Industrial-Scale Study of the Chemical Composition of Olive Oil Process-Derived Matrices
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Solvent-Extraction of the Polar Fraction
2.2.1. Extraction of Polar Fraction from omww
2.2.2. Extraction of Polar Fraction from Olive Pomace and Paste
2.2.3. Extraction of Polar Fraction from Virgin Olive Oil Samples
2.3. Quality Indices
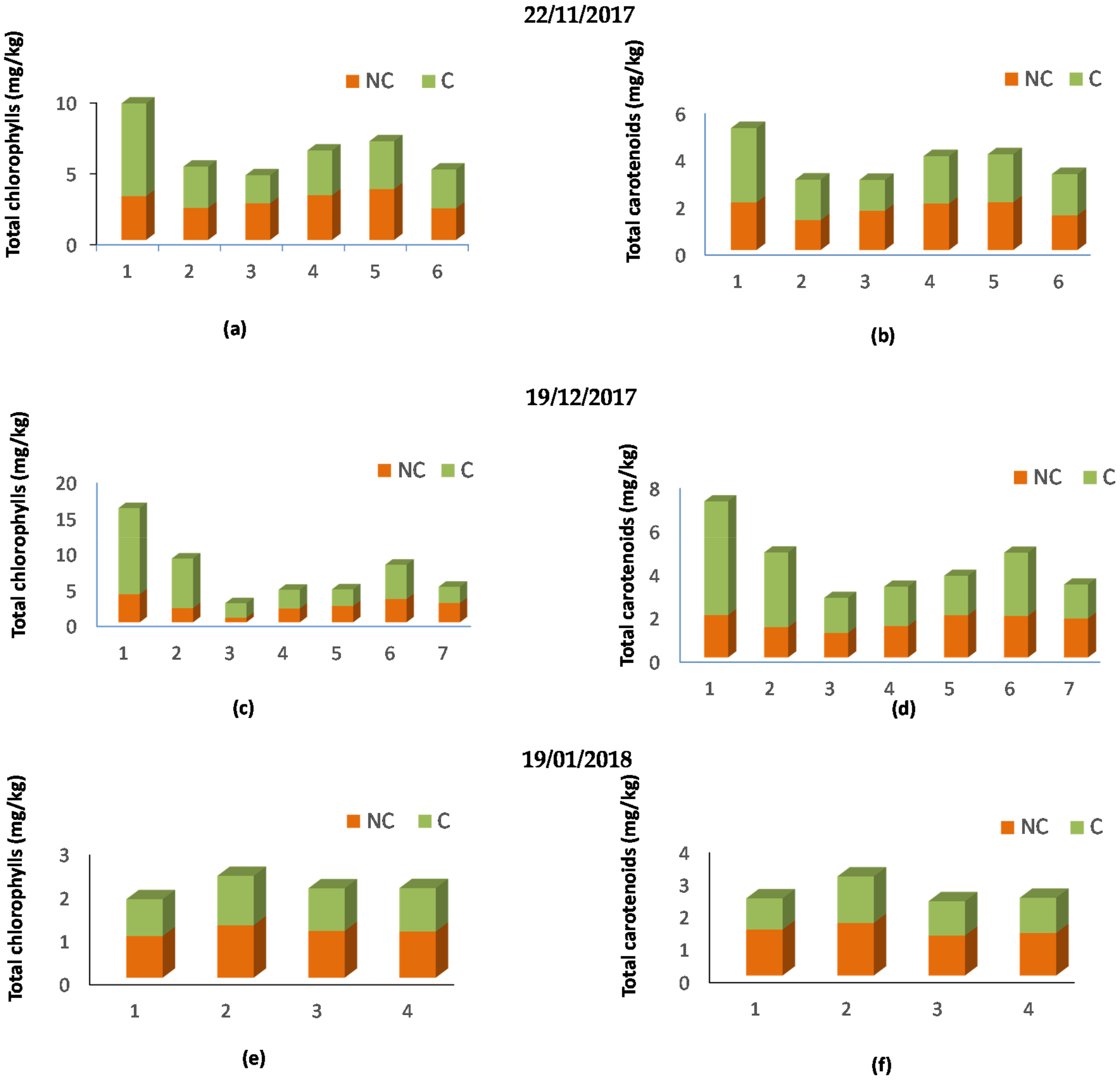
2.4. Pigment Content
2.5. Trolox Equivalent Antioxidant Capacity (TEAC)
2.6. Determination of Total Polyphenols
2.7. HPLC-MS Analysis of the Polar Fraction
2.8. Analysis of Fatty Acids
2.9. Statistical Analysis
3. Results
3.1. Quality Indices, Pigments, and Fatty Acid Composition of Olive Oil Samples
3.2. Total Polyphenols in the Extracts
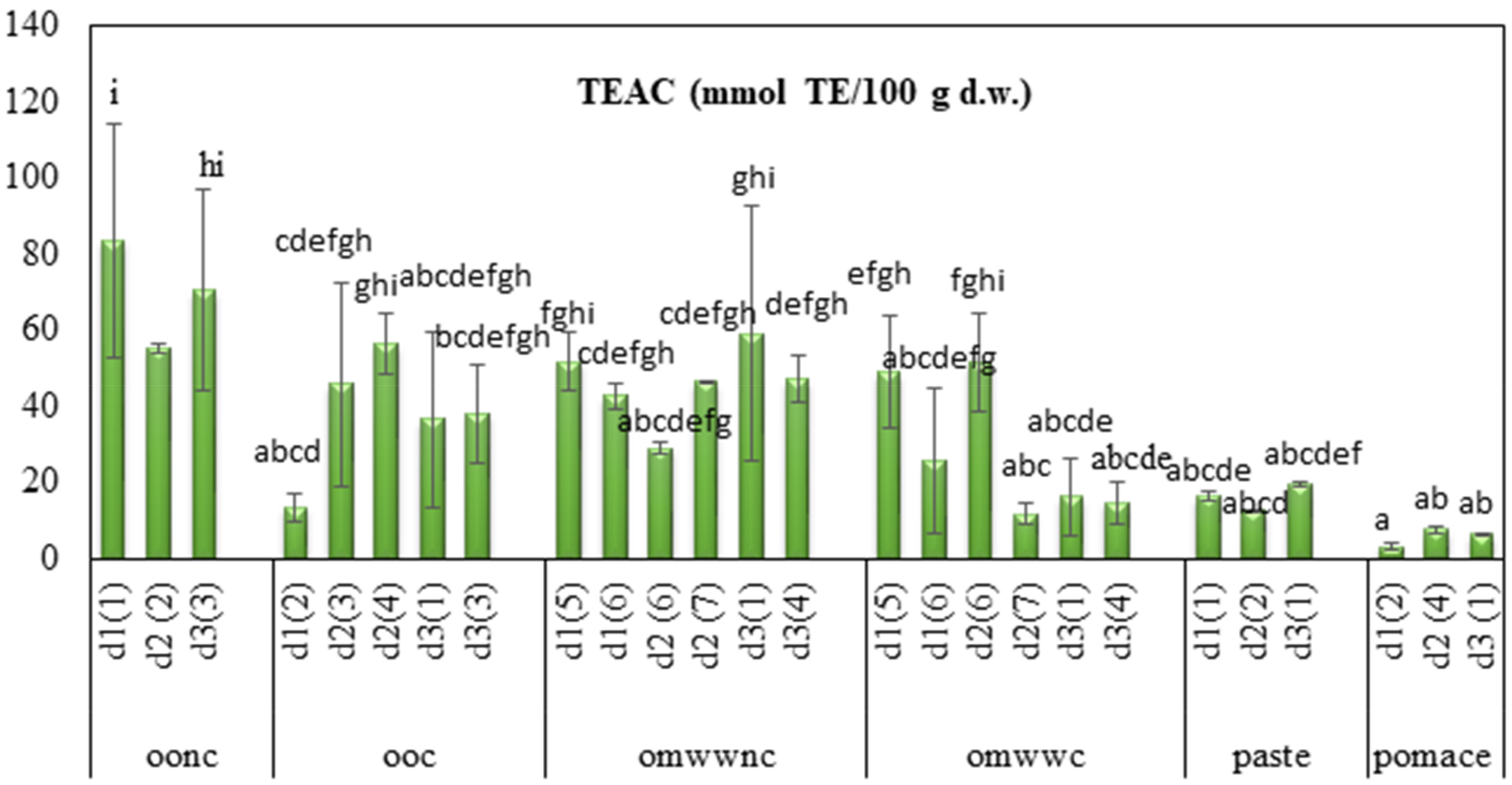
3.3. Evaluation of the Antioxidant Activity: TEAC Assay
3.4. Phenolic Compound Analysis in Olive Paste, Olive Oil and by-Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De La Rosa, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. From olive fruits to olive Oil: Phenolic compound transfer in six different olive cultivars grown under the same agronomical conditions. Int. J. Mol. Sci. 2016, 17, 337. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological Activities of Phenolic Compounds of Extra Virgin Olive Oil. Antioxidants 2014, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Issaoui, M.; Dabbou, S.; Brahmi, F.; Hassine, K.B.; Ellouze, M.H.; Hammami, M. Effect of extraction systems and cultivar on the quality of virgin olive oils. Int. J. Food Sci. Technol. 2009, 44, 1713–1720. [Google Scholar] [CrossRef]
