Experimental Study on Hydrothermal Carbonization of Lignocellulosic Biomass with Magnesium Chloride for Solid Fuel Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass
2.2. Chemical and Physical Analysis
2.3. Hydrothermal Carbonization
3. Results
3.1. Influence of Time and Temperature on Hydrochar Properties
3.2. Effect of Magnesium Chloride on Mass Yield
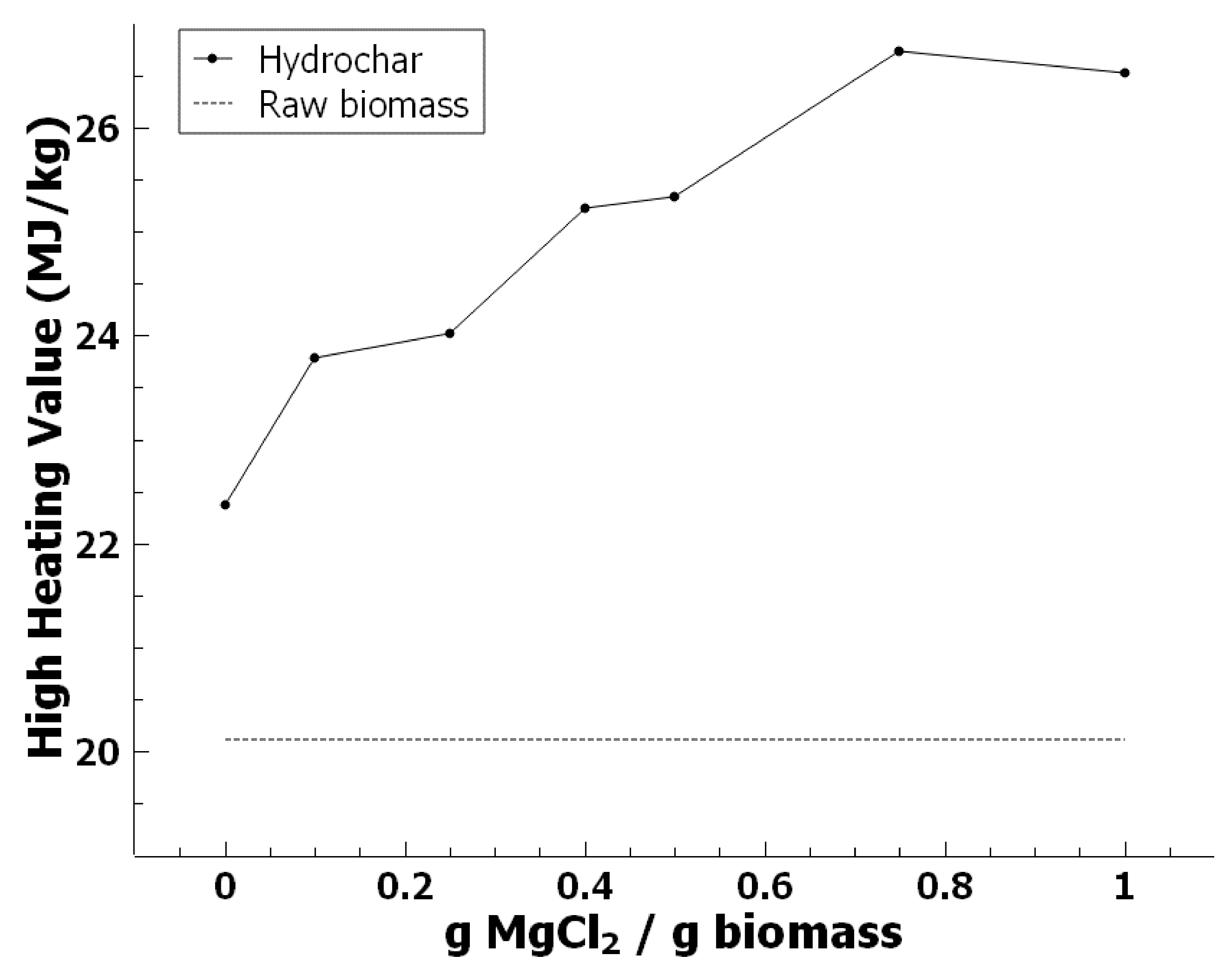
3.3. Effect of Magnesium Chloride on HHV
3.4. Effect of Magnesium Chloride on Energy Yield
3.5. Effect of Magnesium Chloride on Elemental Fractions
3.6. Effect of Magnesium Chloride on the Volatile Matter, Ash and Fixed Carbon Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Díaz-Robles, L.A.; Fu, J.S.; Vergara-Fernández, A.; Etcharren, P.; Schiappacasse, L.N.; Reed, G.D.; Silva, M.P. Health risks caused by short term exposure to ultrafine particles generated by residential wood combustion: A case study of Temuco, Chile. Environ. Int. 2014, 66, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Lalak, J.; Martyniak, D.; Kasprzycka, A.; Zurek, G.; Moroń, W.; Chmielewska, M.; Wiacek, D.; Tys, J. Comparison of selected parameters of biomass and coal. Int. Agrophys. 2016, 30, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Cereceda-Balic, F.; Toledo, M.; Vidal, V.; Guerrero, F.; Diaz-Robles, L.A.; Petit-Breuilh, X.; Lapuerta, M. Emission factors for PM2.5, CO, CO2, NOx, SO2 and particle size distributions from the combustion of wood species using a new controlled combustion chamber 3CE. Sci. Total Environ. 2017, 584–585, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Robles, L.A.; Ortega, J.C.; Fu, J.S.; Reed, G.D.; Chow, J.C.; Watson, J.G.; Moncada-Herrera, J.A. A hybrid ARIMA and artificial neural networks model to forecast particulate matter in urban areas: The case of Temuco, Chile. Atmos. Environ. 2008, 42, 8331–8340. [Google Scholar] [CrossRef] [Green Version]
- Sanhueza, P.A.; Torreblanca, M.A.; Diaz-Robles, L.A.; Schiappacasse, L.N.; Silva, M.P.; Astete, T.D. Particulate air pollution and health effects for cardiovascular and respiratory causes in Temuco, Chile: A wood-smoke-polluted urban area. J. Air Waste Manag. Assoc. 2009, 59, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Starfelt, F.; Daianova, L.; Yan, J. Influence of drying process on the biomass-based polygeneration system of bioethanol, power and heat. Appl. Energy 2012, 90, 32–37. [Google Scholar] [CrossRef]
- Miranda, M.T.; Arranz, J.I.; Román, S.; Rojas, S.; Montero, I.; López, M.; Cruz, J.A. Characterization of grape pomace and pyrenean oak pellets. Fuel Process. Technol. 2011, 92, 278–283. [Google Scholar] [CrossRef]
