1. Introduction
The unique physical and chemical properties of cobalt make this metal an important raw material in the fields of aerospace, petrochemicals, glass manufacturing, and medicine. Cobalt plays an important role in the development of strategic emerging industries. The content of cobalt in the crust is very low, and 90% of cobalt in the crust is in a dispersed state. In nature, cobalt is mostly associated with copper, nickel, iron, and other minerals, and there is no separate cobalt deposit. Both the United States and the European Union have included cobalt in the list of key minerals and materials that affect national and regional security and future economic development. According to the research of the China Mining Federation, cobalt will become one of the nine minerals in serious shortage by 2020. China is one of the world’s major producers and consumers of refined cobalt, but China’s cobalt reserves only account for 1.1% of the total global reserves. A large number of cobalt raw materials rely on imports. Most of the cobalt minerals found on the earth are associated with copper sulfide ore, nickel sulfide ore, pyrite, and other minerals, and only about 2% of the global cobalt production comes from independent cobalt mines. According to the statistics of USGS 2019, the proven global land cobalt resources are 25 million tons and reserves are 6.88 million tons [
1]. Based on the global cobalt production of 136,000 tons in 2018, the static guarantee period is 50 years. More than 120 million tons of cobalt resources have been found at the bottom of the Atlantic Ocean, the Indian Ocean, and the Pacific Ocean. Because of technical and economic reasons, these resources have not been exploited up to now. The global land cobalt resources are widely distributed. Cobalt resources mainly occur in sedimentary stratiform copper cobalt deposits in the Congo and Zambia; laterite nickel cobalt deposits in Australia, Cuba, the Philippines, and Madagascar; and magmatic nickel copper sulfide deposits in Australia, Canada, and Russia. Although cobalt ore is widely distributed, except that the bouazzer cobalt ore in Morocco is a separate cobalt ore with arsenic cobalt as the main mineral, the rest of the world’s cobalt ore is produced as a co-associated mineral of copper, nickel, and other minerals. At present, only a few countries, such as the Congo, Australia, Cuba, Canada, and Russia, can use the cobalt ore economically. There are more than 100 kinds of minerals containing cobalt in nature, and more than 59 kinds of minerals contain cobalt as a basic element. However, at present, there are mainly three kinds of cobalt minerals with economic significance: arsenides, sulfides, and oxides. Common cobalt minerals in the industry include cobaltite, glaucodot, linnetie, smaltite, safflorite, and erthrite as well as asbolite and heterogenite in supergene minerals [
2]. The main common cobalt minerals and their properties are shown in
Table 1.
Cobalt-sulfur concentrate is the most common raw material for industrial cobalt extraction. Pyrite is the most important sulfur-bearing mineral in cobalt sulfur concentrate. Because the cobalt-sulfur concentrate mainly contains pyrite, pyrrhotite, and some gangue minerals such as talcum, quartz, and chlorite, it is difficult to obtain separate cobalt and sulfur concentrates. Most cobalt minerals are associated with other metal minerals. At present, the main cobalt minerals to be processed include cobaltite, skutterudite, thiocopper cobalt, thiocobaltite, nickel cobalt, cobaltite, iron manganese combined cobalt, etc. The most common process to recover cobalt minerals is flotation, which also includes manual separation and gravity separation. Single cobalt minerals, such as cobaltite and thiocobaltite, can be directly obtained from cobalt concentrate by flotation; for cobalt existing in pyrite and chalcopyrite, carrier flotation is generally used, and cobalt concentrate cannot be directly obtained. Recovery of associated cobalt in V-Ti-magnetite is essentially a mixture of pyrite, pyrrhotite, cobaltite, and thiocobaltite. The recovery of valuable metals from cobalt-bearing sulfur concentrate is usually carried out by the combination of pyrometallurgy and hydrometallurgy [
