Green Corrosion Inhibitors from Agri-Food Wastes: The Case of Punica granatum Extract and Its Constituent Ellagic Acid. A Validation Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Solvents
2.2. Extraction Procedure and Extract Characterization
2.3. Spectrophotometry
2.4. Corrosion Tests
2.4.1. Mass Loss Investigation
2.4.2. Potentiodynamic Polarization
3. Results and Discussion
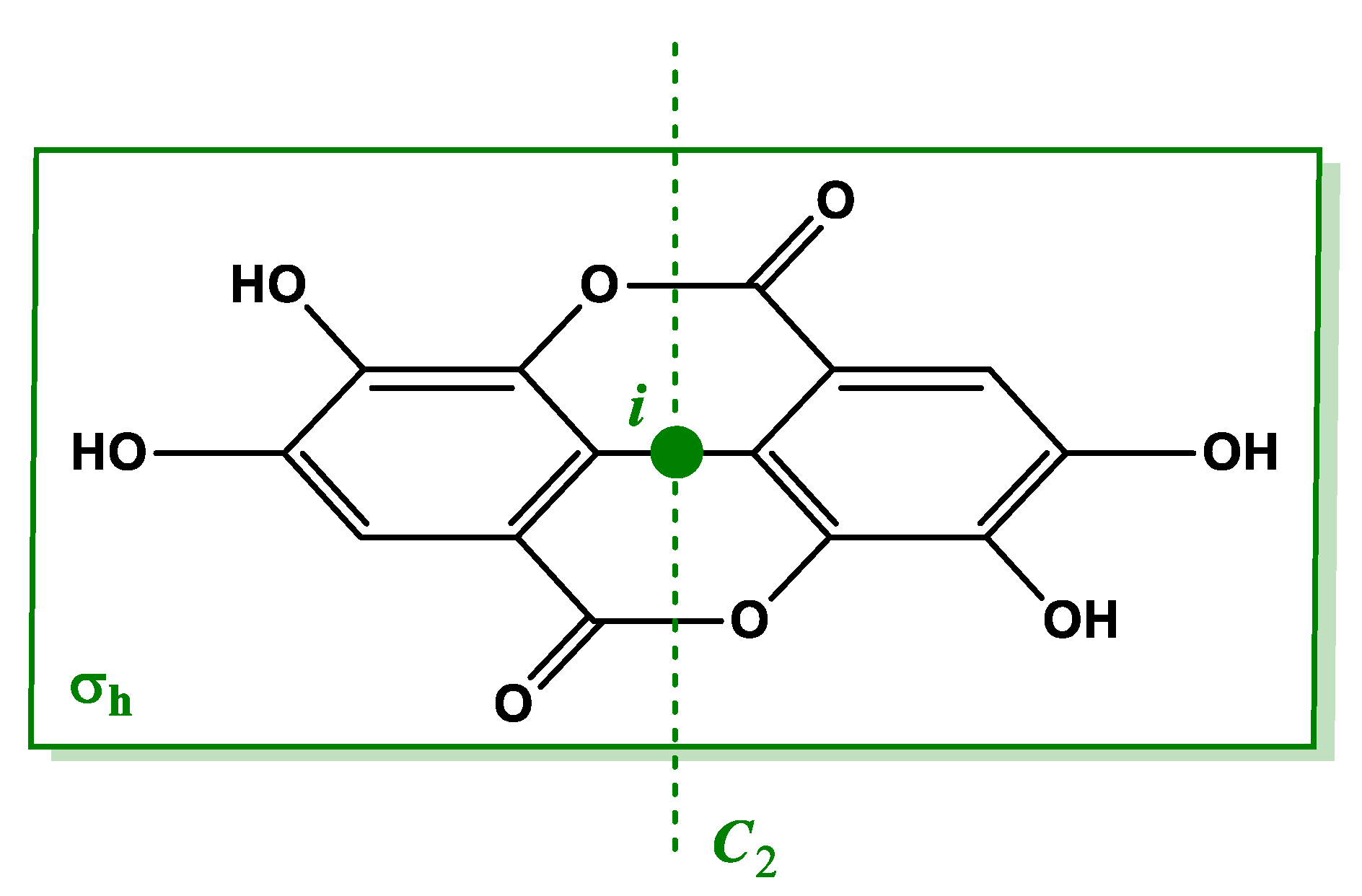
3.1. Spectrophotometric Investigation
3.2. Corrosion Inhibition of Iron in Hydrochloric Acid Solution
3.2.1. Choice of a Co-Solvent for Performing Corrosion Inhibition Tests
3.2.2. Investigation of the Performance of EA and FPW Extract as Corrosion Inhibitors
Mass Loss Test
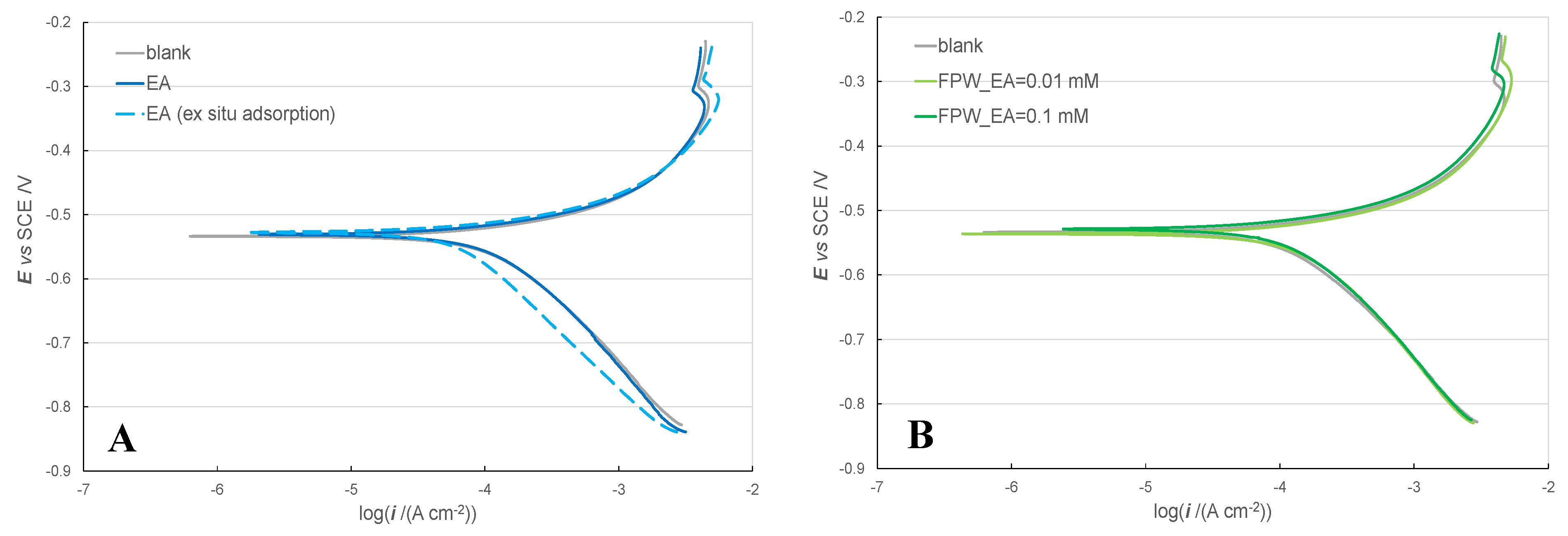
Potentiodynamic Polarization
3.3. EA and FPW Extract as Corrosion Inhibitors of Iron in Chloride-Based Near Neutral Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Putilova, I.N.; Balezin, S.A.; Barannik, P.V. Metallic Corrosion Inhibitors; Pergamon Press: London, UK; New York, NY, USA, 1960. [Google Scholar]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green corrosion inhibitors from natural sources and biomass wastes. Molecules 2019, 24, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, S.; Luo, N.-Q.; Li, N.-B. Plant extracts as “green” corrosion inhibitors for steel in sulphuric acid. Chem. Pap. 2016, 70, 1131–1143. [Google Scholar] [CrossRef]
