Phenolic Compounds Extraction of Arbutus unedo L.: Process Intensification by Microwave Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
Moisture Content Determination
2.2. Chemicals
2.3. Extraction Procedures
2.3.1. Conventional Solid–Liquid Extractions
2.3.2. Microwave Pretreatment
2.4. Extract Characterization
2.4.1. Total Mass Yield
2.4.2. Total Phenolic Content (TPC)
2.4.3. Anthocyanin Content
2.5. Analysis of Phytochemical Composition of Extracts by HPLC-DAD
2.6. Analysis of Phytochemical Composition of Extracts by HPLC-MS/MS
2.7. Data Treatment
3. Results
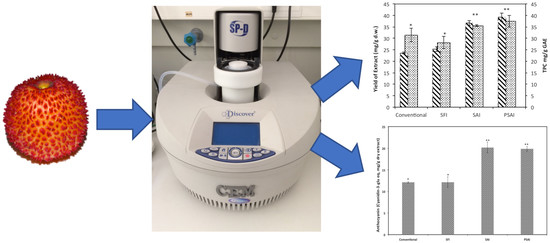
3.1. Extract Mass Yield and TPC
Kinetic Extraction of TPC
3.2. Anthocyanin Content
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fortalezas, S.; Tavares, L.; Pimpão, R.; Tyagi, M.; Pontes, V.; Alves, P.M.; Mcdougall, G.; Stewart, D.; Ferreira, R.B.; Santos, C.N. Antioxidant properties and neuroprotective capacity of strawberry tree fruit (Arbutus unedo). Nutrients 2010, 2, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Pawlowska, A.M.; De Leo, M.; Braca, A. Phenolics of Arbutus unedo L. (Ericaceae) fruits: Identification of anthocyanins and gallic acid derivatives. J. Agric. Food Chem. 2006, 54, 10234–10238. [Google Scholar] [CrossRef] [PubMed]
- Alarcão-E-Silva, M.L.C.M.M.; Leitão, A.E.B.; Azinheira, H.G.; Leitão, M.C.A. The Arbutus Berry: Studies on its Color and Chemical Characteristics at Two Mature Stages. J. Food Compos. Anal. 2001, 14, 27–35. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Kucukislamoglu, M.; Reunanen, M. Sugar, Non-volatile and Phenolic Acids Composition of Strawberry Tree (Arbutus unedo L. var. Ellipsoidea) Fruits. J. Food Compos. Anal. 2000, 13, 171–177. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Fernández-Ruiz, V.; Sánchez-Mata, M.S.O.S.C.C.; Cámara, M.; Morales, R.; Tardío, J. Wild edible fruits as a potential source of phytochemicals with capacity to inhibit lipid peroxidation. Eur. J. Lipid Sci. Technol. 2013, 115, 176–185. [Google Scholar] [CrossRef]
- Kivçak, B.; Mert, T. Quantitative determination of α-tocopherol in Arbutus unedo by TLC-densitometry and colorimetry. Fitoterapia 2001, 72, 656–661. [Google Scholar] [CrossRef]
- Delgado-pelayo, R.; Hornero-méndez, D. Carotenoid composition from strawberry tree (Arbutus unedo L.) fruits. Food Chem. 2010, 199, 165–175. [Google Scholar]
- Pallauf, K.; Rivas-Gonzalo, J.C.; del Castillo, M.D.; Cano, M.P.; de Pascual-Teresa, S. Characterization of the antioxidant composition of strawberry tree (Arbutus unedo L.) fruits. J. Food Compos. Anal. 2008, 21, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, A.M.R.C.; Matias, A.; Duarte, C.M.M.; Bronze, M.R. High-pressure CO2assisted extraction as a tool to increase phenolic content of strawberry-tree (Arbutus unedo) extracts. J. CO2 Util. 2018, 27, 73–80. [Google Scholar] [CrossRef]
- Simonetti, M.S.; Damiani, F.; Gabrielli, L.; Cossignani, L.; Blasi, F.; Marini, F.; Montesano, D.; Maurizi, A.; Ventura, F.; Bosi, A.; et al. Characterization of triacylglycerols in Arbutus unedo L. seeds. Ital. J. Food Sci. 2008, 20, 49–56. [Google Scholar]
- Alexandre, A.M.R.C.; Serra, A.T.; Matias, A.A.; Duarte, C.M.M.; Bronze, M.R. Supercritical fl uid extraction of Arbutus unedo distillate residues—Impact of process conditions on antiproliferative response of extracts. J. CO2 Util. 2020, 37, 29–38. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Dugay, A.; Acquaviva, R.; Malfa, G.A.; Bonesi, M.; Bouzidi, C.; Tundis, R. Contribution of Flavonoids and Iridoids to the Hypoglycaemic, Antioxidant, and Nitric Oxide (NO) Inhibitory Activities of Arbutus unedo L. Antioxidants 2020, 9, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Mustapa, A.N.; Martin, A.; Gallego, J.R.; Mato, R.B.; Cocero, M.J. Microwave-assisted extraction of polyphenols from Clinacanthus nutans Lindau medicinal plant: Energy perspective and kinetics modeling. Chem. Eng. Process. Process Intensif. 2015, 97, 66–74. [Google Scholar] [CrossRef]
