Cold Sintering as a Cost-Effective Process to Manufacture Porous Zinc Electrodes for Rechargeable Zinc-Air Batteries
Abstract
1. Introduction
2. Results
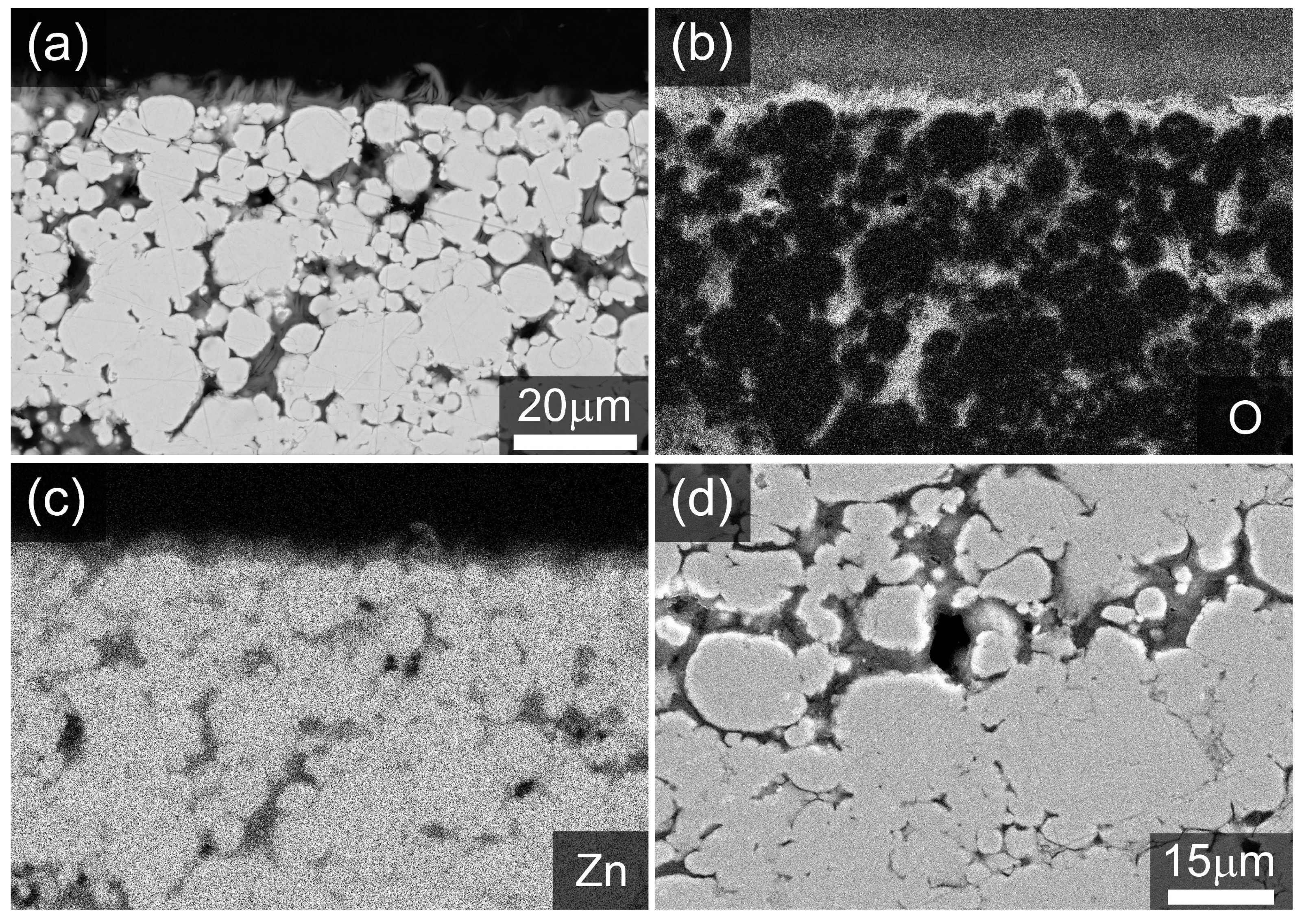
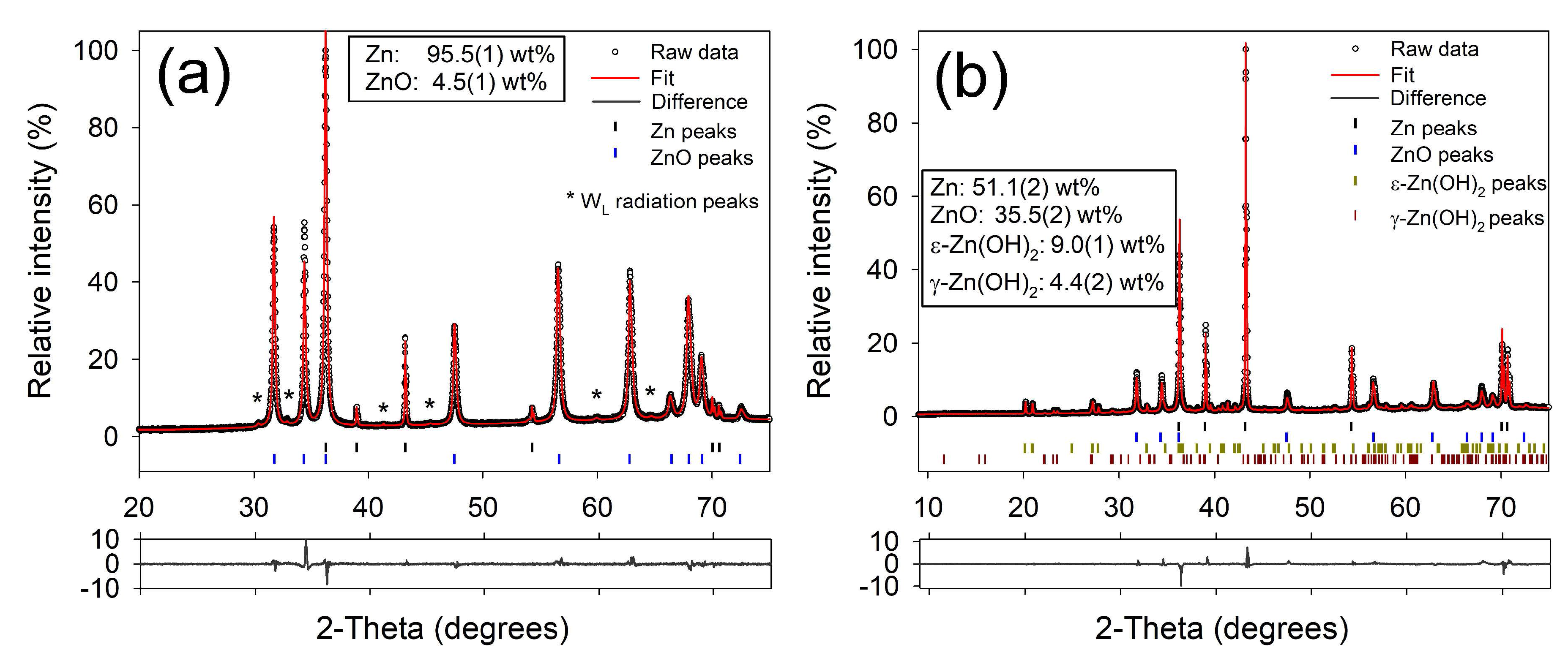
2.1. Physical Characterization
2.2. Electrochemical Characterization
3. Discussion
3.1. Performance Analysis
3.2. Techno-Economic Analysis
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BSE | Backscattered electron |
| CSP | Cold sintering process |
| EDS | Energy dispersive X-ray spectroscopy |
| OAc | Acetate |
| OCV | Open circuit voltage |
| OER | Oxygen evolution reaction |
| ORR | Oxygen reduction reaction |
| PTFE | Polytetrafluoroethylene |
| RTE | Round trip efficiency |
| SE | Secondary electron |
| SEM | Scanning electron microscope |
| TSP | Thermal sintering process |
| XRD | X-ray diffraction |
| ZAB | Zinc-air battery |
References
- Gu, P.; Zheng, M.; Zhao, Q.; Xiao, X.; Xue, H.; Pang, H. Rechargeable zinc-air battery: A promising way to green energy. J. Mater. Chem. A 2017, 5, 7651–7666. [Google Scholar] [CrossRef]
- Mainar, A.R.; Iruin, E.; Colmenares, L.C.; Kvasha, A.; de Meatza, I.; Bengoechea, M.; Leonet, O.; Boyano, I.; Zhang, Z.; Blazquez, J.A. An overview of progress in electrolytes for secondary zinc-air batteries and other storage systems based on zinc. J. Energy Storage 2018, 15, 304–328. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2020; U.S. Geological Survey: Reston, VA, USA, 2020; p. 200.