- Chandra, M.; Sathiavelu, S. Waste management in the olive oil industry in the Mediterranean region by composting. Clean Technol. Environ. Policy 2009, 293–298. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Russo, M.; Bonaccorsi, I.L.; Cacciola, F.; Dugo, L.; De Gara, L.; Dugo, P.; Mondello, L. Distribution of bioactives in entire mill chain from the drupe to the oil and wastes. Nat. Prod. Res. 2020, 0, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Roig, A.; Cayuela, M.L.; Sa, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Azaizeh, H.; Halahlih, F.; Najami, N.; Brunner, D.; Faulstich, M.; Tafesh, A. Antioxidant activity of phenolic fractions in olive mill wastewater. Food Chem. 2012, 134, 2226–2234. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Fidalgo, A.; Ilharco, L.M.; Pagliaro, M. Extraction, benefits and valorization of olive polyphenols. Eur. J. Lipid Sci. Technol. 2016, 118, 503–511. [Google Scholar] [CrossRef]
- Frankel, E.; Bakhouche, A.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Literature review on production process to obtain extra virgin olive oil enriched in bioactive compounds. Potential use of byproducts as alternative sources of polyphenols. J. Agric. Food Chem. 2013, 61, 5179–5188. [Google Scholar] [CrossRef]
- Ventura, G.; Calvano, C.D.; Abbattista, R.; Bianco, M.; De Ceglie, C.; Losito, I.; Palmisano, F.; Cataldi, T.R.I. Characterization of bioactive and nutraceutical compounds occurring in olive oil processing wastes. Rapid Commun. Mass Spectrom. 