- Shi, J.; Nawaz, H.; Pohorly, J.; Mittal, G.; Kakuda, Y.; Jiang, Y. Extraction of polyphenolics from plant material for functional foods—Engineering and technology. Food Rev. Int. 2005, 21, 139–166. [Google Scholar] [CrossRef]
- Mason, J.T.; Chemat, F.; Vinatoru, M. The Extraction of Natural Products using Ultrasound or Microwaves. Curr. Org. Chem. 2011, 15, 237–247. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Pap, N.; Beszédes, S.; Pongrácz, E.; Myllykoski, L.; Gábor, M.; Gyimes, E.; Hodúr, C.; Keiski, R.L. Microwave-Assisted Extraction of Anthocyanins from Black Currant Marc. Food Bioprocess Technol. 2013, 6, 2666–2674. [Google Scholar] [CrossRef]
- Sólyom, K.; Solá, R.; Cocero, M.J.; Mato, R.B. Thermal degradation of grape marc polyphenols. Food Chem. 2014, 159, 361–366. [Google Scholar] [CrossRef]
- Hernández, T.; Estrella, I.; Carlavilla, D.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V. Characterisation of phenolic acid derivatives and flavonoids from different morphological parts of Helichrysum obconicum by a RP-HPLC-DAD-(-)-ESI-MS n method. Anal. Chim. Acta 2006, 563, 116–125. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.T.; Anastas, P.T. Introduction: Green chemistry. Chem. Rev. 2007, 107, 2167–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero, M.; Castro-Puyana, M.; Mendiola, J.A.; Ibañez, E. Compressed fluids for the extraction of bioactive compounds. TrAC Trends Anal. Chem. 2013, 43, 67–83. [Google Scholar] [CrossRef]
- Vardanega, R.; Prado, J.M.; Meireles, M.A.A. Adding value to agri-food residues by means of supercritical technology. J. Supercrit. Fluids 2015, 96, 217–227. [Google Scholar] [CrossRef]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-Assisted Extraction of Natural Products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave assisted extraction—An innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry Theory and Practice; Oxford University Press Inc.: Oxford, UK, 1998. [Google Scholar]
- Ekezie, F.G.C.; Sun, D.W.; Cheng, J.H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Ganzler, K.; Salgó, A.; Valkó, K. Microwave extraction. A novel sample preparation method for chromatography. J. Chromatogr. A 1986, 371, 299–306. [Google Scholar] [CrossRef]
- Galan, A.M.; Calinescu, I.; Trifan, A.; Winkworth-Smith, C.; Calvo-Carrascal, M.; Dodds, C.; Binner, E. New insights into the role of selective and volumetric heating during microwave extraction: Investigation of the extraction of polyphenolic compounds from sea buckthorn leaves using microwave-assisted extraction and conventional solvent extraction. Chem. Eng. Process. Process Intensif. 2017, 116, 29–39. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-Tixier, A.S.; Vian, M.A.; Chemat, F. Solvent-free microwave extraction of bioactive compounds provides a tool for green analytical chemistry. TrAC Trends Anal. Chem. 2013, 47, 1–11. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Prieto, M.A.; Barreiro, M.F.; Rodrigues, A.; Curran, T.P.; Barros, L.; Ferreira, I.C.F.R. Catechin-based extract optimization obtained from Arbutus unedo L. fruits using maceration/microwave/ultrasound extraction techniques. Ind. Crops Prod. 2017, 95, 404–415. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, B.R.; Prieto, M.A.; Vazquez, J.A.