3,
4,
5,
6]. For example, the main disadvantage of direct sulfuric acid roasting in the Shandong Zibo cobalt plant is that the treatment capacity is low (3.0 t/m
2·d), and the SO
2 content in the flue gas is only 4–5%, which is not conducive to acid production. Aiming at the roasting leaching of cobalt sulfur concentrate with high cobalt content, low sulfur content, and a considerable amount of copper, the effects of roasting temperature, roasting time, additive dosage, leaching time, leaching temperature, and liquid–solid ratio were analyzed to investigate the influence of the leaching rates of copper and cobalt. After the cobalt-sulfur concentrate was mixed evenly, the temperature was raised to 620 °C at 2.7 °C/min, calcined for 3 h, and the calcine was leached at 80 °C with 33% slurry concentration of 30 g/L sulfuric acid for 2 h, the results showed leaching rates of Co and Cu of 91% and 90% respectively. The cobalt sulfur concentrate was oxidized roasted at 850–900 °C, and the cinder was mixed with some cobalt sulfur concentrate and chlorinating agent to carry out medium-temperature chlorination sulfuric acid roasting at about 650 °C. This way had a high bed capacity (17–18 t/m
2·d), but the SO
2 concentration in the flue gas was only 1–2%, which is difficult for acid production, and there was a certain amount of hydrogen chloride gas in the flue gas, which will lead to serious corrosion of the equipment [
7,
8,
9,
10].
There is a large amount of valuable mineral resources in the Panxi area of China, and the proven reserves of V-Ti magnetite are 10 billion tons. Among them, iron resources account for 20% of the domestic iron ore reserves, vanadium resources account for 62% of the national vanadium reserves, and titanium resources account for 90.5% of the national titanium reserves. In addition, there are 900,000 tons of cobalt, 700,000 tons of nickel, 250,000 tons of scandium, 180,000 tons of gallium, and other resources. The comprehensive utilization rate of nonferrous metal resources in the Panxi area of China is very low. Because of the low grade and scattered distribution, it is difficult to recover the sulfur and cobalt resources directly from the raw ore. Most of the associated valuable metal cobalt in V-Ti magnetite occurs in the form of pyrite, pyrrhotite, and cobaltite, and a very small amount of cobalt occurs in the form of thiocobaltite. Because the floatability difference among pyrite, pyrrhotite, cobalt nickel pyrite, and thiocobaltite is small, they basically exhibit the surface properties of pyrite minerals, so it is difficult to recover separate minerals by flotation.
At present, iron concentrate, titanium concentrate, and cobalt-bearing sulfur concentrate are obtained from V-Ti magnetite ores in the Panxi area of China by magnetic separation and flotation. In the process of flotation, cobalt enters into sulfur concentrate, which is closely related to pyrite. Generally, Co-bearing sulfur concentrate with a cobalt grade of about 0.6% can be enriched by flotation. It is difficult to further separate cobalt and sulfur using flotation or other physical beneficiation. In this paper, a technology of two-stage roasting and magnetic separation is adopted to extract cobalt and iron from the complex Co-bearing sulfur concentrate in the Panxi area of China. This will provide an important research basis for the efficient utilization of Co-bearing sulfur concentrate resources in Panxi [
11,
12,
13,
14].
2. Materials and Methods
2.1. Sampling
The Co-bearing sulfur concentrate sample was collected from a V-Ti magnetite dressing plant by flotation in the Panzhihua area of China. The Co-bearing sulfur concentrate contained Co 0.68%, Fe 33.26%, and S 36.58%. Pyrite was the main sulfide ore. Co-pyrite and linneite were Co-bearing minerals. Gangue minerals were mica, chlorite, feldspar, calcite, etc., in the Co-bearing concentrate. The main chemical composition analysis of the sample is shown in
Table 2. The main mineral analysis of the sample is shown in
Table 3, a grain-sized analysis of the sample is shown in
Table 4, and the XRD analysis of the sample is shown in
Figure 1.
