Synthesis and Characterization of New Lithium and Boron Based Metal Organic Frameworks with NLO Properties for Application in Neutron Capture Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of LiM2B and Li5T2B
2.2. Single Crystal X-ray Diffraction (XRD)
2.2.1. Crystal Data for Li[(C6H12O6)2B]·2H2O (LiM2B)
2.2.2. Crystal Data for Li5[(C4H2O6)2B]·5.5 H2O (Li5T2B)
2.3. Computational Methods
2.4. Second Harmonic Generation Measurements
2.5. The e_LiBANS Neutron Irradiation Facility
3. Results and Discussion
3.1. Crystal Structures
3.2. Computational Results and SHG Measurements
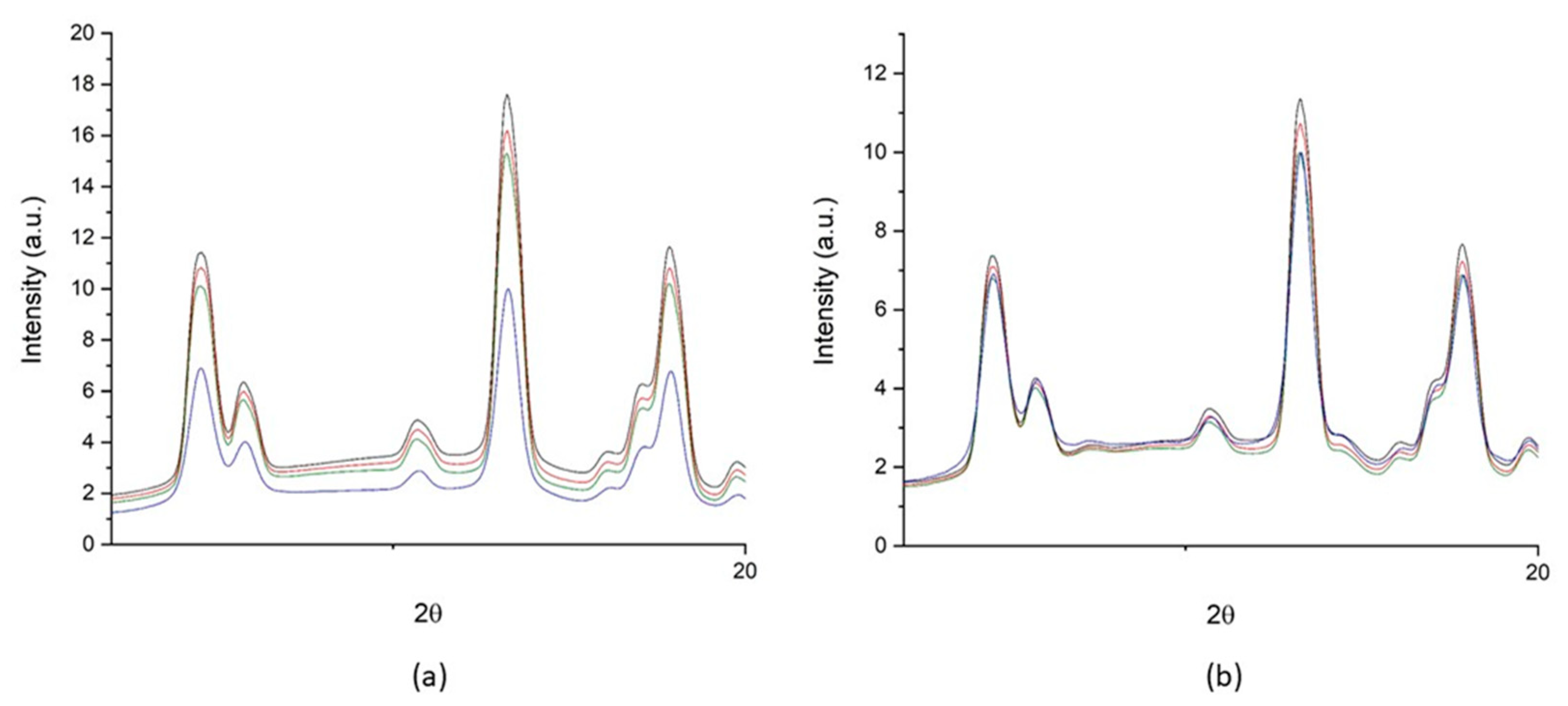
3.3. The Neutron Irradiation Campaign
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nedunchezhian, K.; Aswath, N.; Thiruppathy, M.; Thirugnanamurthy, S. Boron Neutron Capture Therapy—A Literature Review. J. Clin. Diagn. Res. 2016, 10, 2E01–2E04. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.L. Critical review, with an optimistic outlook, on Boron Neutron Capture Therapy (BNCT). Appl. Rad. Isot. 2014, 88, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Marabello, D.; Antoniotti, P.; Benzi, P.; Beccari, P.; Canepa, C.; Barge, A.; Boscaro, V.; Gallicchio, M.; Peira, E. Synthesis, characterization and cell uptake of nanoparticles for a novel approach to radionuclide therapy: A feasibility study. Int. J. Res. Pharm. Nano Sci. 2019, 8, 230–240. Available online: http://www.ijrpns.com/archives1.php?volume=8&issue=5 (accessed on 5 July 2019).
