Ion Exchange Dialysis for Aluminium Transport through a Face-Centred Central Composite Design Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Experimental Design and Statistical Analysis
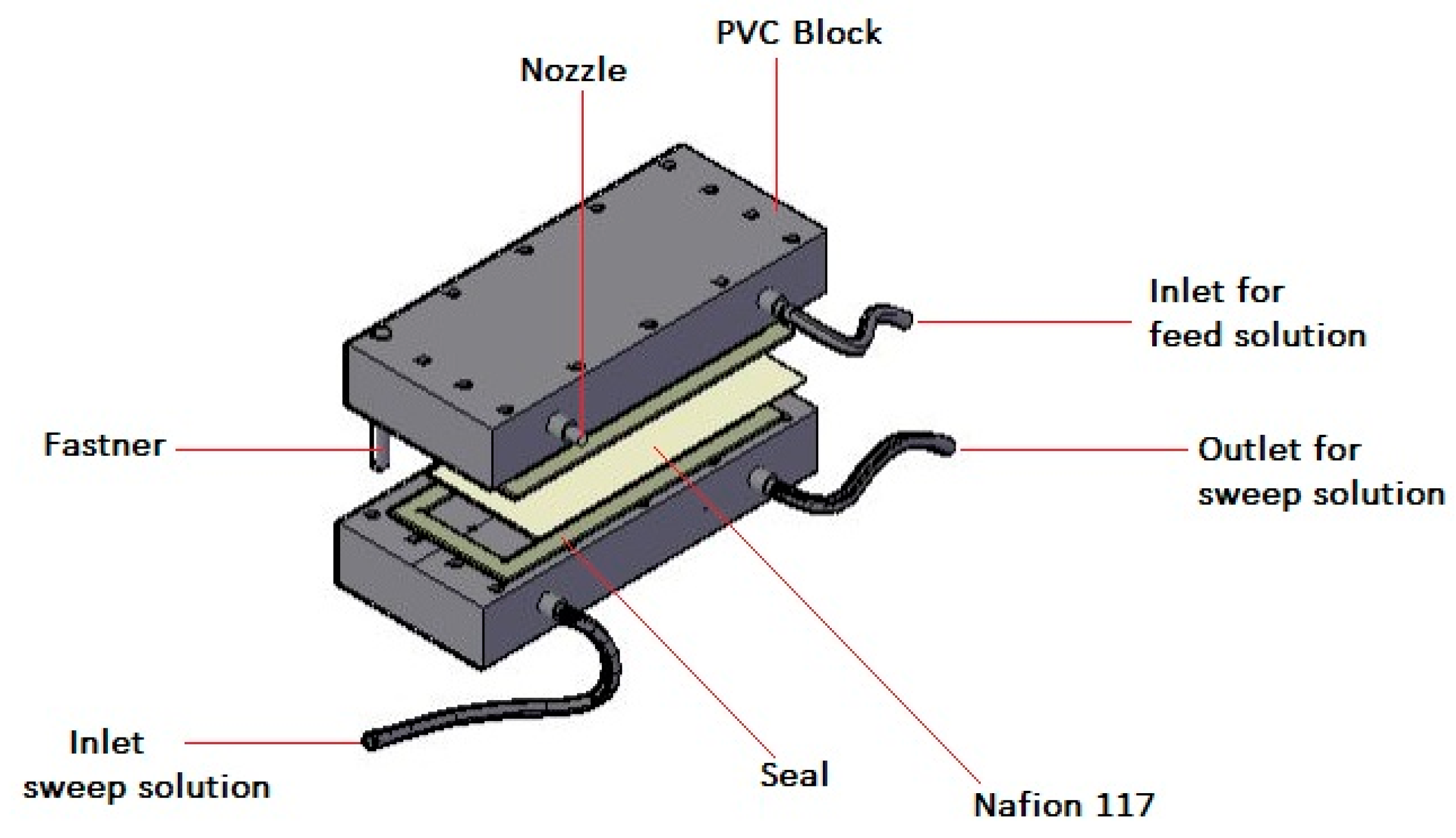
2.3. Ion Exchange Dialysis Set-Up
2.4. Analytical
3. Results
4. Discussion
4.1. Regression Models and Statistical Testing
4.1.1. Analysis of Variance (ANOVA)
4.1.2. Diagnostic Plots
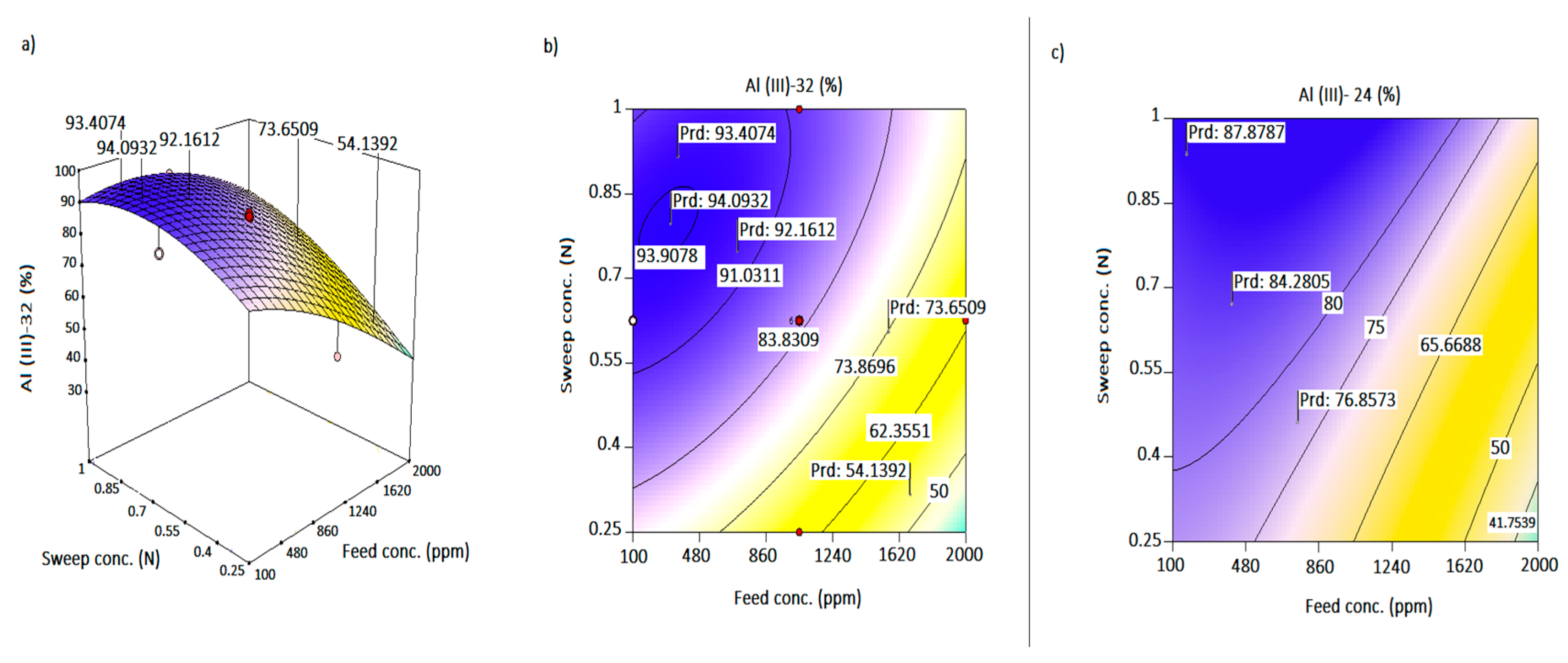
4.2. Combined Effects of Operating Parameters on the Response
4.3. Enrichment Effect
4.4. Desirability
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bobadilla, M.C.; Lorza, R.L.; García, R.E.; Gómez, F.S.; González, E.P.V. Coagulation: Determination of key operating parameters by multi-response surface methodology using desirability functions. Water 2019, 11, 398. [Google Scholar] [CrossRef] [Green Version]
- Barrera-Díaz, C.E.; Balderas-Hernández, P.; Bilyeu, B. Electrocoagulation: Fundamentals and Prospectives. Electrochem. Water Wastewater Treat. 2018, 61–76. [Google Scholar] [CrossRef]
- Kweinor Tetteh, E.; Rathilal, S. Application of Organic Coagulants in Water and Wastewater Treatment. Org. Polym. 2019, 13. [Google Scholar] [CrossRef] [Green Version]
- Keeley, J.; Jarvis, P.; Judd, S.J. An economic assessment of coagulant recovery from water treatment residuals. Desalination 2012, 287, 132–137. [Google Scholar] [CrossRef]
- Nesterenko, P.N. Ion Exchange-Overview, 3rd ed; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Keeley, J.; Jarvis, P.; Judd, S.J. Coagulant Recovery from Water Treatment Residuals: A Review of Applicable Technologies. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2675–2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlowski, S.; Crespo, J.G.; Velizarov, S. Profiled Ion Exchange Membranes: A Comprehensible Review. Int. J. Mol. Sci. 2019, 20, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassanvand, A.; Wei, K.; Talebi, S.; Chen, G.; Kentish, S. The Role of Ion Exchange Membranes in Membrane Capacitive Deionisation. Membranes 2017, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Hagesteijn, K.F.L.; Jiang, S.; Ladewig, B.P. A review of the synthesis and characterization of anion exchange membranes. J. Mater. Sci. 2018, 53, 11131–11150. [Google Scholar] [CrossRef] [Green Version]