2019, 33, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- El-Abbassi, A.; Kiai, H.; Hafidi, A. Phenolic profile and antioxidant activities of olive mill wastewater. Food Chem. 2012, 132, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Achmon, Y.; Fishman, A. The antioxidant hydroxytyrosol: Biotechnological production challenges and opportunities. Appl. Microbiol. Biotechnol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Santos, S.A.O.; Guerra, Â.R.; Guerreiro, O.; Felício, L.; Jerónimo, E.; Silvestre, A.J.D.; Neto, C.P.; Duarte, M. Valorization of olive mill residues: Antioxidant and breast cancer antiproliferative activities of hydroxytyrosol-rich extracts derived from olive oil by-products. Ind. Crops Prod. 2013, 46, 359–368. [Google Scholar] [CrossRef]
- Nunzio, M.D.; Picone, G.; Pasini, F.; Caboni, F.; Gianotti, A.; Bordoni, A.; Capozzi, F. Olive oil industry by-products. Effects of a polyphenol-RICH extract on the metabolome and response to inflammation in cultured intestinal cell. Food Res. Int. 2018. [Google Scholar] [CrossRef]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and fractionation of phenolic compounds extracted from olive oil mill wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- Abaza, B.L.; Youssef, N.B.; Manai, H.; Haddada, F.M. Chétoui olive leaf extracts: Influence of the solvent type on phenolics and antioxidant activities. Grasas Aceites 2011, 62, 96–104. [Google Scholar] [CrossRef]
- Norme Commerciale Applicable Aux Huiles D’Olive Et Aux Huiles De Grignons D’Olive. Cons. Oleic. Int. 2015, COI/T.15, 1–18.