- Boyd, R.W. Nonlinear Optics; Academic Press—An imprint of Elsevier Science: San Diego, CA, USA, 2003; ISBN 0-12-121682-9. [Google Scholar]
- Monti, V.; Costa, M.; Durisi, E.; Mafucci, E.; Menzio, L.; Sans-Planell, O.; Visca, L.; Bedogni, R.; Treccani, M.; Pola, A.; et al. The e LiBANS facility: A new compact thermal neutron source based on a medical electron, LINAC. Nuclear Instrum. Methods Phys. Sect. A 2020, 953, 163154. [Google Scholar] [CrossRef] [Green Version]
- CrysAlisPro 1.171.39.46 (Rigaku Oxford Diffraction, 2018). Available online: https://www.rigaku.com/products/smc/crysalis (accessed on 5 July 2019).
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Jiang, J.S.; Brünger, A.T. Protein hydration observed by X-ray diffraction. J. Mol. Biol. 1994, 243, 100–115. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Schlegel, H.B.; Daudel, C. Computational Theoretical Organic Chemistry; Reidel Publ, Co.: Dordrecht, The Netherlands, 1981. [Google Scholar]
- Schlegel, H.B. An efficient algorithm for calculating ab initio energy gradients using s, p Cartesian Gaussians. J. Chem. Phys. 1982, 77, 3676–3681. [Google Scholar] [CrossRef]
- Schlegel, H.B. Optimization of equilibrium geometries and transition structures. J. Comput. Chem. 1982, 3, 214–218. [Google Scholar] [CrossRef]
- Schlegel, H.B.; Binkley, J.S.; Pople, J.A. First and second derivatives of two electron integrals over Cartesian Gaussians using Rys polynomials. J. Chem. Phys. 1984, 80, 1976–1981. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Densityfunctional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Hehre, W.J.; Radom, L.; Schleyer, P.R.; Pople, J.A. Ab Initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Hrobáriková, V.; Hrobárik, P.; Gajdos, P.; Fitilis, I.; Fakis, M.; Persephonis, P.; Zahradník, P. Benzothiazole-Based Fluorophores of Donor-π-Acceptor-π-Donor Type Displaying High Two-Photon Absorption. J. Org. Chem. 2010, 75, 3053–3068. [Google Scholar] [CrossRef] [PubMed]
- Hrobárik, P.; Sigmundová, I.; Zahradník, P.; Kasák, P.; Arion, V.; Franz, E.; Clays, K. Molecular Engineering of Benzothiazolium Salts with Large Quadratic Hyperpolarizabilities: Can Auxiliary Electron-Withdrawing Groups Enhance Nonlinear Optical Responses? J. Phys. Chem. C 2010, 114, 22289–22302. [Google Scholar] [CrossRef]
- Marabello, D.; Antoniotti, P.; Benzi, P.; Canepa, C.; Diana, D.; Operti, L.; Mortati, L.; Sassi, M.P. Non Linear Optical Properties of β-D-Fructopyranose Calcium Chloride MOFs: An Experimental and Theoretical Approach. J. Mater. Sci. 2015, 50, 4330–4341. [Google Scholar] [CrossRef]
- Marabello, D.; Antoniotti, P.; Benzi, P.; Canepa, C.; Mortati, L.; Sassi, M.P. Synthesis, structure and non-linear optical properties of new isostructural β-D-fructopyranose alkaline halide metal–organic frameworks: A theoretical and an experimental study. Acta Crystallogr. 2017, B73, 737–743. [Google Scholar] [CrossRef]
- Marabello, D.; Antoniotti, P.; Benzi, P.; Cariati, E.; Lo Presti, L.; Canepa, C. Developing new SrI2 and β-D-fructopyranose-based metal–organic frameworks with nonlinear optical properties. Acta Crystallogr. 2019, B75, 210–218. [Google Scholar] [CrossRef]
