Abstract
Tissue adhesives have been widely used in surgical procedures. Compared to traditional surgical sutures, tissue adhesives provide fast bonding experiences and full closure of wounds. However, current tissue adhesives are mostly fossil-based synthetic products. Therefore, it is of great significance to explore the use of natural materials in tissue adhesives. Whey is a low-end byproduct of cheese manufacturing. Whey protein, a group of small globular proteins, can exhibit adhesive properties if their structures are modified by physical or chemical means. The objectives of this study were to investigate the functional and structural properties of whey protein-based tissue adhesive, along with the antibacterial effect of totarol, a natural antimicrobial agent. Whey protein isolate (WPI) solutions (25%–33% protein) were mixed with different levels (0.1%–0.3% w/w) of totarol. The mixtures were analyzed for total plate count and yeast and mold count. The lap-shear bonding strength was tested after the WPI-totarol solutions were mixed with a crosslinking agent, glutaraldehyde (GTA). The lap-shear bonding strength of the tissue adhesive was about 20 kPa, which is comparable to that of a commercial BioGlue®. The microstructures of the mixtures were analyzed by scanning electron microscopy (SEM).
1. Introduction
In modern medicine, suture is the most common and basic method for wound closure. However, the disadvantages of suture include physical pain, long healing time, and the possibility of infection [1], and have led to the emergence of alternatives like surgical staples, tapes, and tissue adhesives. Like sutures, tissue adhesives can be used to seal wounds and stop hemorrhages. The advantages of tissue adhesives include no need for removal after use, no risk of needle stick injury to the patients or surgeons, significantly reducing the pain of suture, and providing the possibility of a surgery without the development of scars afterwards. Furthermore, most tissue adhesives are very convenient for clinical use and do not require professional surgical training [2,3].
Protein-based tissue adhesives have low toxicity and can be absorbed by human and animal tissues during the healing process [4]. The principle of protein adhesives is to use a protein polymer reacted with a crosslinking agent to adhere to tissue surfaces. Glutaraldehyde (GTA) is a commonly used crosslinker in tissue adhesives. The cross-linking of the protein-based tissue adhesive to the tissue is the result of the reaction between the amino groups of tissue proteins and the terminal aldehyde groups, which are provided by the crosslinker. Proteins can form a soft and ductile barrier moving with the human body [5]. After bonding, the protein-based tissue adhesive can be degraded by proteolysis [6]. One of the tissue adhesives approved by the Food and Drug Administration (FDA) in the United States is BioGlue®. Its major components are bovine serum albumin (BSA) (45% w/w) as the protein polymer and glutaraldehyde (10% w/w) as the crosslinker [1], and it provides a lap-shear bonding strength of 40.1 ± 12.2 kPa [7].
Whey protein is a mixture of various secreted proteins. The major whey proteins are β-lactoglobulin (β-LG), α-lactalbumin (α-LA), and BSA [8]. β-LG and α-LA are rich in ε-amino groups just as BSA, so we hypothesize that whey protein could be a suitable protein polymer for tissue adhesives as well.
Whey is the by-product of cheese making, it is obtained by separating casein from bovine milk. Whey protein is obtained through whey concentrating and drying techniques. United States generated over 490 thousand tons of dry whey in 2018, according to the Dairy Products Annual Summary of USDA. For a long time, the main applications of whey have been animal feed, fertilizers, and ingredient for human foods. Other than food applications, whey protein can also be used to manufacture many biobased products such as wood adhesives [9] and paper adhesives [10]. The development of a tissue adhesive would be another value-added application of whey protein
Totarol is a naturally occurring phenolic diterpenoid isolated from Podocarpus totara [11], a conifer native to New Zealand. Totarol contains a single phenolic moiety with an isopropyl group ortho to the hydroxyl group [12]. It exhibits a strong antibacterial activity against several gram-positive bacteria, including Streptococcus mutans, Bacillus subtilis, Brevibacterium ammoniagenes [13], Staphylococcus aureus (both penicillin-susceptible and penicillin-resistant strains), and Mycobacterium tuberculosis [14,15]. The minimum inhibitory concentration (MIC) value of totarol against S. aureus strains was 2 µg/mL [16]. In search for the explanation of the mechanism of totarol’s antimicrobial activity, it was proposed that the mode of action of totarol was the alteration in cytoplasmic membrane’s integrity and permeability, which led to the leakage of cellular materials [17]. Another study proposed that totarol functions by compromising the functional integrity of cell membranes [12]. Haraguchi et al. [18] investigated totarol’s effects on Pseudomonas and found that it affects the oxidation of NADH in membrane preparation by inhibiting several NADH-related enzymes. Evans et al. [19] suggested that totarol disrupts bacterial energy metabolism at high concentrations. Smith et al. [20] argued that totarol was a potent efflux pump inhibitor in S. aureus and could reduce the biofilm formation. For its antibacterial activity, totarol has been approved for application as an antimicrobial additive in several consumer products, including toothpaste and acne creams [21]. Therefore, totarol could be fit for use as an antimicrobial agent in the whey protein-based tissue adhesive.
2. Materials and Methods
2.1. Preparation of Adhesive Components
Whey protein isolate (WPI, 92% whey protein) (FonterraTM, Auckland, New Zealand) powder was slowly dissolved in deionized water. The solution’s whey protein content was 40% (w/w). The solution was then sealed and stored in the refrigerator overnight for defoaming. Totarol (1.21%, w/w) (Shanxi Undersun Biomedtech Co., Ltd., Xi’an, China) was then added into the WPI solution. The samples’ final whey protein concentrations were 25%, 27%, 29%, 31%, and 33% (w/w) and totarol concentrations were 0.1%, 0.2%, and 0.3% (w/w), respectively. The WPI-totarol solutions were mixed to reach homogeneity and stored for testing. The process was conducted under sterile conditions to avoid contamination. When testing the adhesive property, GTA (Fisher Scientific Inc., Pittsburgh, PA, USA) was diluted to 6%, 8%, 10%, 12%, and 14% (w/w), and the WPI-totarol solutions were mixed with the GTA solutions, respectively.