- Tan, H.; Wang, S. Experimental study of the effect of acid-washing pretreatment on biomass pyrolysis. J. Fuel Chem. Technol. 2009, 37, 668–672. [Google Scholar] [CrossRef]
- Narayanan, K.V.; Natarajan, E. Experimental studies on cofiring of coal and biomass blends in India. Renew. Energy 2007, 32, 2548–2558. [Google Scholar] [CrossRef]
- Chen, W.-H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Tekin, K.; Karagöz, S.; Bektaş, S. A review of hydrothermal biomass processing. Renew. Sustain. Energy Rev. 2014, 40, 673–687. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal carbonization (HTC) of lignocellulosic biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Zielinska, B.; Felix, L. Hydrothermal carbonization (HTC) of selected woody and herbaceous biomass feedstocks. Biomass Convers. Biorefin. 2012, 3, 113–126. [Google Scholar] [CrossRef]
- Sabio, E.; Álvarez-Murillo, A.; Román, S.; Ledesma, B. Conversion of tomato-peel waste into solid fuel by hydrothermal carbonization: Influence of the processing variables. Waste Manag. 2015, 47. [Google Scholar] [CrossRef] [PubMed]
- Benavente, V.; Fullana, A.; Berge, N.D. Life cycle analysis of hydrothermal carbonization of olive mill waste: Comparison with current management approaches. J. Clean. Prod. 2017, 142, 2637–2648. [Google Scholar] [CrossRef] [Green Version]
- Yahav Spitzer, R.; Mau, V.; Gross, A. Using hydrothermal carbonization for sustainable treatment and reuse of human excreta. J. Clean. Prod. 2018, 205, 955–963. [Google Scholar] [CrossRef]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal carbonization (HTC) of wheat straw: Influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef]
- Pala, M.; Kantarli, I.C.; Buyukisik, H.B.; Yanik, J. Hydrothermal carbonization and torrefaction of grape pomace: A comparative evaluation. Bioresour. Technol. 2014, 161, 255–262. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, E. Treatment of urban sludge by hydrothermal carbonization. Bioresour. Technol. 2017, 238, 182–187. [Google Scholar] [CrossRef]
- Lynam, J.G.; Coronella, C.J.; Yan, W.; Reza, M.T.; Vasquez, V.R. Acetic acid and lithium chloride effects on hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 6192–6199. [Google Scholar] [CrossRef]
- Lynam, J.G.; Toufiq Reza, M.; Vasquez, V.R.; Coronella, C.J. Effect of salt addition on hydrothermal carbonization of lignocellulosic biomass. Fuel 2012, 99, 271–273. [Google Scholar] [CrossRef]
- Rayner-Canham, G. Isodiagonality in the periodic table. Found. Chem. 2011, 13, 121–129. [Google Scholar] [CrossRef]