- Parsons, D.F.; Ninham, B.W. Ab initio molar volumes and Gaussian radii. J. Phys. Chem. A 2009, 113, 1141–1150. [Google Scholar] [CrossRef]
- Kurtz, S.K.; Perry, T.T. A powder technique for the evaluation of nonlinear optical materials. J. Appl. Phys. 1968, 39, 3798–3813. [Google Scholar] [CrossRef]
- Davis, H.B.; Mott, C.J.B. Interaction of Boric Acid and Borates with Carbohydrates and Related Substances. J. Chem. Soc. Faraday 1 1980, 76, 1991–2002. [Google Scholar] [CrossRef]
- Celeste, M.; Avezedo, C.; Cavailero, A.M.V. The Acid–Base Titration of a Very Weak Acid: Boric Acid. J. Chem. Educ. 2012, 89, 767–770. [Google Scholar]
- Pizer, R.; Ricatto, P.J. Ternary alkaline earth metal complex ions in the M2+/borate/tartrate system as studied by 11B NMR. Inorg. Chem. 1994, 33, 4985–4990. [Google Scholar] [CrossRef]
- Kustin, K.; Pizer, R. Temperature-Jump Study of the Rate and Mechanism of the Boric Acid-Tartaric Acid Complexation. J. Am. Chem. Soc. 1969, 91, 317–322. [Google Scholar] [CrossRef]
- Kanis, D.R.; Ratner, M.A.; Marks, T.J. Design and construction of molecular assemblies with large second-order optical nonlinearities.Quantum chemical aspects. Chem. Rev. 1994, 94, 195–242. [Google Scholar] [CrossRef]
- Kyrill, Y.; Suponitsky, K.Y.; Tafur, S.; Masunov, A.E. Applicability of hybrid density functional theory methods to calculation of molecular hyperpolarizability. J. Chem. Phys. 2008, 129, 044109. [Google Scholar]





| LiM2B | Li5T2B | |||
|---|---|---|---|---|
| XRD | B3LYP/6-31G(d) | XRD | B3LYP/6-31G(d) | |
| Li-O | 1.944 | 2.011 | 2.096 | 2.201 |
| Li-OW | 1.929 | 1.987 | 1.941 | 1.942 |
| B-O | 1.471 | 1.483 | 1.474 | 1.476 |
| C-O | 1.426 | 1.431 | 1.305 | 1.317 |
| C-C | 1.526 | 1.536 | 1.535 | 1.544 |
| Li···B | 2.682 | 2.588 | ||
| Li···Li | 2.998 | 3.209 | ||
| Li···C | 2.715 | 2.775 | ||
| LiM2B | Li5T2B | |
|---|---|---|
| µ | 14.469 | 19.196 |
| <α> | 840.337 | 719.428 |
| β | 3.9 | 8.0 |
| χ(2) | 0.65 | 1.53 |
| χ(2)/χ(2)sucrose | 0.4 | 0.8 |
| I2ω/I2ωsucrose (measured) | 0.6 | 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marabello, D.; Benzi, P.; Beccari, F.; Canepa, C.; Cariati, E.; Cioci, A.; Costa, M.; Durisi, E.A.; Monti, V.; Sans Planell, O.; et al. Synthesis and Characterization of New Lithium and Boron Based Metal Organic Frameworks with NLO Properties for Application in Neutron Capture Therapy. Processes 2020, 8, 558. https://doi.org/10.3390/pr8050558
Marabello D, Benzi P, Beccari F, Canepa C, Cariati E, Cioci A, Costa M, Durisi EA, Monti V, Sans Planell O, et al. Synthesis and Characterization of New Lithium and Boron Based Metal Organic Frameworks with NLO Properties for Application in Neutron Capture Therapy. Processes. 2020; 8(5):558. https://doi.org/10.3390/pr8050558
Chicago/Turabian StyleMarabello, Domenica, Paola Benzi, Fabio Beccari, Carlo Canepa, Elena Cariati, Alma Cioci, Marco Costa, Elisabetta Alessandra Durisi, Valeria Monti, Oriol Sans Planell, and et al. 2020. "Synthesis and Characterization of New Lithium and Boron Based Metal Organic Frameworks with NLO Properties for Application in Neutron Capture Therapy" Processes 8, no. 5: 558. https://doi.org/10.3390/pr8050558