2.2. Microbial Analysis
The totarol content of the final adhesive formulation was determined through microbial analysis. The WPI solutions and the WPI-totarol solutions (containing 0.1%, 0.2%, and 0.3% of totarol) were tested for microorganisms by 3MTM Aerobic Count Petrifilm and 3MTM Yeast and Mold Count Petrifilm (3MTM, St. Paul, MN, USA). The samples were diluted to 100, 10−1, 10−2, and 10−3 with sterile buffer. Each dilution was applied on the Petrifilms according to the manufacturer’s instructions. The top of the films was lifted and 1 mL of each sample was applied on the Petrifilms. The top was gradually rolled down and samples distributed evenly by a plastic spreader. Gas bubbles were also excluded by this process. After a few minutes, the Aerobic Count Petrifilms were put into the incubator at 37 °C for 48 h and the Yeast and Mold Count Petrifilms were put at room temperature for at least 48 h. Triplicates of each sample were tested for both aerobe count and yeast and mold count. On the first day of testing, the tests for aerobic bacteria were conducted every 2 h to investigate the time needed for totarol to show antimicrobial effect.
2.3. Lap-Shear Bonding Strength Tests
The whey protein and GTA content of the final adhesive formulation were determined through lap-shear bonding strength tests. Bonding strength is mainly affected by the types of adhesives, surface of adherends and bonding process conditions [22]. In this study, ASTM standard (ASTM F2255-05) [23] was adapted and the Instron 5566 testing machine (Instron Corporation, Norwood, MA, USA) was used to measure the lap-shear bonding strength. Porcine skin was proven to be most similar to human skin. It is structurally similar to human epidermal thickness and dermal-epidermal thickness ratios. Pigs have similar hair follicle and blood vessel patterns to humans. Pigs also contain dermal collagen and elastic content that is more similar to humans than other laboratory animals. In addition, pigs have similar physical and molecular responses to various growth factors [24]. Therefore, porcine skin was used as an alternative to human skin. Porcine skin used in this study was purchased from a local market (South Burlington, VT, USA), sealed and stored at −20 °C.
According to the ASTM standard, tissue substrate materials should be kept moist at all times with phosphate buffered saline. The substrate will be cut to required dimensions and adhered to a test fixture. After the adhesive is applied and two sides of the tissue substrate are bonded together, a force of approximately 1–2 N should be applied to the bonding area until the adhesive sets. The specimens are placed in the grips of the testing machine and are loaded to failure at a constant cross-head speed. The load at failure (maximum load) is record and the shear strength is calculated as the maximum load divided by the bond area in Pascals (Pa).
During the trials, frozen porcine skin was thawed at 23 °C for 2 h and cut into 50 mm × 20 mm × 3 mm pieces, and then wrapped by gauzes soaked with phosphate buffered saline (PBS) (Fisher Scientific, Fair Lawn, NJ, USA) to keep moist. The experiment was carried out in the environment chamber at 23 °C and 40% of humidity. SciencewareTM Super Glue (Bel-ArtTM, Wayne, NJ, USA) was used to hold the porcine skin firmly on the aluminum blocks. A total of 112 µL WPI-totarol solution and 28 µL GTA solution was applied and mixed on the dermal side of the porcine skin. The mixture was spread evenly in the bonding area (50 mm × 20 mm). Two skin pieces were lapped together and held for 45 s. Once again, the specimens were wrapped with gauzes soaked with phosphate buffered saline to keep moist. The Instron 5566 was set at a mobile rate of 12.70 mm/min. The gripping force is supplied by two tightening knobs on the sides of the grips. These tightening knobs are hand-operated, and the jaw faces are self-aligning. The clamping force is adjustable by how hard the knobs are tightened. Specimens are fixed tightly in the loading cell and the maximum load (N) is recorded. The test ran until the two porcine skin pieces separated. Three replicates were made for each sample. Bonding strength is calculated by dividing the maximum load (N) by the bonding area (50 mm × 20 mm).
2.4. Shelf Life Tests
After the formulation of whey protein-based adhesive was determined, the samples were stored at room temperature for one month. During storage, the microbial tests were conducted every other day and tests for lap-shear bonding strength were conducted each week. Gelation was also observed every other day during storage. If the WPI-totarol solutions gelled, they are not suitable for use of tissue adhesives.
2.5. Microstructure Analysis
Scanning electron microscopy (SEM) was conducted to examine the microstructures of WPI solution, WPI-totarol mix, WPI-totarol crosslinked with GTA, and porcine skins bonded by the adhesive.
The samples of WPI solution and WPI-totarol solution were placed onto plastic coverslips and allowed to settle for 1 h at room temperature. Then they are gently covered in Karnovsky’s fixative and allowed to fix for 1 h at room temperature. The samples were rinsed for 15 min for three times in 0.1 M cacodylate buffer and stored in buffer at 4 °C. Open ended beam capsules were placed in the center of wells of 6-well plate. The capsule was surrounded with warmed 2% SeaPrep agarose. The beam capsule would make a well in the agarose. Agarose is then chilled for 1.5 h at 4 °C. The beam capsule was removed to reveal the well, then placed 1–2 drops of agarose into well to make a base of agarose. Then, 1–2 drops of the samples were placed on top of the agarose base in the well and allowed to chill for 10 min at 4 °C. A few drops of agarose were placed on top of samples and allowed to chill overnight at 4 °C. Agarose was submerged with samples in Karnovsky’s fixative and stored at 4 °C overnight. The samples were rinsed for 15 min for three times in cacodylate buffer and stored in buffer at 4 °C. For WPI-totarol crosslinked with GTA, the sample was submerged in Karnovsky’s fixative and chilled at 4 °C for 1 h, then rinsed for 15 min for three times in 0.1 M cacodylate buffer and stored in buffer at 4 °C. For the porcine skin with bonded adhesive, the whole piece was submerged in Karnovsky’s fixative and stored at 4 °C for 5 h and then rinsed for 15 min for three times in 0.1 M cacodylate buffer and stored in buffer at 4 °C.
All samples were treated with 1% OsO4 for 60 min at 4 °C and then rinsed for 15 min for three times in cacodylate buffer. The samples were then dehydrated in 35%, 50%, 70%, 85%, and 95% ethanol for 15 min each and in 100% ethanol for 30 min. Then samples went through for critical point drying with liquid CO2 and mounted onto aluminum stubs with carbon tape and colloidal graphite adhesive. Samples were sputter coated with gold and palladium and imaged at 10 kV with JSM-6060 Scanning Electron Microscope (JEOL USA Inc., Peabody, MA, USA). The SEM images were obtained from the University of Vermont Scanning Electron Microscopy Center (Burlington, VT, USA).