- Bdiri, M.; Dammak, L.; Larchet, C.; Hellal, F.; Porozhnyy, M.; Nevakshenova, E.; Pismenskaya, N.; Nikonenko, V. Characterization and cleaning of anion-exchange membranes used in electrodialysis of polyphenol-containing food industry solutions; comparison with cation-exchange membranes. Sep. Purif. Technol. 2019, 210, 636–650. [Google Scholar] [CrossRef]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Sarkar, S.; SenGupta, A.K.; Prakash, P. The Donnan Membrane Principle: Opportunities for Sustainable Engineered Processes and Materials. Environ. Sci. Technol. 2010, 44, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Vanoppen, M.; Stoffels, G.; Demuytere, C.; Bleyaert, W.; Verliefde, A.R.D. Increasing RO efficiency by chemical-free ion-exchange and Donnan dialysis: Principles and practical implications. Water Res. 2015, 80, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.; SenGupta, A.K. Selective Coagulant Recovery from Water Treatment Plant Residuals Using Donnan Membrane Process. Environ. Sci. Technol. 2003, 37, 4468–4474. [Google Scholar] [CrossRef]
- Kim, D.; Judy, J.W. Analysis of Donnan-dialyzer irreproducibility and experimental study of a microfluidic parallel-plate membrane-separation module for total analysis systems. J. Membr. Sci. 2014, 460, 148–159. [Google Scholar] [CrossRef]
- Çengeloǧlu, Y.; Kir, E.; Ersöz, M. Recovery and concentration of Al(III), Fe(III), Ti(IV), and Na(I) from red mud. J. Colloid Interface Sci. 2001, 244, 342–346. [Google Scholar] [CrossRef]
- Çengeloǧlu, Y.; Kir, E.; Ersoz, M.; Buyukerkek, T.; Gezgin, S. Recovery and concentration of metals from red mud by Donnan dialysis. Colloids Surf. A Physicochem. Eng. Asp. 2003, 223, 95–101. [Google Scholar] [CrossRef]
- Sonoc, A.C.; Jeswiet, J.; Murayama, N.; Shibata, J. A study of the application of Donnan dialysis to the recycling of lithium ion batteries. Hydrometallurgy 2018, 175, 133–143. [Google Scholar] [CrossRef]
- Ping, Q.; Abu-Reesh, I.M.; He, Z. Boron removal from saline water by a microbial desalination cell integrated with donnan dialysis. Desalination 2015, 376, 55–61. [Google Scholar] [CrossRef]
- Okada, T.; Xie, G.; Gorseth, O.; Kjelstrup, S.; Nakamura, N.; Arimura, T. Ion and water transport characteristics of Nafion membranes as electrolytes. Electrochim. Acta 1998, 43, 3741–3747. [Google Scholar] [CrossRef]
- Miyoshi, H. Diffusion coefficients of ions through ion excange membrane in Donnan dialysis using ions of different valence. J. Membr. Sci. 1998, 141, 101–110. [Google Scholar] [CrossRef]