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Park, J.; Park, M.; Nam, G.; Lee, J.S.; Cho, J. All-solid-state cable-type flexible zinc-air battery. Adv. Mater. 2015, 27, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Mainar, A.R.; Leonet, O.; Bengoechea, M.; Boyano, I.; de Meatza, I.; Kvasha, A.; Guerfi, A.; Blazquez, J.A. Alkaline aqueous electrolytes for secondary zinc-air batteries: An overview. Int. J. Energy Res. 2016, 40, 1032–1049. [Google Scholar] [CrossRef]
- Stamm, J.; Varzi, A.; Latz, A.; Horstmann, B. Modeling nucleation and growth of zinc oxide during discharge of primary zinc-air batteries. J. Power Sources 2017, 360, 136–149. [Google Scholar] [CrossRef]
- Yi, J.; Liang, P.; Liu, X.; Wu, K.; Liu, Y.; Wang, Y.; Xia, Y.; Zhang, J. Challenges, mitigation strategies and perspectives in development of zinc-electrode materials and fabrication for rechargeable zinc-air batteries. Energy Environ. Sci. 2018, 11, 3075–3095. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Q.; Tang, Y.; Zhang, L.; Li, Y. Zinc-air batteries: Are they ready for prime time? Chem. Sci. 2019, 10, 8924–8929. [Google Scholar] [CrossRef]
- Stock, D.; Dongmo, S.; Janek, J.; Schröder, D. Benchmarking Anode Concepts: The Future of Electrically Rechargeable Zinc-Air Batteries. ACS Energy Lett. 2019, 4, 1287–1300. [Google Scholar] [CrossRef]
- Heise, G.W.; Cahoon, N.C. Dry Cells of the Leclanche Type, 1902–1952—A Review. J. Electrochem. Soc. 1952, 99, 179C–187C. [Google Scholar] [CrossRef]
- Horn, Q.C.; Shao-Horn, Y. Morphology and Spatial Distribution of ZnO Formed in Discharged Alkaline Zn/MnO2 AA Cells. J. Electrochem. Soc. 2003, 150, A652–A658. [Google Scholar] [CrossRef]
- Arlt, T.; Schröder, D.; Krewer, U.; Manke, I. In operando monitoring of the state of charge and species distribution in zinc air batteries using X-ray tomography and model-based simulations. Phys. Chem. Chem. Phys. 2014, 16, 22273–22280. [Google Scholar] [CrossRef] [PubMed]
- Mainar, A.R.; Colmenares, L.C.; Blázquez, J.A.; Urdampilleta, I. A brief overview of secondary zinc anode development: The key of improving zinc-based energy storage systems. Int. J. Energy Res. 2017, 42, 903–918. [Google Scholar] [CrossRef]
- Schmitt, T.; Arlt, T.; Manke, I.; Latz, A.; Horstmann, B. Zinc Electrode Shape-Change in Secondary Air Batteries: A 2D Modeling Approach. J. Power Sources 2019, 432, 119–132. [Google Scholar] [CrossRef]
- Kelly, F.J.; Przybyla, F. Method of Making a Sintered Zinc Battery Anode Structure. US Patent 3,384,482, 21 May 1968. [Google Scholar]
- Himy, A. Development of Improved Zinc Electrodes for Secondary Batteries; Technical Report; NASA: Newport Beach, CA, USA, 1969.