2.6. Statistical Analysis
IBM SPSS® Statistics 25 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The data were the averages of three tests and were processed by one-way ANOVA analysis. The p-value less than 0.05 was considered as significant differences.
3. Results and Discussion
3.1. Preliminary Results of Microbial Analysis and Lap-Shear Bonding Strength Tests
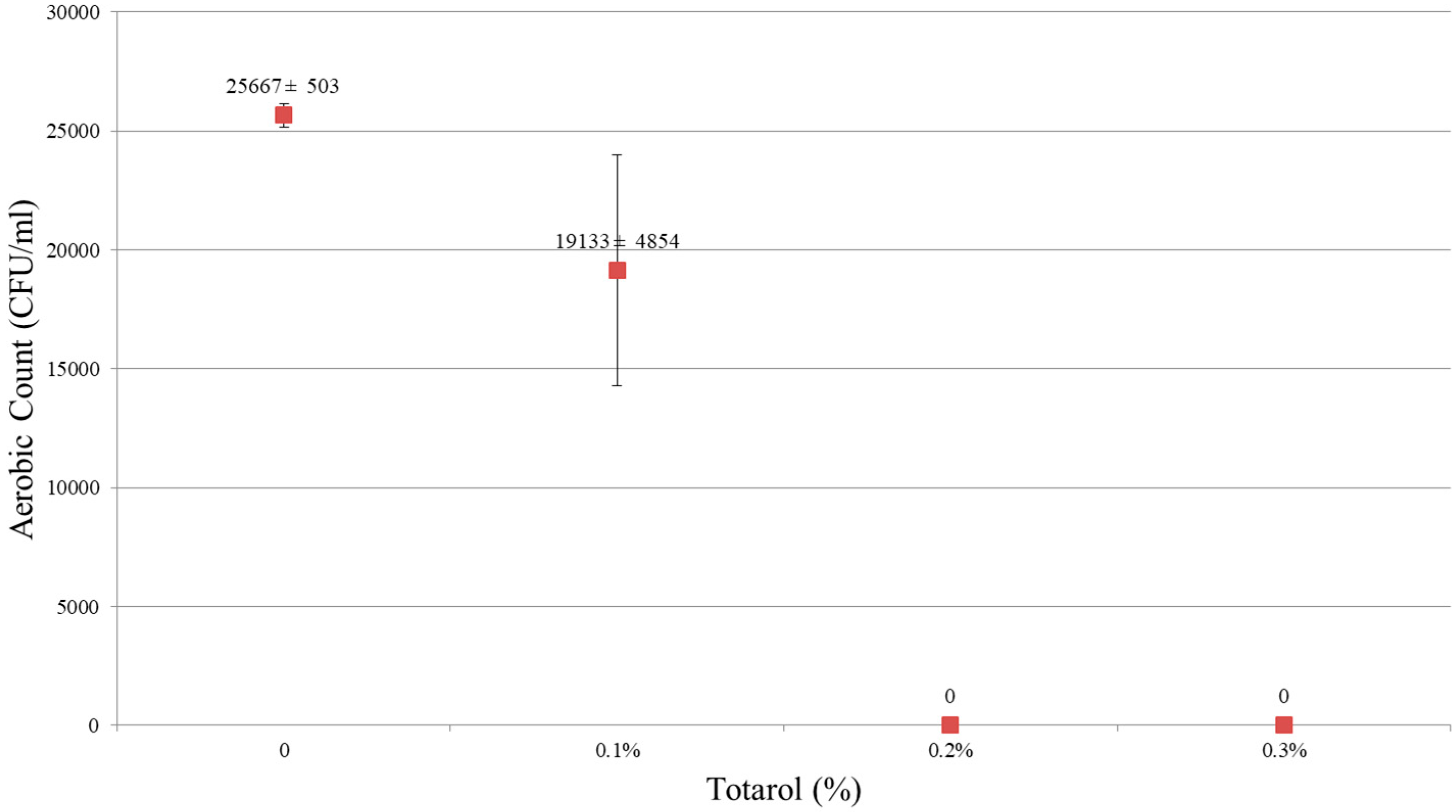
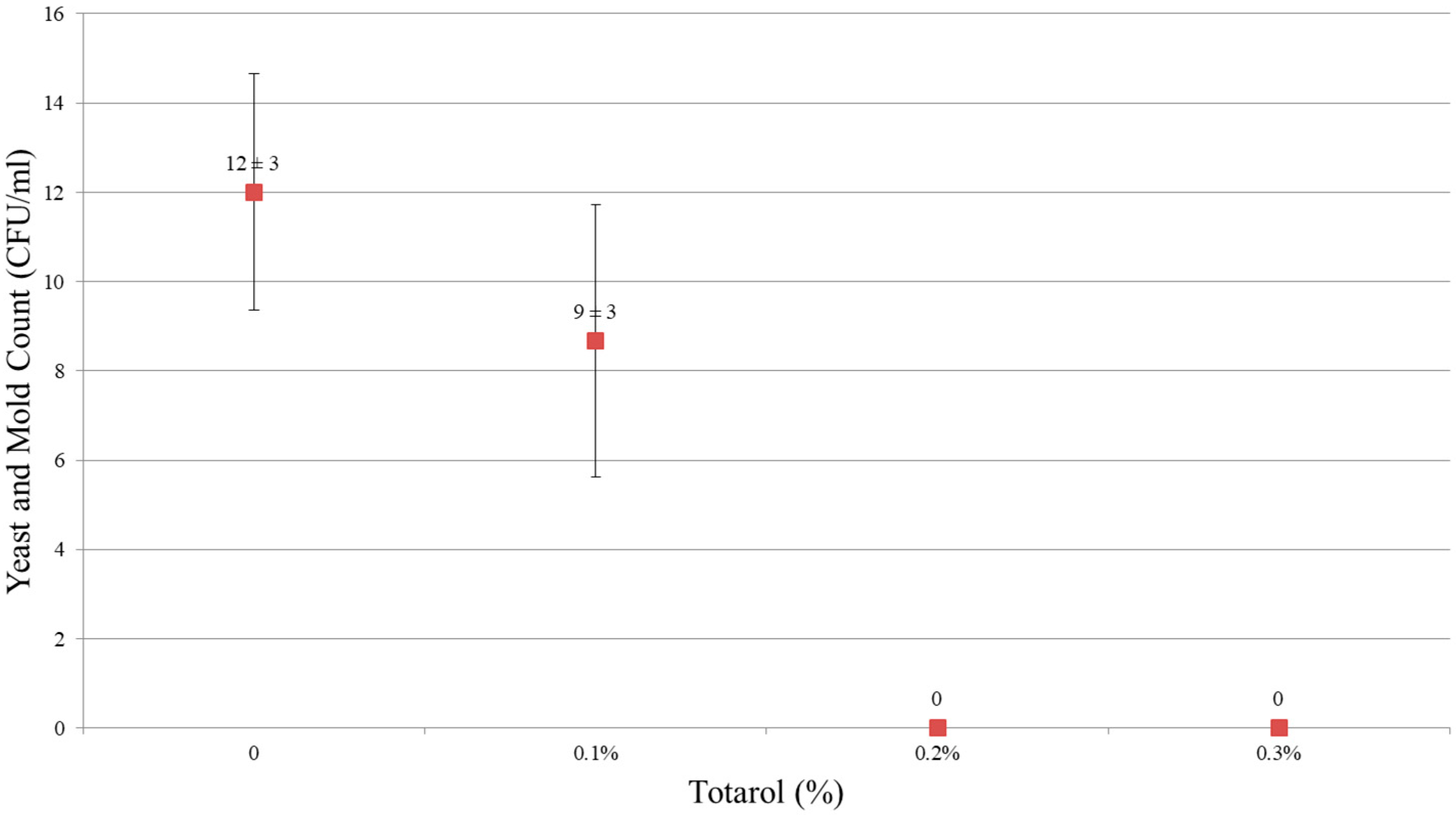
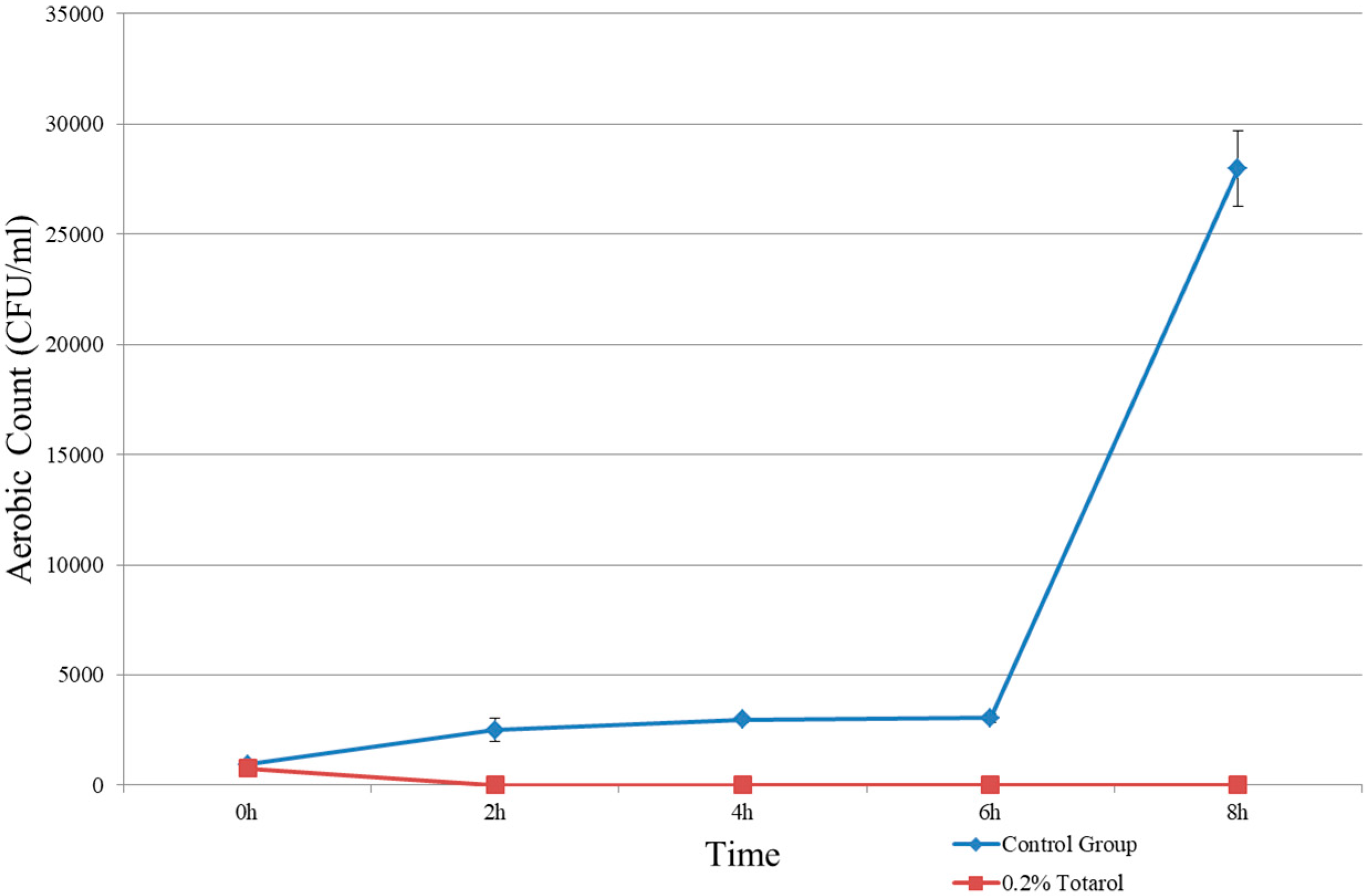
The results showed that 0.2% and 0.3% (w/w) of totarol had a potent antimicrobial effect. As shown in Figure 1 and Figure 2, no microorganism was detected in samples containing 0.2% and 0.3% totarol. In samples without totarol, the results of colonies on aerobic count were 25,667 ± 503 CFU/mL and the yeast and mold count was 12 ± 3 CFU/mL. In samples containing 0.1% (w/w) of totarol, the results were 19,133 ± 4854 CFU/mL for aerobic count and 9 ± 3 CFU/mL for yeast and mold count. It is also indicated that 0.2% (w/w) totarol could eliminate the presence of viable microorganisms in 2 h (Figure 3). The samples with 0.2% (w/w) totarol were tested for aerobic count immediately after the addition of totarol and the result was 770 ± 95 CFU/mL. Thereafter, there was no microorganisms detected in these samples. Meanwhile, the aerobic count of samples without totarol (control) increased over time.