- Pessoa-Lopes, M.; Crespo, J.G.; Velizarov, S. Arsenate removal from sulphate-containing water streams by an ion-exchange membrane process. Sep. Purif. Technol. 2016, 166, 125–134. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, H.; Ni, J. Arsenate removal by Donnan dialysis: Effects of the accompanying components. Sep. Purif. Technol. 2010, 72, 250–255. [Google Scholar] [CrossRef]
- Velizarov, S. Transport of arsenate through anion-exchange membranes in Donnan dialysis. J. Membr. Sci. 2013, 425–426, 243–250. [Google Scholar] [CrossRef]
- Wiśniewski, J.A.; Kabsch-Korbutowicz, M.; Łakomska, S. Ion-exchange membrane processes for Br- and BrO3-Ion removal from water and for recovery of salt from waste solution. Desalination 2014, 342, 175–182. [Google Scholar] [CrossRef]
- Marzouk, I.; Dammak, L.; Chaabane, L.; Hamrouni, B. Optimization of Chromium (Vi) Removal by Donnan Dialysis. Am. J. Anal. Chem. 2013, 4, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Turki, T.; Amor, M.B. Nitrate removal from natural water by coupling adsorption and Donnan dialysis. Water Sci. Technol. Water Supply 2017, 17, 771–779. [Google Scholar] [CrossRef]
- Oehmen, A.; Valerio, R.; Llanos, J.; Fradinho, J.; Serra, S.; Reis, M.A.M.; Crespo, J.G.; Velizarov, S. Arsenic removal from drinking water through a hybrid ion exchange membrane—Coagulation process. Sep. Purif. Technol. 2011, 83, 137–143. [Google Scholar] [CrossRef]
- Ayyildiz, H.F.F.; Kara, H. Boron removal by ion exchange membranes. Desalination 2005, 180, 99–108. [Google Scholar] [CrossRef]
- Szczepański, P.; Szczepańska, G. Donnan dialysis—A new predictive model for non−steady state transport. J. Membr. Sci. 2017, 525, 277–289. [Google Scholar] [CrossRef]
- Agarwal, C.; Chaudhury, S.; Pandey, A.K.; Goswami, A. Kinetic aspects of Donnan dialysis through Nafion-117 membrane. J. Membr. Sci. 2012, 415–416, 681–685. [Google Scholar] [CrossRef]
- Agarwal, C.; Goswami, A. Nernst Planck approach based on non-steady state flux for transport in a Donnan dialysis process. J. Membr. Sci. 2016, 507, 119–125. [Google Scholar] [CrossRef]
- Wang, Q.; Lenhart, J.J.; Walker, H.W. Recovery of metal cations from lime softening sludge using Donnan dialysis. J. Membr. Sci. 2010, 360, 469–475. [Google Scholar] [CrossRef]
- Prakash, P.; Hoskins, D.; SenGupta, A.K. Application of homogeneous and heterogeneous cation-exchange membranes in coagulant recovery from water treatment plant residuals using Donnan membrane process. J. Membr. Sci. 2004, 237, 131–144. [Google Scholar] [CrossRef]