- De Príncipe, V.; Nunzio, M.D.; Toselli, M.; Verardo, V.; Caboni, F.; Bordoni, A. Counteraction of oxidative damage by pomegranate juice: Influence of the cultivar. J. Sci. Food Agric. 2013. [Google Scholar] [CrossRef]
- Tomás-Menor, L.; Barrajón-Catalán, E.; Segura-Carretero, A.; Martí, N.; Saura, D.; Menéndez, J.A.; Joven, J.; Micol, V. The promiscuous and synergic molecular interaction of polyphenols in bactericidal activity: An opportunity to improve the performance of antibiotics? Phyther. Res. 2015, 29, 466–473. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De la Rosa, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of phenolic compounds of “Sikitita” olive leaves by HPLC-DAD-TOF-MS. Comparison with its parents “Arbequina” and “Picual” olive leaves. LWT—Food Sci. Technol. 2014, 58, 28–34. [Google Scholar] [CrossRef]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Saura, D.; Guillén, E.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food Chem. Toxicol. 2010, 48, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, P.; Termentzi, A.; Michel, T.; Gikas, E.; Halabalaki, M.; Skaltsounis, A.L. From olive drupes to olive Oil An HPLC-orbitrap-based qualitative and quantitative exploration of olive key metabolites. Planta Med. 2013, 79, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Ammar, S.; Contreras, M.d.M.; Gargouri, B.; Segura-Carretero, A.; Bouaziz, M. RP-HPLC-DAD-ESI-QTOF-MS based metabolic profiling of the potential Olea europaea by-product “wood” and its comparison with leaf counterpart. Phytochem. Anal. 2017, 28, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, W.; Liu, Q.; Xu, J.; Bao, B.; Shan, M.; Cao, Y.; Cheng, F.; Ding, A.; Zhang, L. Application of UHPLC-ESI-Q-TOF-MS to identify multiple constituents in processed products of the herbal medicine ligustri lucidi fructus. Molecules 2017, 22, 689. [Google Scholar] [CrossRef]
- Shaoping, F.; Segura Carretero, A.; Arraez Roman, D. Tentative Characterization of Novel Phenolic Compounds in Extra Virgin Olive Oils by Rapid-Resolution Liquid Chromatography Coupled with Mass Spectrometry. J. Agric. Food Chem. 2009, 11140–11147. [Google Scholar] [CrossRef]