2.2. Chemical Reagent and Equipment
The main chemical reagents used in this test were calcium chloride and sodium chloride with analytical purity from Guangzhou Chemical Regent, Co., Ltd., Guangzhou China. The coke from Shanxi Coking Coal Group Co. Ltd. (Taiyuan, China) was crushed and divided into five particle sizes of −1 mm, −0.8 mm, −0.6 mm, −0.4 mm, and −0.2 mm as reducing agents in the process of segregation roasting. The entire quality analysis of coke is shown in
Table 5.
The main equipment used in the experiment included an electric atmosphere tube furnace (Model: SX-6-16, Changsha Kehui Furnace Technology Co., Ltd., Changsha, China), muffle furnace (≤1300 °C, Shanghai Shiyan Electric Furnace Co., Ltd. Shanghai, China), cone ball mill (XMQ—Φ 240 × 90 mm, Jilin Exploration Machinery Factory, Changchun, China), Disc grinder (Φ 300 × 150 mm, Jilin Exploration Machinery Factory, Changchun, China), drying box (Shanghai Shiyan Yan Electric Furnace Co., Ltd. Shanghai China), and vacuum filter (Φ 300, Southwest Chengdu Experimental Equipment Co., Ltd. Chendu, China).
2.3. Oxidizing Roasting Test
Oxidizing roasting tests were conducted in an electric atmosphere tube furnace, and Co-bearing sulfur concentrate was put into a corundum dry pot and put into the electric atmosphere tube furnace. The temperature was set to 600–1000 °C in an oxygen atmosphere. When the temperature of the electric atmosphere tube furnace rose to the set temperature, timing started. After roasting for 1–3 h, the oxygen supply was stopped, and the power supply was turned off. Meanwhile, the oxygen-passing tube was pulled out, and the roasting product was taken out for cooling, manual crushing, and grinding to <0.100 mm for segregation roasting. The oxidizing roasting conditions were confirmed to be reasonable according to the analysis of cobalt, iron, and sulfur contents in oxidizing roasting ores. Calculation of the desulfurization rate is shown in Formula (1).
Here, Q1 is the weight of Co-bearing sulfur concentrate, g; Q2 is the weight of oxidizing roasting ore, g; α is the sulfur content of Co-bearing sulfur concentrate, %; and β is the sulfur content of oxidizing roasting ore, %.
2.4. Segregation Roasting–Magnetic Separation Test
The Co-bearing concentrate, the chlorinating agent, and reducing agent were mixed and put into the roasting furnace. These mixtures were heated to a certain temperature (400–1100 °C) under a neutral or weak reducing atmosphere, where the chlorinating agent was used to form volatile metal chlorides. For each magnetic test, segregation roasting ores were put into a 6.25 dm
3Φ 240 × 90 conical ball mill. The grinding density was set at 60%. The pulp was then placed in a XCGS-13 (Φ 50, Overall dimensions 1000 × 800 × 500 mm) Davis Magnetic Tube (Jilin Exploration Machinery Plant, Shenyang China) with special magnetic field intensity. The products were filtered, dried, weighed, sampled, and tested for further evaluation based on the grade and recovery of cobalt and iron in magnetic products. The calculation formulas of cobalt recovery and iron recovery are shown in Formula (2).
Here, Q3 is the weight of cobalt concentrate, g; Q4 is the weight of iron concentrate, g; Q0 is the weight of segregation roasting ores, g; β1 is the cobalt grade of cobalt concentrate, %; β2 is the iron grade of cobalt concentrate, %; α1 is the cobalt grade of segregation roasting ores, %; and α2 is the iron grade of segregation roasting ores, %.
2.5. Procedure
The Co-bearing sulfur concentrate (mass of 100 g for each test) was placed in a tubular atmosphere furnace for oxidizing roasting. The reaction of the sulfide ore and oxygen was reversed to oxidize the ore, and the metals such as iron and cobalt were transformed into iron oxide and cobalt oxide. Oxidizing roasting ore (mass of 50 g for each test) after cooling, chlorinating agent, and coke were mixed and put into the muffle furnace for segregation chlorination roasting, which turned cobalt into a new metal cobalt mineral phase and iron into a new magnetite mineral phase. Metal cobalt is a ferromagnetic metal, and magnetite is a highly magnetic minerals. The specific magnetic susceptibility of cobalt metal is greater than the magnetic susceptibility of magnetite. Cobalt was recovered from segregation after grinding with a magnetic separator; iron was recovered from magnetic separation tailings by two-stage magnetic separation. The process of oxidizing roasting–segregation roasting–magnetic separation is shown in
Figure 2.