- Adesina, O.A.; Abdulkareem, F.; Yusuff, A.S.; Lala, M.; Okewale, A. Response surface methodology approach to optimization of process parameter for coagulation process of surface water using Moringa oleifera seed. S. Afr. J. Chem. Eng. 2019, 28, 46–51. [Google Scholar] [CrossRef]
- Tetteh, E.; Amano, K.O.A.; Asante-Sackey, D.; Armah, E. Response Surface Optimisation of Biogas Potential in Co-Digestion of Miscanthus Fuscus and Cow Dung. Int. J. Technol. 2018, 9, 944. [Google Scholar] [CrossRef] [Green Version]
- John, B.D.; King, P.; Prasanna, K.Y. Optimization of Cu (II) biosorption onto sea urchin test using response surface methodology and artificial neural networks. Int. J. Environ. Sci. Technol. 2019, 16, 1885–1896. [Google Scholar] [CrossRef]
- Taran, M.; Aghaie, E. Designing and optimization of separation process of iron impurities from kaolin by oxalic acid in bench-scale stirred-tank reactor. Appl. Clay Sci. 2015, 107, 109–116. [Google Scholar] [CrossRef]
- Asante-Sackey, D.; Rathilal, S.; Pillay, L.; Tetteh, E.K. Effect of ion exchange dialysis process variables on aluminium permeation using response surface methodology. Environ. Eng. Res. 2019. [Google Scholar] [CrossRef]
- Kleijnen, J.P.C. Response surface methodology. Int. Ser. Oper. Res. Manag. Sci. 2015, 216, 81–104. [Google Scholar]
- Sahoo, P.; Barman, T.K. ANN modelling of fractal dimension in machining. Mechatron. Manuf. Eng. 2012, 159–226. [Google Scholar] [CrossRef]
- Iqbal, M.; Iqbal, N.; Bhatti, I.A.; Ahmad, N.; Zahid, M. Response surface methodology application in optimization of cadmium adsorption by shoe waste: A good option of waste mitigation by waste. Ecol. Eng. 2016, 88, 265–275. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S. Effects of a polymeric organic coagulant for industrial mineral oil wastewater treatment using response surface methodology (Rsm). Water SA 2018, 44, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Owolabi, R.U.; Usman, M.A.; Kehinde, A.J. Modelling and optimization of process variables for the solution polymerization of styrene using response surface methodology. J. King Saud Univ. Eng. Sci. 2018, 30, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Siegel, A.F. Chapter 12-Multiple Regression: Predicting One variable from several others. Pract. Bus. Stat. 2012, 347–416. [Google Scholar] [CrossRef]
- Nair, A.T.; Ahammed, M.M. The reuse of water treatment sludge as a coagulant for post-treatment of UASB reactor treating urban wastewater. J. Clean. Prod. 2015, 96, 272–281. [Google Scholar] [CrossRef]