Figure 1.
Effect of totarol level on aerobic counts.

Figure 2.
Effect of totarol level on yeast and mold counts.

Figure 3.
Time needed for antimicrobial effect of totarol.
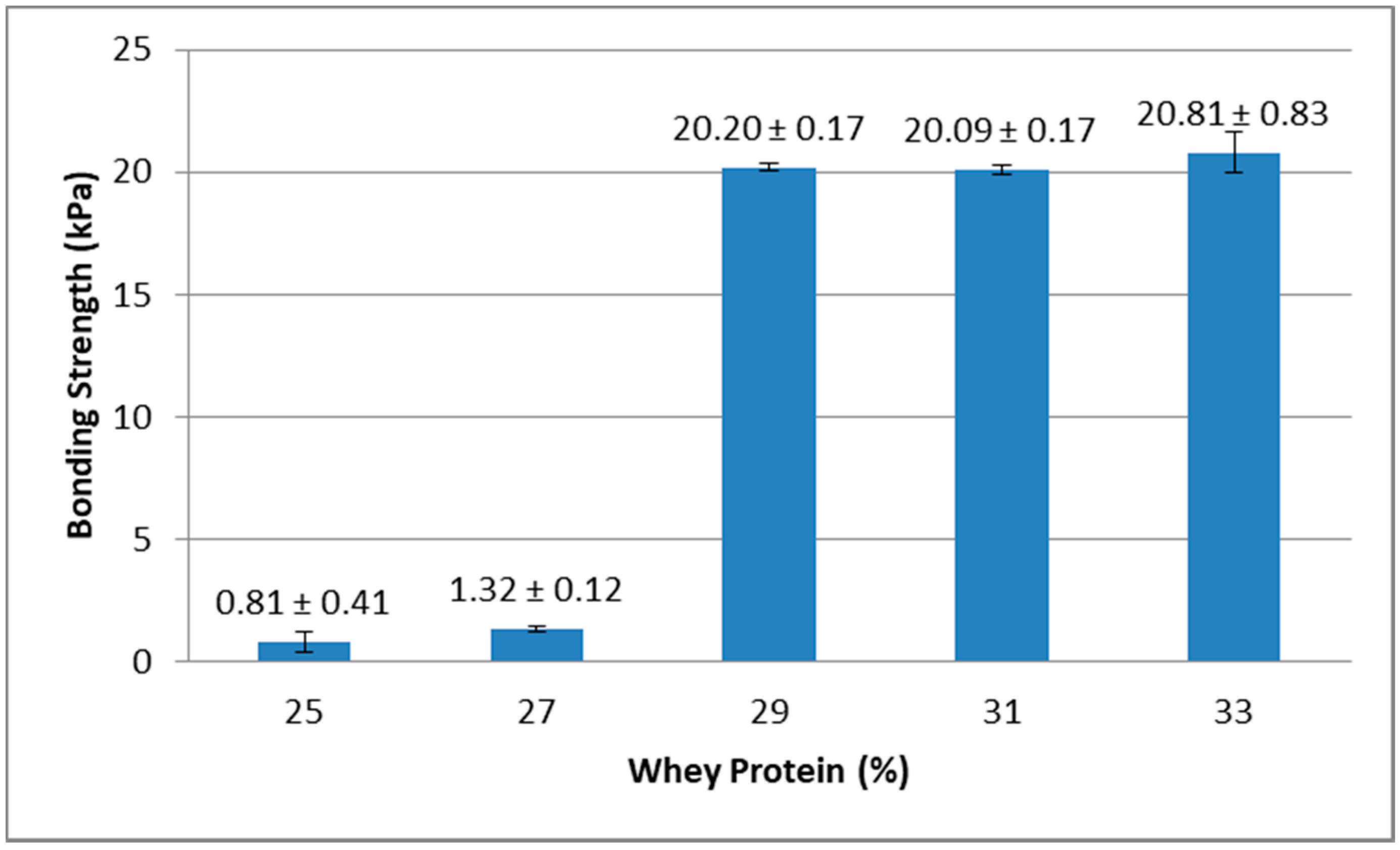
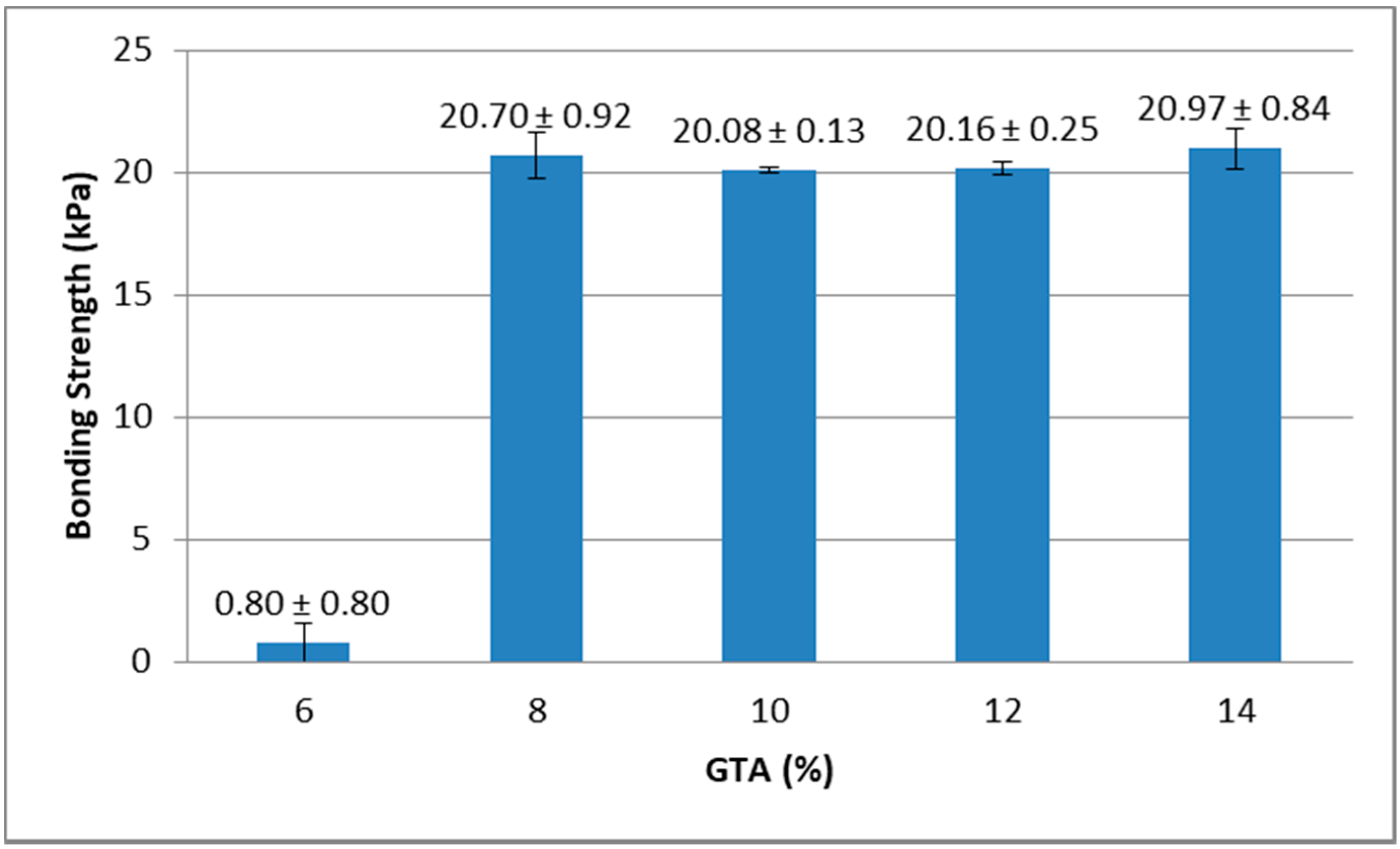
Results from the lap-shear bonding strength tests indicated that lap-shear bonding strength generally increased with higher concentration of whey protein and also with higher concentration of GTA. As shown in Figure 4, when GTA concentration was 14%, the bonding strength of samples containing 25% and 27% of protein was lower than samples containing 29%, 31%, and 33% of protein. Figure 5 showed that when whey protein concentration was 33%, the bonding strength of samples containing 6% of GTA was lower than samples containing 8%, 10%, 12%, and 14% of GTA.

Figure 4.
Effect of whey protein concentration on bonding strength (glutaraldehyde (GTA) at 14%).

Figure 5.
Effect of GTA levels on bonding strength (protein at 33%).