- Arrance, F.C.; Berger, C. Process of Forming a Sintered Zinc Electrode. US Patent 3,617,592, 2 November 1971. [Google Scholar]
- Weller, R.D. Process for the Preparation of Sintered Zinc Powder Battery Electrodes. US Patent 3,663,297, 16 May 1972. [Google Scholar]
- Drillet, J.F.; Adam, M.; Barg, S.; Herter, A.; Koch, D.; Schmidt, V.M.; Wilhelm, M. Development of a novel zinc/air fuel cell with a Zn foam anode, a PVA/KOH membrane and a MnO2/SiOC-based air cathode. ECS Trans. 2010, 28, 13–24. [Google Scholar]
- Parker, J.F.; Chervin, C.N.; Nelson, E.S.; Rolison, D.R.; Long, J.W. Wiring zinc in three dimensions re-writes battery performance—Dendrite-free cycling. Energy Environ. Sci. 2014, 7, 1117–1124. [Google Scholar] [CrossRef]
- Parker, J.F.; Nelson, E.S.; Wattendorf, M.D.; Chervin, C.N.; Long, W.; Rolison, D.R. Retaining the 3D Framework of Zinc Sponge Anodes upon Deep Discharge in Zn-Air Cells. ACS Appl. Mater. Interfaces 2014, 6, 19471–19476. [Google Scholar] [CrossRef]
- Parker, J.F.; Chervin, C.N.; Pala, I.R.; Machler, M.; Burz, M.F.; Long, J.W.; Rolison, D.R. Rechargeable nickel–3D zinc batteries: An energy-dense, safer alternative to lithium-ion. Science 2017, 356, 415–418. [Google Scholar] [CrossRef]
- Stock, D.; Dongmo, S.; Walther, F.; Sann, J.; Janek, J.; Schröder, D. Homogeneous coating with anion-exchange ionomer improves the cycling stability of secondary batteries with zinc anode. ACS Appl. Mater. Interfaces 2018, 10, 8640–8648. [Google Scholar] [CrossRef]
- Liu, P.; Ling, X.; Zhong, C.; Deng, Y.; Han, X.; Hu, W. Porous Zinc Anode Design for Zn-air Chemistry. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Ko, J.S.; Geltmacher, A.B.; Hopkins, B.J.; Rolison, D.R.; Long, J.W.; Parker, J.F. Robust 3D Zn sponges enable high-power, energy-dense alkaline batteries. ACS Appl. Energy Mater. 2019, 2, 212–216. [Google Scholar] [CrossRef]
- Hopkins, B.J.; Sassin, M.B.; Chervin, C.N.; DeSario, P.A.; Parker, J.F.; Long, J.W.; Rolison, D.R. Fabricating architected zinc electrodes with unprecedented volumetric capacity in rechargeable alkaline cells. Energy Storage Mater. 2020, 27, 370–376. [Google Scholar] [CrossRef]
- Kränzlin, N.; Niederberger, M. Controlled fabrication of porous metals from the nanometer to the macroscopic scale. Mater. Horiz. 2015, 2, 359–377. [Google Scholar] [CrossRef]
- Lee, Y.K.; Kim, J.; Kim, Y.; Kwak, J.W.; Yoon, Y.; Rogers, J.A. Room Temperature Electrochemical Sintering of Zn Microparticles and Its Use in Printable Conducting Inks for Bioresorbable Electronics. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Smith, R.; Martell, A. Critical Stability Constants; Springer: New York, NY, USA, 1976; Volume 4. [Google Scholar]
- Zhang, Y.; Muhammed, M. Critical evaluation of thermodynamics of complex formation of metal ions in aqueous solutions—VI. Hydrolysis and hydroxo-complexes of Zn2+ at 298.15 K. Hydrometallurgy 2001, 60, 215–236. [Google Scholar] [CrossRef]
- Mahlendorf, F.; Fuchs, D. ZnMobil Abschlussbericht; Technical Report; Universitaet Duisburg-Essen: Duisburg-Essen, Germany, 2019. [Google Scholar]