- Aerts, S.; Haesbroeck, G.; Ruwet, C. Multivariate coefficients of variation: Comparison and influence functions. J. Multivar. Anal. 2015, 142, 183–198. [Google Scholar] [CrossRef]
- Meloun, M.; Militký, J. Statistical analysis of multivariate data. Stat. Data Anal. 2011, 151–403. [Google Scholar] [CrossRef]
- Luis, P. Introduction. Fundam. Model. Memb. Syst. 2018, 1–23. [Google Scholar] [CrossRef]
- Davis, T.A. Donnan Dialysis. In Membrane Processes; Academic Press: Cambridge, MA, USA, 2000; Volume 2, pp. 1701–1707. [Google Scholar]
- Mohapatra, T.; Sahoo, S.S.; Padhi, B.N. Analysis, prediction and multi-response optimization of heat transfer characteristics of a three fluid heat exchanger using response surface methodology and desirability function approach. Appl. Therm. Eng. 2019, 151, 536–555. [Google Scholar] [CrossRef]





| Symbol | Variable | Coded Levels of Variables | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| X1 | Feed concentration (ppm) | 100 | 1050 | 2000 |
| X2 | Feed flowrate (%) | 25 | 55 | 85 |
| X3 | Sweep flowrate (%) | 25 | 55 | 85 |
| X4 | Sweep concentration (N) | 0.25 | 0.625 | 1 |
| Run Order | Variable Level | Response (%) | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | 24 h | 32 h | |
| 1 | 1 | −1 | −1 | −1 | 28.55 | 35.95 |
| 2 | 1 | 1 | 1 | −1 | 33.35 | 45.65 |
| 3 | −1 | 1 | −1 | −1 | 75.90 | 84.10 |
| 4 | 1 | −1 | 1 | 1 | 61.6 | 71.85 |
| 5 | −1 | −1 | −1 | 1 | 70.2 | 78.25 |
| 6 | 0 | 0 | 0 | 0 | 79.1 | 86.00 |
| 7 | 1 | 1 | −1 | 1 | 64.25 | 73.45 |
| 8 | −1 | −1 | 1 | −1 | 58.15 | 61.60 |
| 9 | −1 | 1 | 1 | 1 | 86.95 | 93.55 |
| 10 | 0 | 0 | 0 | 0 | 78.82 | 86.05 |
| 11 | −1 | 1 | −1 | 1 | 87.50 | 94.85 |
| 12 | 0 | 0 | 0 | 0 | 78.36 | 85.96 |
| 13 | 0 | 0 | 0 | 0 | 78.62 | 85.85 |
| 14 | 1 | 1 | 1 | 1 | 51.60 | 63.85 |
| 15 | −1 | 1 | 1 | −1 | 81.40 | 90.00 |
| 16 | −1 | −1 | 1 | 1 | 57.95 | 68.75 |
| 17 | 1 | 1 | −1 | −1 | 32.55 | 32.85 |
| 18 | 1 | −1 | −1 | 1 | 56.95 | 66.95 |
| 19 | −1 | −1 | −1 | −1 | 58.80 | 65.85 |
| 20 | 1 | −1 | 1 | −1 | 30.25 | 34.50 |
| 21 | 0 | 0 | 0 | 0 | 78.98 | 86.01 |
| 22 | 0 | −1 | 0 | 0 | 52.57 | 60.52 |
| 23 | −1 | 0 | 0 | 0 | 78.55 | 84.98 |
| 24 | 0 | 0 | 0 | 1 | 84.81 | 90.19 |
| 25 | 0 | 1 | 0 | 0 | 72.19 | 80.71 |
| 26 | 0 | 0 | 1 | 0 | 66.95 | 77.33 |
| 27 | 0 | 0 | 0 | 0 | 78.99 | 87.12 |
| 28 | 0 | 0 | −1 | 0 | 75.90 | 81.90 |