The results indicated that higher concentrations of whey protein could provide more amino groups and higher concentrations of GTA introduced more terminal aldehyde groups. These are the two functional groups that could crosslink and form bonding strength.
Based on these preliminary study results, the final whey protein-based adhesive formulation is consisted of 33% (w/w) whey protein, 0.2% (w/w) totarol, and 14% (w/w) glutaraldehyde.
3.2. Changes in Aerobic Count, Yeast and Mold Count, and Lap-Shear Bonding Strength during Storage
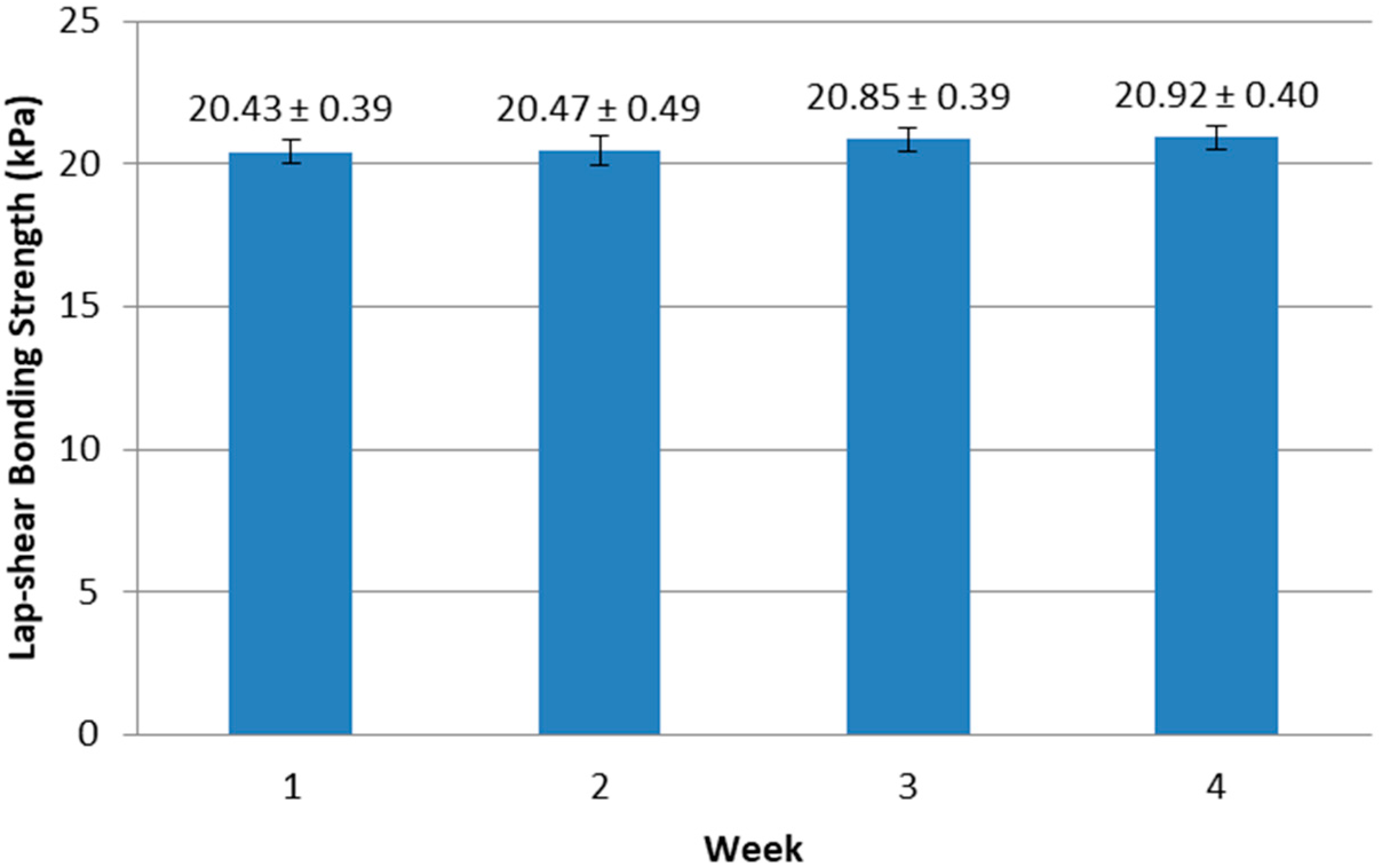
During the one-month storage, there was no microorganism found in samples containing 0.2% (w/w) totarol for both aerobic count and yeast and mold count. There was no significant change (p = 0.406) in lap-shear bonding strength (Figure 6) during storage. In addition, the samples did not gel during storage. These results indicated that the whey protein-based tissue adhesive with totarol as an antimicrobial agent was stable for at least one month at room temperature.