- Kirmizibekmez, H.; Montoro, P.; Piacente, S.; Pizza, C.; Dönmez, A.; Çaliş, I. Identification by HPLC-PAD-MS and quantification by HPLC-PAD of phenylethanoid glycosides of five Phlomis species. Phytochem. Anal. 2005, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; De Simón, B.F.; Cadahía, E.; Esteruelas, E.; Muñoz, A.M.; Hernández, T.; Estrella, I.; Pinto, E. LC-DAD/ESI-MS/MS study of phenolic compounds in ash (Fraxinus excelsior L. and F. americana L.) heartwood. Effect of toasting intensity at cooperage. J. Mass Spectrom. 2012, 47, 905–918. [Google Scholar] [CrossRef]
- Cardinali, A.; Pati, S.; Minervini, F.; D’Antuono, I.; Linsalata, V.; Lattanzio, V. Verbascoside, isoverbascoside, and their derivatives recovered from olive mill wastewater as possible food antioxidants. J. Agric. Food Chem. 2012, 60, 1822–1829. [Google Scholar] [CrossRef]
- Altieri, G.; Di Renzo, G.C.; Genovese, F.; Tauriello, A.; D’Auria, M.; Racioppi, R.; Viggiani, L. Olive oil quality improvement using a natural sedimentation plant at industrial scale. Biosyst. Eng. 2014, 122, 99–114. [Google Scholar] [CrossRef]
- Alowaiesh, B.; Singh, Z.; Fang, Z.; Kailis, S.G. Harvest time impacts the fatty acid compositions, phenolic compounds and sensory attributes of Frantoio and Manzanilla olive oil. Sci. Hortic. 2018, 234, 74–80. [Google Scholar] [CrossRef]
- Barbera, A.C.; Maucieri, C.; Cavallaro, V.; Ioppolo, A.; Spagna, G. Effects of spreading olive mill wastewater on soil properties and crops, a review. Agric. Water Manag. 2013, 119, 43–53. [Google Scholar] [CrossRef]
- Sellami, F.; Jarboui, R.; Hachicha, S.; Medhioub, K.; Ammar, E. Co-composting of oil exhausted olive-cake, poultry manure and industrial residues of agro-food activity for soil amendment. Bioresour. Technol. 2008, 99, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Jerman Klen, T.; Mozetič Vodopivec, B. The fate of olive fruit phenols during commercial olive oil processing: Traditional press versus continuous two- and three-phase centrifuge. LWT Food Sci. Technol. 2012, 49, 267–274. [Google Scholar] [CrossRef]
- Artajo, L.S.; Romero, M.P.; Suárez, M.; Motilva, M.J. Partition of phenolic compounds during the virgin olive oil industrial extraction process. Eur. Food Res. Technol. 2007, 225, 617–625. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Carrasco-Pancorbo, A.; Simal-Gándara, J.; Giampieri, F.; et al. Characterization of Phenolic Extracts from BRAVA Extra Virgin Olive Oils and Their Cytotoxic Effects on MCF-7 Breast Cancer Cells. Food Chem. Toxicol. 2018, 119, 73–85. [Google Scholar] [CrossRef]