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Recovery of bioactive compounds from Arbutus unedo L. fruits: Comparative optimization study of maceration/microwave/ultrasound extraction techniques. Food Res. Int. 2018, 109, 455–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sólyom, K.; Mato, R.B.; Pérez-Elvira, S.I.; Cocero, M.J. The influence of the energy absorbed from microwave pretreatment on biogas production from secondary wastewater sludge. Bioresour. Technol. 2011, 102, 10849–10854. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. Current Protocols in Food Analytical Chemistry. Curr. Protocol. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Romero-Díez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodríguez-Rojo, S.; Cocero, M.J.; Matias, A.A. Microwave and ultrasound pre-treatments to enhance anthocyanins extraction from different wine lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Motilva, M.J. Understanding of human metabolic pathways of different sub-classes of phenols from Arbutus unedo fruit after an acute intake. Food Funct. 2016, 7, 1700–1710. [Google Scholar] [CrossRef]




| Extracts | ||||
|---|---|---|---|---|
| mAU × min | Conventional | SFI | MAI | PMAI |
| 280 nm | 265.3 | 398.8 | 1027.6 | 1743.8 |
| 520 nm | - | 0.081 | 0.617 | 0.909 |
| Peak No. | Rt (min) | λmax (nm) | Putative Identification |
|---|---|---|---|
| 1 | 7.3 | 226 | Glucaric acid |
| 2 | 8.5 | 227 | Quinic acid |
| 3 | 9.2 | 227 | n.i. |
| 4 | 9.6 | 240 | Malic acid |
| 5 | 9.9 | 229 | Ascorbic acid |
| 6 | 11.12 | 238 | Quinic acid derivative |
| 7 | 17.5 | 227 | Galloyl-hexoside |
| 8 | 18.5 | 268 | Gallic acid glucoside |
| 9 | 19.4 | 274 | 5-O-Galloylquinic acid |
| 10 | 22.1 | 227 | Galloyl-hexoside |
| 11 | 23.5 | 274 | Galloyl shikimic acid |
| 12 | 24.3 | 280 | Protocatechuic acid |
| 13 | 26.7 | 276 | 3,5-Di-O-galloylquinic acid |
| 14 | 26.9 | 278 | Procyanidin B2 |
| 15 | 28.3 | 278 | Catechin |
| 16 | 29.6 | 276 | Strictinin elagitannin |
| 17 | 276 | Digalloyl shikimic acid | |
| 18 | 30.5 | 525 | Delphinidin-3-O-glucoside |
| 19 | 30.7 | 277 | Coumaric acid derivative |
| 20 | 32.5 | 513 | Cyanidin-3-O-glucoside |
| 21 | 33.9 | 278 | n.i. |
| 22 | 38.8 | 277 | Gallotannin |
| 23 | 40.8 | 271 | Ellagic acid |
| 24 | 46.6 | 360 | Ellagic acid xyloside |
| Retention Time (min) | λmax (nm) | [M + H]+ m/z | MS/MS | Putative Identification | Reference |
|---|---|---|---|---|---|
| 25.9 | 525 | 433 | 301 | Peonidin arabinoside | [40] |
| 26.5 | 525 | 433 | 271 | Pelargonidin glucoside | [40] |
| 26.9 | 525 | 463 | 301 | Peonidin glucoside | [40] |
| 29.9 | 555 | 403 | 271 | Pelargonidin arabinoside | [40] |
| 30.5 | 525 | 465 | 303 | Delphinidin glucoside | [1] |
| 32.5 | 513 | 449 | 287 | Cyanidin glucoside | [1] |
| 35.4 | 513 | 419 | 287 | Cyanidin arabinoside | [1] |
| 33.6 | 525 | 435 | 303 | Delphinidin arabinoside | [1] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandre, A.M.R.C.; Matias, A.A.; Bronze, M.R.; Cocero, M.J.; Mato, R. Phenolic Compounds Extraction of Arbutus unedo L.: Process Intensification by Microwave Pretreatment. Processes 2020, 8, 298. https://doi.org/10.3390/pr8030298
Alexandre AMRC, Matias AA, Bronze MR, Cocero MJ, Mato R. Phenolic Compounds Extraction of Arbutus unedo L.: Process Intensification by Microwave Pretreatment. Processes. 2020; 8(3):298. https://doi.org/10.3390/pr8030298
Chicago/Turabian StyleAlexandre, Agostinho M. R. C., Ana A. Matias, Maria Rosário Bronze, Maria Jose Cocero, and Rafael Mato. 2020. "Phenolic Compounds Extraction of Arbutus unedo L.: Process Intensification by Microwave Pretreatment" Processes 8, no. 3: 298. https://doi.org/10.3390/pr8030298
APA StyleAlexandre, A. M. R. C., Matias, A. A., Bronze, M. R., Cocero, M. J., & Mato, R. (2020). Phenolic Compounds Extraction of Arbutus unedo L.: Process Intensification by Microwave Pretreatment. Processes, 8(3), 298. https://doi.org/10.3390/pr8030298