Figure 6.
Changes in bonding strength of whey protein adhesive during storage at 23 °C.
3.3. Microstructure
The cross-linking of the whey proteins to themselves and to the tissue is the result of the reaction between the amine groups of proteins and the terminal aldehyde groups which are provided by the crosslinking agent GTA.
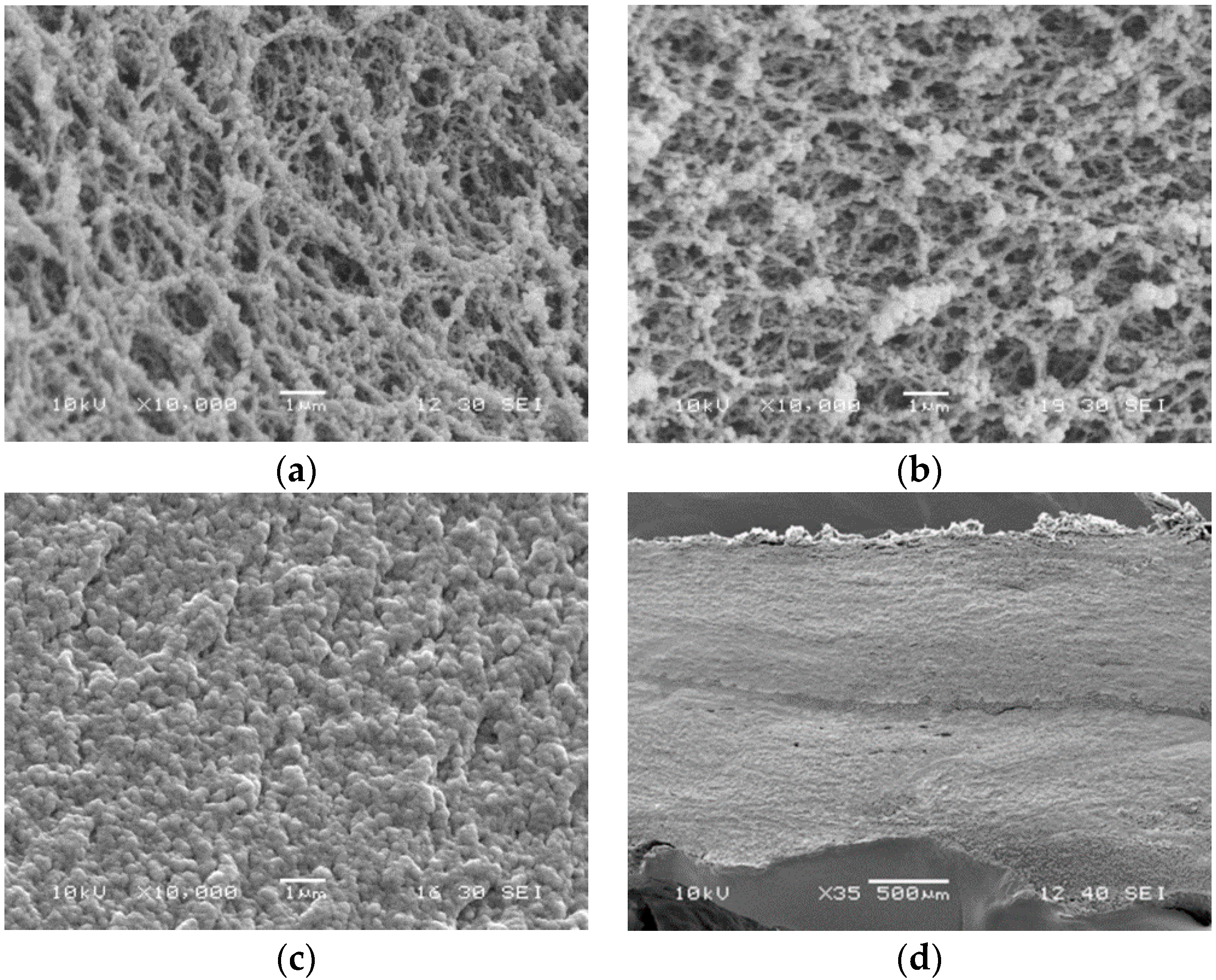
Figure 7a,b showed the micrographs of WPI solution and WPI-totarol mixture. Totarol did not seem to affect the microstructure of whey protein. Figure 7c was the micrograph of WPI-totarol mixture after crosslinking with GTA. GTA reacted with proteins, making them linked together. The protein molecules interacted and formed a uniform and continuous structure. Figure 7d showed the micrograph of the adhesive bonding with two pieces of porcine skin.

Figure 7.
Scanning electron microscopy (SEM) micrographs of whey protein isolate (WPI) solution (33% protein) (a), WPI solution (33% protein) containing 0.2% totarol (b), WPI solution (33% protein) containing 0.2% totarol crosslinked by 14% glutaraldehyde (c) at 10,000× magnification, and porcine skins bonded by tissue adhesive at 35× magnification (d).