- Barrajón-Catalán, E.; Taamalli, A.; Quirantes-Piné, R.; Roldan-Segura, C.; Arráez-Román, D.; Segura-Carretero, A.; Micol, V.; Zarrouk, M. Differential metabolomic analysis of the potential antiproliferative mechanism of olive leaf extract on the JIMT-1 breast cancer cell line. J. Pharm. Biomed. Anal. 2015, 105, 156–162. [Google Scholar] [CrossRef]
- Bouallagui, Z.; Han, J.; Isoda, H.; Sayadi, S. Hydroxytyrosol rich extract from olive leaves modulates cell cycle progression in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2011, 49, 179–184. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Pérez-Sánchez, A.; Herrero, M.; Ibañez, E.; Micol, V.; Zarrouk, M.; Segura-Carretero, A.; et al. Use of advanced techniques for the extraction of phenolic compounds from Tunisian olive leaves: Phenolic composition and cytotoxicity against human breast cancer cells. Food Chem. Toxicol. 2012, 50, 1817–1825. [Google Scholar] [CrossRef]
- Elamin, M.H.; Daghestani, M.H.; Omer, S.A.; Elobeid, M.A.; Virk, P.; Al-Olayan, E.M.; Hassan, Z.K.; Mohammed, O.B.; Aboussekhra, A. Olive oil oleuropein has anti-breast cancer properties with higher efficiency on ER-negative cells. Food Chem. Toxicol. 2013, 53, 310–316. [Google Scholar] [CrossRef] [PubMed]
| 22-11-2017 | |||||
| Sample | FFAs | PV | K232 | K270 | |
| before vertical centrifugation | 1 | 0.4 ± 0.8 a,b | 6 ± 0.8 a, | 1.90 ± 0.02 a | 0.1 ± 0.1 a,b |
| 2 | 0.4 ± 0.1 a,b | 9.70 ± 1.15 a,b,c,d | 1.9 ± 0.4 a | 0.07 ± 0.04 a | |
| 3 | 0.5 ± 0.1 a,b | 16.0 ± 1.5 e | 2.10 ± 0.05 a | 0.10 ± 0.01 a,b | |
| 4 | 0.40 ± 0.02 a,b | 13 ±4.6 d | 2.17 ± 0.10 a | 0.14 ± 0.02 c,d | |
| 5 | 0.4 ± 0.1 a,b | 9 ±0.6 a,b,c | 2.3 ± 0.3 a,b | 0.17 ± 0.04 b,c,d | |
| 6 | 0.40 ± 0.01 a,b | 13.0 ± 1.5 d | 2.13 ± 0.22 a | 0.15 ± 0.03 d | |
| after vertical centrifugation | 1 | 0.43 ± 0.06 a,b | 10 ± 1 b,c,d | 2.12 ± 0.1 a | 0.12 ± 0.02 a,b,c |
| 2 | 0.30 ± 0.01 a | 6.7 ± 2.3 a,b | 1.87 ± 0.03 a | 0.06 ± 0.01 a | |
| 3 | 0.40 ± 0.01 a,b | 10 ± 1 c,d | 2.1 ± 0.2 a | 0.07 ± 0.01 a | |
| 4 | 0.3 ± 0.1 a | 9.00 ± 0.01 a,b,c | 2.6 ± 0.5 a | 0.18 ± 0.10 b,c,d | |
| 5 | 0.40 ± 0.15 a,b | 10 ± 2 cd | 2.3 ± 0.1 a,b | 0.14 ± 0.02 c,d | |
| 6 | 0.3 ± 0.1 a,b | 13.0 ± 2.1 d | 2.6 ± 0.2 b | 0.20 ± 0.04 b,c,d | |
| 19-12-2018 | |||||
| Sample | FFAs | PV | K232 | K270 | |
| before vertical centrifugation | 1 | 0.5 ± 0.0 a,b,c | 7.0 ± 1.2 a,b | 1.9 ± 0.1 a,b | 0.18 ± 0.02 b |
| 2 | 1.2 ± 0.1 e | 9 ± 2 b,c,d | 2.47 ± 0.05 d | 0.3 ± 0.1 c | |
| 3 | 0.4 ± 0.0 a | 5.7 ± 1.5 a | 1.92 ± 0.02 a,b | 0.18 ± 0.01 b | |