- Gopiraman, D.K.M.; Sulochana, N. Green inhibitors for corrosion of metals: A review. Chem. Sci. Rev. Lett. 2012, 1, 1–8. [Google Scholar]
- Heusler, K.E.; Landolt, D.; Trasatti, S. Electrochemical corrosion nomenclature (Recommendations 1988). Pure Appl. Chem. 1989, 61, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Dariva, C.G.; Galio, A.F. Corrosion Inhibitors—Principles, Mechanisms and Applications. In Developments in Corrosion Protection; Aliofkhazraei, M., Ed.; IntechOpen: London, UK, 2014; pp. 365–379. [Google Scholar]
- Chidiebere, M.A.; Ogukwe, C.E.; Oguzie, K.L.; Eneh, C.N.; Oguzie, E.E. Corrosion inhibition and adsorption behavior of punica granatum extract on mild steel in acidic environments: experimental and theoretical studies. Ind. Eng. Chem. Res. 2012, 51, 668–677. [Google Scholar] [CrossRef]
- Behpour, M.M.; Ghoreishi, S.M.; Khayatkashani, M.; Soltani, N. Green approach to corrosion inhibition of mild steel in two acidic solutions by the extract of Punica granatum peel and main constituents. Mater. Chem. Phys. 2012, 131, 621–633. [Google Scholar] [CrossRef]
- Verotta, L.; Panzella, L.; Antenucci, S.; Calvenzani, V.; Tomay, F.; Petroni, K.; Caneva, E.; Napolitano, A. Fermented pomegranate wastes as sustainable source of ellagic acid: Antioxidant properties, anti-inflammatory action, and controlled release under simulated digestion conditions. Food Chem. 2018, 246, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Verotta, L.; Macchi, M.P. Monograph on pomegranate. In Connecting Indian Wisdom and Western Science: Plant Usage For Nutrition and Health, 1st ed.; Verotta, L., Macchi, M.P., Venkatasubranian, P., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 303–307. [Google Scholar]
- Moccia, F.; Flores-Gallegos, A.C.; Chávez-González, M.L.; Sepúlveda, L.; Marzorati, S.; Verotta, L.; Panzella, L.; Ascacio-Valdes, J.A.; Aguilar, C.N.; Napolitano, A. Ellagic acid recovery by solid state fermentation of pomegranate wastes by aspergillus niger and saccharomyces cerevisiae: A comparison. Molecules 2019, 24, 3689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Ravi Kumar, M.N.V. Analytical methods for assay of ellagic acid and its solubility studies. J. Pharm. Biomed. Anal. 2006, 40, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E.E. Organic corrosion inhibitors for industrial cleaning of ferrous and nonferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 565–573. [Google Scholar] [CrossRef]
- Maas, J.L.; Galletta, G.J.; Stoner, G.D. Ellagic acid, an anticarcinogen in fruits, especially in strawberries: A review. HortScience 1991, 26, 10–14. [Google Scholar] [CrossRef]
- Standard Practice for Preparing, Cleaning, and Evaluation Corrosion Test Specimens (G1-90, Reapproved 1999); ASTM International: West Conshohocken, PA, USA, 2014.