- Chang, R. Chemistry, 10th ed.; McGraw-Hill Publishing Company: New York, NY, USA, 2010; ISBN 978-0-07-351109-2. [Google Scholar]
- Berg, A.; Bidart, C.; Espinoza, D.; Flores, M.; Moraga, A.; Müller, N.; Segura, C. Recomendaciones para la elaboración de una Estrategia Nacional de Bioenergía; Unidad de Desarrollo Tecnológico, Universidad de Concepción: Coronel, Chile, 2013. [Google Scholar]
- Heidari, M.; Dutta, A.; Acharya, B.; Mahmud, S. A review of the current knowledge and challenges of hydrothermal carbonization for biomass conversion. J. Energy Inst. 2019, 92, 1779–1799. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.G.; Uddin, M.H.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Jatzwauck, M.; Schumpe, A. Kinetics of hydrothermal carbonization (HTC) of soft rush. Biomass Bioenergy 2015, 75, 94–100. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Álvarez-Murillo, A.; Román, S.; Ledesma, B.; Sabio, E. Study of variables in energy densification of olive stone by hydrothermal carbonization. J. Anal. Appl. Pyrolysis 2015, 113, 307–314. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Graphical-statistical method for the study of structure and reaction processes of coal. Fuel 1950, 29, 269–284. [Google Scholar]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Laca, A.; Laca, A.; Díaz, M. Chapter 8—Hydrolysis: From cellulose and hemicellulose to simple sugars. In Second and Third Generation of Feedstocks; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 213–240. ISBN 978-0-12-815162-4. [Google Scholar]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Purcell, R.; Zielinska, B.; Felix, L.; Irvin, J. Process Development Unit (PDU) for Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Waste Biomass Valorization 2014, 5, 669–678. [Google Scholar] [CrossRef]
- Ushak, S.; Gutierrez, A.; Galleguillos, H.; Fernandez, A.G.; Cabeza, L.F.; Grágeda, M. Thermophysical characterization of a by-product from the non-metallic industry as inorganic PCM. Sol. Energy Mater. Sol. Cells 2015, 132, 385–391. [Google Scholar] [CrossRef] [Green Version]




| Parameter | Units | Value * |
|---|---|---|
| Proximate Analysis | ||
| Volatiles | % | 86.35 ± 0.10 |
| Ash | % | 0.21 ± 0.01 |
| Fixed Carbon (balance) | % | 13.44 |
| Ultimate Analysis | ||
| Carbon | % | 51.55 ± 1.09 |
| Hydrogen | % | 5.95 ± 0.05 |
| Nitrogen | % | 0.06 ± 0.01 |
| Sulphur | % | 0.08 ± 0.00 |
| Chlorine | % | 0.012 ± 0.003 |
| Oxygen (balance) | % | 42.14 |
| Higher Heating Value (HHV) | MJ/kg | 20.11 ± 0.01 |
| Lower Heating Value (LHV) | MJ/kg | 18.81 |
| Experimental Run | Temp °C | Time h | Mass Yield (%) | HHV (MJ/kg) | EDR | EY (%) | Ash (%) |