2.6. Analysis and Characterization
The chemical composition of solid materials was analyzed by a Z-2000 atomic absorption spectrophotometer (Hitachi Co., Ltd. Tokyo, Japan); the direction grating was Zenier-tana type, 1800 lines/mm; the flash wavelength was 200 nm, the wavelength range was 190~900 nm; and the automatic peak seeking setting and the spectral bandwidth was divided into 4 grades (0.2, 0.4, 1.3, and 2.6 nm) to analyze the chemical composition of the mineral.
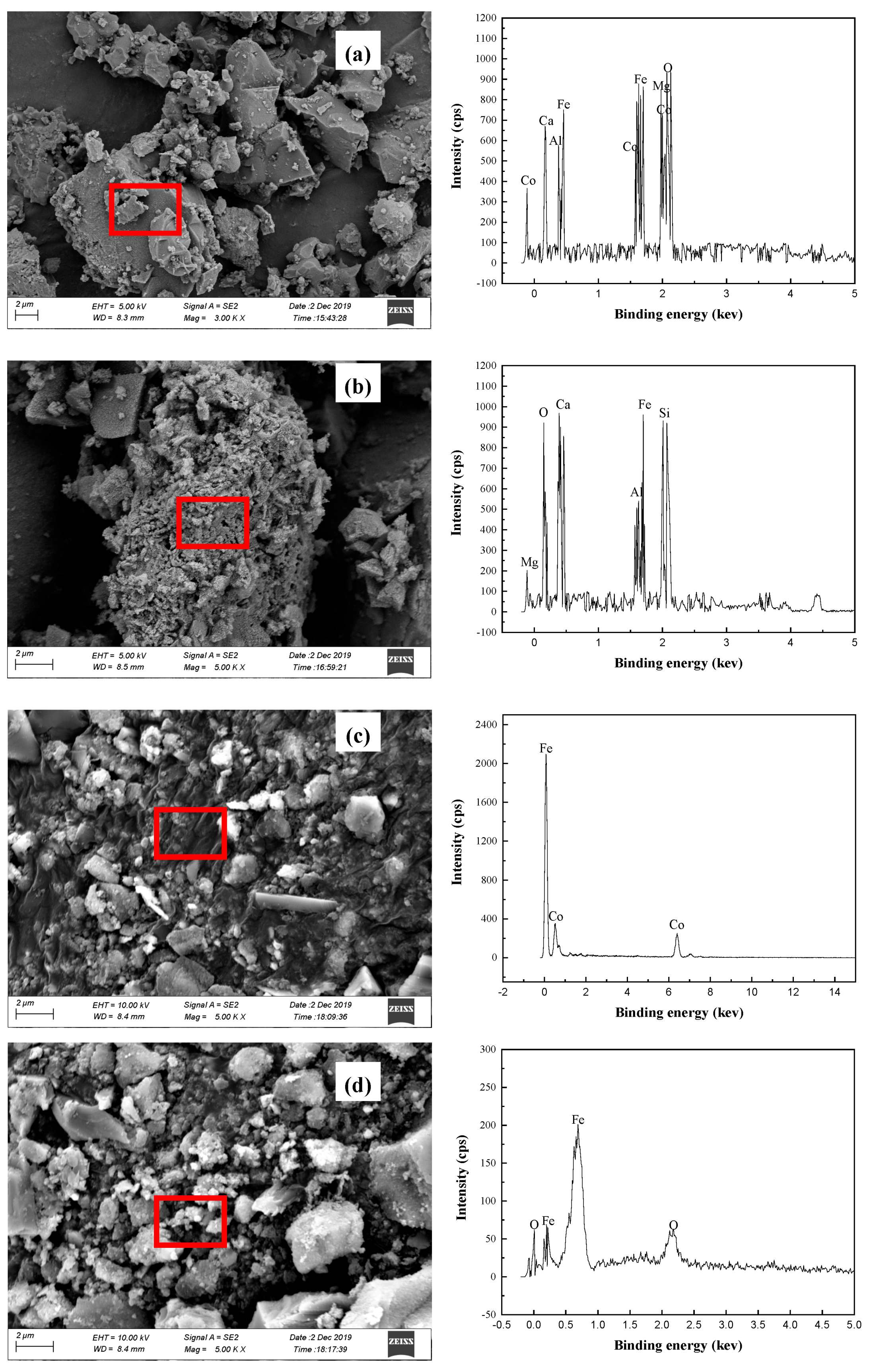
The phase compositions of solid substances (Co-bearing sulfur concentrate, oxidizing roasting ores, segregation roasting ores, cobalt concentrate, iron concentrate, and magnetic separation tailings) were analyzed by X-ray diffraction (XRD, X Pert pro, Panaco, The Netherlands). The microstructure of the solid products was observed by SEM (S440, Hirschmann Laborgerate GmbH & Co. KG, Eberstadt, Germany) equipped with an energy-dispersive X-ray spectroscopy (EDS) detector (UItra55, CarlzeissNTS GmbH, Hirschmann Laborgerate GmbH & Co. KG, Eberstadt, Germany).
4. Conclusions
(1) The refractory Co-bearing sulfur concentrate sample was collected from a V-Ti magnetite dressing plant by flotation in the Panzhihua area of China, and the Co-bearing sulfur concentrate contained Co 0.68%, Fe 33.26%, and S 36.58%. Pyrite was the main sulfide ore; Co-pyrite and linneite were Co-bearing minerals; gangue minerals were mica, chlorite, feldspar, calcite, etc., in Co-bearing concentrate.