| 4 | 0.4 ± 0.1 a,b | 9.0 ± 1.2 b,c | 1.85 ± 0.1 a,b | 0.18 ± 0.01 b | |
| 5 | 0.5 ± 0.1 a,b,c | 18.0 ± 1.5 g | 1.82 ± 0.04 a,b | 0.180 ± 0.004 b | |
| 6 | 0.4 ± 0.1 a,b | 7.0 ± 1.2 a,b | 1.71 ± 0.03 a | 0.19 ± 0.01 b,c | |
| 7 | 0.5 ± 0.2 a,b | 8.0 ± 0.6 a,b | 2.07 ± 0.05 c | 0.25 ± 0.05 c | |
| after vertical centrifugation | 1 | 0.6 ± 0.2 b,c | 12 ± 0.6 e,f | 1.9 ± 0.1 a,b | 0.15 ± 0.01 a,b |
| 2 | 0.9 ± 0.1 d | 13.7 ± 1.5 f | 2.3 ± 0.1 c,d | 0.19 ± 0.01 b,c | |
| 3 | 0.5 ± 0.1 ab | 9 ± 1 b,c,d | 1.99 ± 0.05 a,b | 0.19 ± 0.01 b | |
| 4 | 0.7 ± 0.1 c | 12 ± 0.6 e,f | 1.9 ± 0.1 a,b | 0.11 ± 0.01 a | |
| 5 | 0.6 ± 0.1 a,b,c | 10 ± 0.6 c,d,e | 1.98 ± 0.03 a,b | 0.14 ± 0.03 a,b | |
| 6 | 0.6 ± 0.1 a,b,c | 11 ± 1 d,e | 1.88 ± 0.01 a,b | 0.14 ± 0.02 a,b | |
| 7 | 0.5 ± 0.1 a,b,c | 8 ± 1 b,c | 1.9 ± 0.2 a,b | 0.14 ± 0.03 a,b | |
| 19-01-2018 | |||||
| Sample | FFAs | PV | K232 | K270 | |
| before vertical centrifugation | 1 | 0.4 ± 0.1 a,b | 6.0 ± 1.5 a | 1.8 ± 0.1 a,b | 0.15 ± 0.02 a,b,c |
| 2 | 0.4 ± 0.1 a,b | 4.0 ± 2.3 a | 1.7 ± 0.1 a | 0.11 ± 0.03 a | |
| 3 | 0.4 ± 0.1 a,b | 11 ± 2 b | 1.9 ± 0.1 bc | 0.16 ± 0.01 b,c | |
| 4 | 0.5 ± 0.1 a | 6.0 ± 1.5 a | 1.7 ± 0.2 a | 0.14 ± 0.03 a,b | |
| after vertical centrifugation | 1 | 0.4 ± 0.1 a,b | 6.0 ± 1.5 a | 1.7 ± 0.1 a,b | 0.14 ± 0.01 a,b |
| 2 | 0.4 ± 0.1 a | 4 ± 2.3 a | 1.91 ± 0.13 b,c | 0.16 ± 0.03 b,c | |
| 3 | 0.4 ± 0 a | 12.0 ± 2.1 b | 1.9 ± 0.1 b,c | 0.18 ± 0.01 c | |
| 4 | 0.43 ± 0.06 a,b | 6.0 ± 1.5 a | 2.02 ± 0.04 a | 0.17 ± 0.01 b,c | |
| Sample | C16:0 | C16:1 | C17:0 | C17:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C20:1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| d1 | 1 | 12.87 | 0.59 | 0.13 | 0.21 | 3.00 | 64.6 | 16.31 | - | 0.43 | 0.40 |
| 2 | 12.11 | 0.52 | 0.19 | 0.35 | 2.81 | 65.62 | 15.17 | 0.75 | 0.38 | 0.42 | |
| 3 | 13.70 | 0.56 | 0.29 | 0.40 | 2.98 | 64.54 | 14.62 | 0.51 | 0.45 | 0.36 | |
| 4 | 12.71 | 0.52 | 0.11 | 0.13 | 3.00 | 64.32 | 16.97 | - | 0.45 | 0.36 | |
| 5 | 12.92 | - | 0.16 | - | 2.96 | 64.80 | 17.44 | 0.61 | 0.45 | 0.47 | |
| 6 | 12.05 | 0.39 | 0.47 | 0.21 | 2.92 | 62.88 | 19.17 | - | 0.46 | - | |
| d2 | 1 | 13.07 | 0.51 | - | - | 3.12 | 64.94 | 16.48 | 0.49 | 0.41 | 0.45 |
| 2 | 12.00 | 0.36 | - | - | 3.36 | 63.30 | 19.61 | 0.53 | 0.45 | 0.39 | |
| 3 | 10.69 | 0.27 | 0.16 | - | 3.77 | 65.13 | 18.30 | 0.52 | 0.45 | 0.36 | |
| 4 | 11.31 | 0.29 | - | - | 4.01 | 64.67 | 17.92 | 0.63 | 0.48 | 0.46 | |
