
Figure 7, the Cu(II) concentration did not drop when rinsing was done at pH 4.0, and only slightly decreased when the pH value was lowered to 3.5 (96%) and 3.0 (94%) during rinsing (ѳ = 1.8).
The very high concentration of interfering ions in the preconcentrate requires intense rinsing of the filtration circuit. It was very efficient at the beginning but dilution of the fly ash extract in the filtration circuit resulted in a steady decrease of rinsing efficiency with increasing volume of rinsing water used. High purification of the Cu(II) preconcentrate was accompanied by a high consumption of rinsing water and, due to the application of tangential flow ultrafiltration, energy. Using PAUF for selective enrichment and separation requires processes for further metal recovery that tolerate remaining interfering ions in the PAUF concentrate in order to minimize the required rinsing.
3.5.3. Regeneration of the Polymer
Finally, regeneration of the polymer and gaining a polymer-free Cu(II) concentrate in a batch process were investigated. Cu(II) was released from HB-PEI by the addition of hydrochloric acid to the filtration circuit until pH 1.0 was reached. Unbound Cu(II) was then rinsed from the filtration circuit by water. The discharge of Cu(II) (Δ) from the filtration circuit, depending on the volume of rinsing solution used (ѳ), is described with an adaption of Equation (2):
Experimental and calculated data for the Cu(II) discharge are given in
Figure 8. Cu(II) was efficiently discharged from the pilot plant by rinsing with water, generating a polymer-free Cu(II) concentrate. The regenerated HB-PEI remained in the filtration circuit and was used in further separation cycles.
Due to the decreased Cu(II) concentration in the filtration circuit, Cu(II) concentration in the accumulated polymer-free permeate steadily decreased (
Figure 8, calculated by mass balancing) with increasing volume of rinsing water. As the regenerated polymer solution was supposed to be used for Cu(II) retention again, a sufficient discharge of Cu(II) from the filtration circuit was necessary, as remaining unbound Cu(II) in the polymer solution was recirculated to the retention step, reducing the performance of this step. Depending on the polymer concentration in the filtration circuit, Cu(II) concentrations in a polymer-free concentrate, as given in
Table 7, can be achieved in a batch regeneration process. An initial polymer loading of 250 mg Cu(II)/g polymer, no Cu(II) retention during rinsing, and 90% Cu(II) discharge are assumed.
3.6. Photometric Control of the Cu(II) Enrichment and Release
For pilot scale operation, control of the Cu(II) enrichment and release in the filtration circuit is highly beneficial. An inline measurement of the Cu(II) enrichment enables an automated pH decrease by acid addition as soon as the Cu(II) loading of the polymer is completed. Then again, as soon as the Cu(II) release is completed, the pH is automatically increased and a new enrichment cycle can be started.
Therefore, the specific change in light absorption of PEI depending on its complex formation can be utilized. Due to the formation of Cu(II) complexes with HB-PEI (tetraamminecopper(II)) [
37] and H
2O, photometric control of the enrichment and release during PAUF experiments [
23] with fly ash extract was investigated.
Figure 9a shows the increasing Cu(II) loading of HB-PEI at pH 4.0 (feed solutions in cuvettes 1–10), followed by a clearly visible color change representing the Cu(II) release at pH 1.0 (feed solution in cuvette 11). Decreasing the pH value, the feed solutions turned colorless to light blue again, indicating the formation of hexaaquacopper(II) and thus the regeneration of HB-PEI.
Figure 9b shows the UV-vis spectra of the PAUF feed samples with increasing Cu(II) concentration from gray to black to green graphs. The prepared solution of [Cu(NH
3)
4]
2+ (
Figure 9b, blue graph) has an absorption maximum at 605 nm. The copper(II) ammine complex formed with HB-PEI absorbs at very similar wavelengths. The intensity of the absorption bands increases until the maximum loading capacity is reached at around 976 mg L
−1 of Cu(II). Additional Cu(II) forms Cu(II) aqua complexes. Therefore, UV-vis spectra are shifted more to the right toward the absorption maximum of [Cu(H
2O)
6]
2+ (810 nm, red graph) and a mixture of ammine and aqua complexes occurs in the feed solution. At pH 1.0, Cu(II) is released from HB-PEI and the Cu(II) aqua complex exists exclusively, showing an absorption maximum at 830 nm (
Figure 9b, orange graph).
It is easy to distinguish between the absorption maxima of the ammine and aqua complexes formed within the Cu(II) enrichment and those obtained during the release from HB-PEI. This enables calibrated photometric control of the selective Cu(II) separation from real fly ash extract by PAUF.