(2) A new process of oxidizing roasting–segregation roasting–magnetic separation is used to process the complex Co-bearing sulfur concentrate. The cobalt concentrate with Co of 15.15%, Fe 71.22%, and cobalt recovery of 90.81% as well as iron concentrate with Fe of 60.06%, Co of 0.11%, and iron recovery of 76.23% were obtained under the comprehensive conditions of oxidizing roasting temperature of 900 ℃, oxidizing roasting time of 3.0 h, oxygen content of 80%, segregation roasting time of 950 °C, segregation roasting time of 75 min, calcium chloride dosage of 12%, coke dosage of 10%, coke size of <0.4 mm, magnetic separation grinding fineness of <60 μm occupying 90%, cobalt magnetic separation magnetic field intensity of H1 = 0.08 T, and iron magnetic separation intensity of H2 = 0.14 T. Extraction of cobalt and iron is obvious.
(3) The results of cobalt and iron phase transformations show that cobalt is transformed from Co-pyrite and linneite to a Co2FeO4-dominated new cobalt mineral phase. Iron is transformed from pyrite to Fe2O3 and an Fe3O4-dominated new iron mineral phase after oxidizing roasting. Sulfur is produced in the form of SO2 after oxidizing roasting; cobalt changed from CoFe2O4 to a new cobalt mineral phase dominated by [Co]Fe solid solution. Iron changed from Fe2O3 to a new iron mineral phase dominated by metal Fe and Fe3O4 after segregation roasting.
(4) SEM, EDS, and XRD analyses of segregation roasting ores, cobalt concentrate, and iron concentrate show that the main minerals in cobalt concentrate are Fe, [Co] Fe, and a small amount of Fe3O4; the main minerals in iron concentrate are Fe3O4 and FeO; and there was a small amount of gangue minerals such as SiO2, Ca2Si2O4, and Ca2Al2O4 in the cobalt concentrate and iron concentrate because of mechanical entrainment.