|---|---|---|---|---|---|---|---|
| Raw biomass | - | - | - | 20.11 | - | - | 0.21% |
| H_220_30 | 220 | 0.5 | 66.76% | 22.19 | 1.10 | 73.67% | 0.08% |
| H_250_30 | 250 | 0.5 | 45.71% | 22.87 | 1.14 | 51.98% | 0.07% |
| H_270_30 | 270 | 0.5 | 54.90% | 23.55 | 1.17 | 64.29% | 0.07% |
| H_220_60 | 220 | 1.0 | 66.43% | 22.72 | 1.13 | 75.05% | 0.04% |
| H_250_60 | 250 | 1.0 | 50.11% | 23.17 | 1.15 | 57.73% | 0.03% |
| H_270_60 | 270 | 1.0 | 48.86% | 23.42 | 1.16 | 56.90% | 0.04% |
| MgCl2 Dose g MgCl2/g Biomass | %C | %H | %O | %N | %S |
|---|---|---|---|---|---|
| 0.0 | 54.83 ± 0.84 | 6.16 ± 0.03 | 38.42 | 0.12 ± 0.01 | 0.01 ± 0.00 |
| 0.1 | 60.01 ± 0.08 | 5.28 ± 0.03 | 33.29 | 0.13 ± 0.02 | 0.02 ± 0.00 |
| 0.25 | 62.14 ± 1.21 | 5.43 ± 0.21 | 30.32 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| 0.4 | 62.69 ± 0.23 | 5.32 ± 0.00 | 30.59 | 0.10 ± 0.01 | 0.01 ± 0.00 |
| 0.5 | 72.80 ± 0.17 | 5.82 ± 0.02 | 16.68 | 0.05 ± 0.02 | 0.03 ± 0.00 |
| 0.75 | 75.25 ± 0.22 | 5.59 ± 0.01 | 16.21 | 0.03 ± 0.01 | 0.03 ± 0.00 |
| 1 | 73.04 ± 0.32 | 5.67 ± 0.01 | 19.21 | 0.20 ± 0.02 | 0.03 ± 0.00 |
| MgCl2 Dose g MgCl2/g Biomass | %Volatile Matter | %Ash | %Fixed Carbon * |
|---|---|---|---|
| 0 | 78.14 ± 0.06 | 0.03 ± 0.01 | 21.83 |
| 0.1 | 69.86 ± 0.11 | 0.39 ± 0.04 | 29.75 |
| 0.25 | 63.47 ± 0.13 | 1.22 ± 0.01 | 35.31 |
| 0.4 | 62.26 ± 0.15 | 0.54 ± 0.02 | 37.20 |
| 0.5 | 56.43 ± 0.07 | 1.76 ± 0.01 | 41.81 |
| 0.75 | 51.53 ± 0.35 | 1.18 ± 0.01 | 47.29 |
| 1 | 50.52 ± 0.32 | 0.73 ± 0.01 | 48.76 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco, S.; Silva, J.; Pino-Cortés, E.; Gómez, J.; Vallejo, F.; Díaz-Robles, L.; Campos, V.; Cubillos, F.; Pelz, S.; Paczkowski, S.; et al. Experimental Study on Hydrothermal Carbonization of Lignocellulosic Biomass with Magnesium Chloride for Solid Fuel Production. Processes 2020, 8, 444. https://doi.org/10.3390/pr8040444
Carrasco S, Silva J, Pino-Cortés E, Gómez J, Vallejo F, Díaz-Robles L, Campos V, Cubillos F, Pelz S, Paczkowski S, et al. Experimental Study on Hydrothermal Carbonization of Lignocellulosic Biomass with Magnesium Chloride for Solid Fuel Production. Processes. 2020; 8(4):444. https://doi.org/10.3390/pr8040444
Chicago/Turabian StyleCarrasco, Samuel, Javier Silva, Ernesto Pino-Cortés, Jaime Gómez, Fidel Vallejo, Luis Díaz-Robles, Valeria Campos, Francisco Cubillos, Stefan Pelz, Sebastian Paczkowski, and et al. 2020. "Experimental Study on Hydrothermal Carbonization of Lignocellulosic Biomass with Magnesium Chloride for Solid Fuel Production" Processes 8, no. 4: 444. https://doi.org/10.3390/pr8040444