- Queimada, A.J.; Mota, F.L.; Pinho, S.P.; Macedo, E.A. Solubilities of biologically active phenolic compounds: measurements and modeling. J. Phys. Chem. B 2009, 113, 3469–3476. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kataoka, M. Molecular structure and electronic spectrum of ellagic acid: Semiempirical molecular orbital calculations. J. Heterocycl. Chem. 1997, 34, 665–667. [Google Scholar] [CrossRef]
- Hasegawa, M.; Terauchi, M.; Kikuchi, Y.; Nakao, A.; Okubo, J.; Yoshinaga, T.; Hiratsuka, H.; Kobayashi, M.; Hoshi, T. Deprotonation processes of ellagic acid in solution and solid states. Mon. Chem. 2003, 134, 811–821. [Google Scholar] [CrossRef]
- Simic, A.Z.; Verbic, T.Z.; Sentic, M.N.; Vojic, M.P.; Juranic, I.O.; Manojlovic, D.D. Study of ellagic acid electro-oxidation mechanism. Mon. Chem. 2013, 144, 121–128. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Ghalebsaz-Jeddi, N.; Hashemzadeh, F.; Jahani, H. Corrosion inhibition of carbon steel in hydrochloric acid by some polyethylene glycols. Electrochim. Acta 2006, 51, 3848–3854. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods. Fundamentals and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Stern, M.; Geary, A.L. Electrochemical Polarization. I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing (G3-14); ASTM International: West Conshohocken, PA, USA, 2014.
- Stern, M. A method for determining corrosion rates from linear polarization data. Corrosion 1958, 14, 440t–443t. [Google Scholar] [CrossRef]
- Tamayo-Sepúlveda, J.A.; Vásquez-Arroyave, F.A.; Calderón-Gutiérrez, J.A. Effect of aeration on Tafelian behavior of the carbon steel corrosion in acid sulfate medium. Rev. Fac. Ing. Univ. Antioq. 2017, 83, 36–42. [Google Scholar] [CrossRef] [Green Version]


| Solution | Co-solvent | vW /(mg cm−2 h−1) a | v /(mm y−1) b | IE% c |
|---|---|---|---|---|
| 0.05 M HCl | - | 0.8 ± 0.1 | 9 ± 1 | - |
| 0.05 M HCl + 1 vol% co-solvent (blank) | PEG300 | 0.17 ± 0.03 | 1.9 ± 0.3 | 79 ± 5 d |
| Methanol e | 1.0 ± 0.1 | 11 ± 1 | −25 ± 20 d | |
| blank + 10 µM EA | PEG300 | 0.14 ± 0.03 | 1.5 ± 0.3 | 83 ± 4 d |
| methanol | 0.72 ± 0.07 | 8.0 ± 0.8 | 28 ± 10 | |
| blank + 8 mg/dm3 FPW extract (ca. 10 µM EA) | methanol | 0.86 ± 0.03 | 9.6 ± 0.3 | 14 ± 9 |
| blank + 80 mg/dm3 FPW extract (ca. 100 µM EA) | methanol | 0.80 ± 0.04 | 8.9 ± 0.4 | 20 ± 9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magni, M.; Postiglione, E.; Marzorati, S.; Verotta, L.; Trasatti, S.P. Green Corrosion Inhibitors from Agri-Food Wastes: The Case of Punica granatum Extract and Its Constituent Ellagic Acid. A Validation Study. Processes 2020, 8, 272. https://doi.org/10.3390/pr8030272
Magni M, Postiglione E, Marzorati S, Verotta L, Trasatti SP. Green Corrosion Inhibitors from Agri-Food Wastes: The Case of Punica granatum Extract and Its Constituent Ellagic Acid. A Validation Study. Processes. 2020; 8(3):272. https://doi.org/10.3390/pr8030272
Chicago/Turabian StyleMagni, Mirko, Ester Postiglione, Stefania Marzorati, Luisella Verotta, and Stefano P. Trasatti. 2020. "Green Corrosion Inhibitors from Agri-Food Wastes: The Case of Punica granatum Extract and Its Constituent Ellagic Acid. A Validation Study" Processes 8, no. 3: 272. https://doi.org/10.3390/pr8030272
APA StyleMagni, M., Postiglione, E., Marzorati, S., Verotta, L., & Trasatti, S. P. (2020). Green Corrosion Inhibitors from Agri-Food Wastes: The Case of Punica granatum Extract and Its Constituent Ellagic Acid. A Validation Study. Processes, 8(3), 272. https://doi.org/10.3390/pr8030272