4. Conclusions
The results indicated that whey protein may be suitable for tissue adhesive formulation. Totarol is an effective antimicrobial agent for the whey protein-based tissue adhesive system. SEM micrographs of the adhesive showed that the whey protein-based adhesive and the porcine skin could form a smooth and uniform structure. Future work on this study would be to find a way to lower totarol concentration without damaging the antimicrobial property. Totarol used in this study is a 1.21% solution and it would lower the concentration of whey protein when added into the system. Purer totarol could be used to further increase the concentration of whey protein. In addition, a longer testing period may be needed so that the shelf life of this adhesive could be extended.
Author Contributions
Data curation, Y.H., X.Z., and C.W.; Formal analysis, Y.H.; Investigation, Y.H., X.Z., and C.W.; Methodology, Y.H., X.Z., C.W., and M.G.; Project administration, M.G.; Supervision, M.G.; Writing—original draft, Y.H.; Writing—review & editing, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This Hatch project was supported by USDA-NIFA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Quinn, J.V. Tissue Adhesives in Clinical Medicine, 2nd ed.; BC Decker Inc.: Hamilton, ON, Canada, 2005. [Google Scholar]
- Quinn, J.; Wells, G.; Sutcliffe, T.; Jarmuske, M.; Maw, J.; Stiell, I.; Johns, P. Tissue adhesive versus suture wound repair at 1 year: Randomized clinical trial correlating early, 3-month, and 1-year cosmetic outcome. Ann. Emerg. Med. 1998, 32, 645–649. [Google Scholar] [CrossRef]
- Webster, J.; Alghamdi, A. Use of Plastic Adhesive Drapes during Surgery for Preventing Surgical Site Infection; Cochrane Wounds Group: Manchester, UK, 2007; p. CD006353. [Google Scholar]
- Hidas, G.; Mullerad, A.K.M.; Shental, J.; Moskovitz, B.; Nativ, O. Sutureless nephron-sparing surgery: Use of albumin glutaraldelhyde tissue adhesive. Urology 2006, 67, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Walt, D.; Agayn, V. The chemistry of enzyme and protein immobilization with glutaraldehyde. Trend Anal. Chem. 1994, 13, 425–430. [Google Scholar] [CrossRef]
- Ryou, M.; Thompson, C. Tissue adhesives: A review. Tech. Gastrointest. Endosc. 2005, 12, 33–37. [Google Scholar] [CrossRef]
- Wang, G.R.; Liu, N.; Guo, M.R. Use of whey protein as a natural polymer for tissue adhesive: Preliminary formulation and evaluation in vitro. Polymers 2018, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Smithers, G.W.; Ballard, F.J.; Copeland, A.D.; De Silva, K.J.; Dionysius, D.A.; Francis, G.L.; Goddard, C.; Grieve, P.A.; Mcintosh, G.H.; Mitchell, I.R. New opportunities from the isolation and utilization of whey proteins. J. Dairy Sci. 1996, 8, 1454–1459. [Google Scholar] [CrossRef]
- Gao, Z.; Yu, G.; Bao, Y.; Guo, M. Whey-protein based environmentally friendly wood adhesives. Pigm. Resin Technol. 2011, 40, 42–48. [Google Scholar] [CrossRef]
- Wang, G.R.; Guo, M.R. Property and storage stability of whey protein-sucrose based safe paper glue. J. Appl. Polym. 2014, 131. [Google Scholar] [CrossRef]
- Bendall, J.G.; Cambie, R.C. Totarol: A non-conventional diterpenoid. Aust. J. Chem. 1995, 48, 883–917. [Google Scholar] [CrossRef]
- Micol, V.; Mateo, C.R.; Shapiro, S.; Aranda, F.J.; Villalain, J. Effects of totarol, a diterpenoid antibacterial agent, on phospholipid model membranes. Biochim. Biophys. Acta 2001, 1511, 281–290. [Google Scholar] [CrossRef]
- Kubo, I.; Muroi, H.; Himejima, M. Antibacterial activity of totarol and its potentiation. J. Nat. 1992, 10, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Muroi, H.; Kubo, I. Antibacterial activity of anacardic acid and totarol, alone and in combination with methicillin, against methicillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 1996, 80, 387–394. [Google Scholar]
- Constantine, G.H.; Karchesy, J.J.; Franzblau, S.G.; LaFleur, L.E. (+)-Totarol from chamaecyparis nootkatensis and activity against Mycobacterium tuberculosis. Fitoterapia 2001, 72, 572–574. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, X.C.; Li, W.L.; Meng, R.Z.; Liu, Z.H.; Liu, M.Y.; Guo, N.; Yu, L. Inhibitory effect of totarol on exotoxin proteins hemolysin and enterotoxins secreted by Staphylococcus aureus. World J. Urol. 2015, 31, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Che, M.Y.; Zhang, X.W.; Liu, Z.J.; Meng, R.Z.; Bu, X.J.; Ye, H.Q.; Guo, N. Antibacterial activity and mode of action of totarol against Staphylococcus aureus in carrot juice. J. Food Sci. Technol. 2018, 55, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, H.; Oike, S.; Muroi, H.; Kubo, I. Mode of antibacterial action of totarol, a diterpene from Podocarpus nagi. Planta. Med. 1996, 62, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.B.; Furneaux, R.H.; Gainsford, G.J.; Murphy, M.P. The synthesis and antibacterial activity of totarol derivatives, part 3: Modification of ring-B. Bioorg. Med. Chem. 2000, 8, 1663–1675. [Google Scholar] [CrossRef]
- Smith, E.C.; Kaatz, G.W.; Seo, S.M.; Wareham, N.; Williamson, E.M.; Gibbons, S. The phenolic diterpene totarol inhibits multidrug efflux pump activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 4480–4483. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Shi, C.; Wang, C.N.; Guo, M.R. Effects of ultrasound treatment on physiochemical properties and antimicrobial activities of whey protein–totarol nanoparticles. J. Food Prot. 2017, 80, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, S. Building Decorative Materials; Woodhead Publishing: Cambridge, UK, 2011. [Google Scholar]
- ASTM. Standard Test Method for Strength Properties of Tissue Adhesives in Lap-Shear by Tension Loading; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Herron, A.J. Pigs as dermatologic models of human skin disease. In Proceedings of the ACVP/ASVCP Annual Meetings, Monterey, CA, USA, 5–9 December 2009. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).