| 29 | 0 | 0 | 0 | −1 | 48.71 | 54.48 |
| 30 | 1 | 0 | 0 | 0 | 50.65 | 58.75 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value (Prob > F) |
|---|---|---|---|---|---|
| Mean vs. Total | 2130.42 | 1 | 2130.42 | ||
| Linear vs. Block | 24.49 | 4 | 6.12 | 12.09 | <0.0001 |
| 2FI vs. Linear | 4.74 | 6 | 0.7899 | 1.94 | 0.1316 |
| Quadratic vs. 2FI | 5.79 | 4 | 1.45 | 16.72 | <0.0001 |
| Cubic vs. Quadratic | 0.7625 | 8 | 0.0953 | 1.32 | 0.3974 |
| Residual | 0.3623 | 5 | 0.0725 | ||
| Total | 2166.56 | 28 |
| Response | Source | Standard Deviation | Actual R2 | Adjusted R2 | Predicted R2 |
|---|---|---|---|---|---|
| Al3+ transport | Linear | 0.7117 | 0.6776 | 0.6216 | 0.4387 |
| 2FI | 0.6376 | 0.8088 | 0.6963 | 0.3961 | |
| Quadratic | 0.2941 | 0.9689 | 0.9354 | 0.8034 | |
| Cubic | 0.2692 | 0.9900 | 0.9459 | −3.6866 |
| Source | Sum of Squares | Df | Mean Squares | F-Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Regression model | 34.51 | 8 | 4.31 | 50.18 | <0.0001 |
| X1-Feed conc. | 12.74 | 1 | 12.74 | 148.28 | <0.0001 |
| X2-Feed flow | 2.49 | 1 | 2.49 | 28.95 | <0.0001 |
| X4-Sweep conc. | 9.25 | 1 | 9.25 | 107.66 | <0.0001 |
| X1X2 | 1.24 | 1 | 1.24 | 14.38 | 0.0012 |
| X1X4 | 3.01 | 1 | 3.01 | 34.97 | <0.0001 |
| 0.4585 | 1 | 0.4585 | 5.33 | 0.0323 | |
| 0.6027 | 1 | 0.6027 | 7.01 | 0.0159 | |
| 0.4645 | 1 | 0.4645 | 5.40 | 0.0313 | |
| Residuals | 1.63 | 19 | 0.0859 | ||
| Pure Error | 0.0018 | 3 | 0.0006 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asante-Sackey, D.; Rathilal, S.; V. Pillay, L.; Kweinor Tetteh, E. Ion Exchange Dialysis for Aluminium Transport through a Face-Centred Central Composite Design Approach. Processes 2020, 8, 160. https://doi.org/10.3390/pr8020160
Asante-Sackey D, Rathilal S, V. Pillay L, Kweinor Tetteh E. Ion Exchange Dialysis for Aluminium Transport through a Face-Centred Central Composite Design Approach. Processes. 2020; 8(2):160. https://doi.org/10.3390/pr8020160
Chicago/Turabian StyleAsante-Sackey, Dennis, Sudesh Rathilal, Lingham V. Pillay, and Emmanuel Kweinor Tetteh. 2020. "Ion Exchange Dialysis for Aluminium Transport through a Face-Centred Central Composite Design Approach" Processes 8, no. 2: 160. https://doi.org/10.3390/pr8020160
APA StyleAsante-Sackey, D., Rathilal, S., V. Pillay, L., & Kweinor Tetteh, E. (2020). Ion Exchange Dialysis for Aluminium Transport through a Face-Centred Central Composite Design Approach. Processes, 8(2), 160. https://doi.org/10.3390/pr8020160