- Albright, L.F. Albright’s Chemical Engineering Handbook; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Kabekkodu, S. (Ed.) PDF-4+ 2017 (Database); International Centre for Diffraction Data: Newtown Square, PA, USA, 2017. [Google Scholar]
- Ishioka, T.; Murata, A.; Kitagawa, Y.; Nakamura, K.T. Zinc(II) Acetate Dihydrate. Acta Crystallogr. 1997, C53, 1029–1031. [Google Scholar] [CrossRef]
- Hosono, E.; Fujihara, S.; Kimura, T.; Imai, H. Growth of layered basic zinc acetate in methanolic solutions and its pyrolytic transformation into porous zinc oxide films. J. Colloid Interface Sci. 2004, 272, 391–398. [Google Scholar] [CrossRef]
- Stahl, R.; Jung, C.; Lutz, H.; Kockelmann, W.; Jacobs, H. Kristallstrukturen und Wasserstoffbruckenbindungen bei beta-Be(OH)2 und epsilon-Zn(OH)2. Z. Anorg. Chem. 1998, 624, 1130–1136. [Google Scholar] [CrossRef]
- Christensen, A.N. The crystal structure of gamma-Zn(OH)2. Acta Chem. Scand. 1969, 23, 2016–2020. [Google Scholar] [CrossRef]





| Time, min | Power, W | Energy, W h | |
|---|---|---|---|
| Mixing, Zn-water Slurry | 5 | 15.76 | 1.31 |
| Mixing, Emulsified Zn Slurry | 2.5 | 11.16 | 0.47 |
| Mixing, Rest Period | 1440 | 0 | 0 |
| Heat Treatment, 200 bar Pressure | 0.1 | 82.75 | 0.14 |
| Heat Treatment, Heating 2 | 312.5 | 46.23 | 240.77 |
| Heat Treatment, Holding 650 | 90 | 0 * | 0 * |
| Heat Treatment, Cooling 4 | 156.25 | 25.99 | 67.69 |
| Time, min | Power, W | Energy, W h | |
|---|---|---|---|
| Mixing, Zn-ethanol Slurry | 5 | 17.93 | 1.49 |
| Mixing, Rest Period Zn Slurry | 120 | 0 | 0 |
| Pressing, 6 Compression | 2 | 179.95 | 0.05 |
| Drying, Heating 2 | 17.5 | 23.34 | 6.81 |
| Drying, Holding 60 | 900 | 0 * | 0 * |
| Drying, Cooling 4 | 8.75 | 25.99 | 3.79 |
| Thermal Sintering | Cold Sintering | Relative Change | |
|---|---|---|---|
| Time, | 33.4 | 17.6 | −47.50% |
| Energy, | 310.4 | 12.1 | −96.09% |
| Materials Cost, $ | 193.32 | 182.89 | −5.4% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayasayee, K.; Clark, S.; King, C.; Dahl, P.I.; Richard Tolchard, J.; Juel, M. Cold Sintering as a Cost-Effective Process to Manufacture Porous Zinc Electrodes for Rechargeable Zinc-Air Batteries. Processes 2020, 8, 592. https://doi.org/10.3390/pr8050592
Jayasayee K, Clark S, King C, Dahl PI, Richard Tolchard J, Juel M. Cold Sintering as a Cost-Effective Process to Manufacture Porous Zinc Electrodes for Rechargeable Zinc-Air Batteries. Processes. 2020; 8(5):592. https://doi.org/10.3390/pr8050592
Chicago/Turabian StyleJayasayee, Kaushik, Simon Clark, Cara King, Paul Inge Dahl, Julian Richard Tolchard, and Mari Juel. 2020. "Cold Sintering as a Cost-Effective Process to Manufacture Porous Zinc Electrodes for Rechargeable Zinc-Air Batteries" Processes 8, no. 5: 592. https://doi.org/10.3390/pr8050592
APA StyleJayasayee, K., Clark, S., King, C., Dahl, P. I., Richard Tolchard, J., & Juel, M. (2020). Cold Sintering as a Cost-Effective Process to Manufacture Porous Zinc Electrodes for Rechargeable Zinc-Air Batteries. Processes, 8(5), 592. https://doi.org/10.3390/pr8050592