| 5 | 16.44 | 1.28 | - | - | 3.16 | 63.16 | 12.67 | 1.00 | 0.49 | 0.40 | |
| 6 | 12.58 | 1.04 | 0.10 | - | 2.77 | 69.33 | 12.80 | 0.43 | 0.44 | 0.37 | |
| 7 | 16.47 | 0.87 | - | - | 3.18 | 62.45 | 13.86 | 0.41 | 0.46 | 0.46 | |
| d3 | 1 | 11.03 | 0.46 | 0.14 | 3.40 | 67.78 | 15.73 | 0.46 | 0.44 | 0.32 | |
| 2 | 11.69 | 0.24 | - | - | 3.44 | 65.20 | 16.67 | 0.47 | 0.46 | 0.42 | |
| 3 | 9.73 | 0.35 | 0.17 | - | 3.57 | 67.25 | 17.36 | 0.48 | 0.57 | 0.37 | |
| 4 | 11.74 | 0.15 | 0.44 | 0.05 | 3.11 | 65.88 | 16.99 | 0.53 | 0.57 | 0.38 | |
| EVOO (IOC [18]) | 7.5–20 | 3–3.5 | - | - | 0.5–5 | 55–83 | 3.5–21 | ≤1 | ≤0.6 | ≤0.5 |
| Samples | Paste | Oonc | Ooc | Pomace | Omww nc EA | Omwwc EA | Omwwnc MeOH | Omwwc MeOH | |
|---|---|---|---|---|---|---|---|---|---|
| d1 | 1 | 4.9 ± 0.4 a | 45.5 ± 1.4 b | 36.8 ± 1.7 b | 2.2 ± 1.5 a | 13 ± 4 a | 13.6 ± 4.4 a | 2.7 ± 0.4 a | 8.7 ± 2.9 a |
| 2 | 1.2 ± 1.2 a | 35.6 ± 1.8 b,c | 44.5 ± 3.7 c | 2.7 ± 2.0 a | 11.3 ± 3.4 a,b | 9.7 ± 2.6 a,b | 3.2 ± 0.8 a | 3.9 ± 1.7 a | |
| 3 | 2.5 ± 0.4 a | 44.8 ± 2.5 b | 27.6 ± 1.3 b | 2.1 ± 0.8 a | 8.1 ± 1.7 a | 6.95 ± 1.4 a | 3.7 ± 0.6 a | 3.6 ± 1.1 a | |
| 4 | 3.9 ± 0.8 a | 36.9 ± 1.6 b | 35.4 ± 1.7 b | 1.6 ± 0.3 a | 13.4 ± 2.3 a | 11.6 ± 2.3 a | 2.7 ± 0.4 a | 4.4 ± 1.4 a | |
| 5 | 3.5 ± 0.6 a | 37 ± 2 b | 35.5 ± 1.7 b | 1.3 ± 0.2 a | 24.6 ± 6.3 a | 19.4 ± 4.5 a | 6.0 ± 3.1 a | 5.5 ± 1.1 a | |
| 6 | 2.3 ± 0.2 a | 43 ± 2 b | 35.7 ± 1.3 b | 0.7 ± 0.1 a | 20.7 ± 3.5 b,c | 16.7 ± 2.8 a,b | 5.2 ± 1.6 a,b | 5 ± 1.4 a,b | |
| d2 | 1 | 2.8 ± 0.4 a | 37.7 ± 2.0 b | 30.6 ± 4.2 b | - | 10.1 ± 5.1 a | 9.2 ± 2.9 a | 2.9 ± 0.2 a | 4.7 ± 0.8 a |
| 2 | 3.5 ± 0.3 a | 39.4 ± 1.3 b,c | 49.8 ± 4.5 b | - | 29.3 ± 2.9 a,b,c | 22.5 ± 2.1 a,b,c | 7.5 ± 1.8 a,b | 4.8 ± 0.3 a | |
| 3 | 2.1 ± 1.1 a | 38.7 ± 1.1 b | 69.4 ± 2.8 c | - | - | 16.8 ± 2.2 a,b | 5.3 ± 1.8 a | - | |
| 4 | 2.4 ± 0.8 a | 31.5 ± 1.1 b | 68.10 ± 4.05 c | 2 ± 1.1 a | 12.7 ± 2.6 a,b | 10 ± 1.2 a,b | 7.1 ± 2.3 a,b | 4.4 ± 1.4 a,b | |
| 5 | 2.2 ± 0.9 a | 34.21 ± 1.6 b | 61.8 ± 3.3 c | 1.5 ± 0.2 a | 11.5 ± 1.4 a | 10.4 ± 2.3 a | 3.8 ± 1.4 a | 5.44 ± 0.4 a | |
| 6 | 1.02 ± 1.03 a | 34.3 ± 9.1 b | 79.09 ± 4.6 c | 0.06 ± 0.64 a | 15.1 ± 5.2 a,b | 12.1 ± 3.7 a,b | 7.7 ± 2.4 a,b | 5.3 ± 0.9 a,b | |
| 7 | - | 31.1 ± 9.8 b,c | 51.46 ± 2.8 c | 0.3 ± 1.5 a | 16.4 ± 6.3 a,b | - | 7.3 ± 2.7 a,b | - | |
| d3 | 1 | 6.6 ± 0.5 a | 36 ± 1.6 b | 49.3 ± 3.1 b | 1.1 ± 0.1 a | 10.4 ± 2.9 a | 7.8 ± 1.4 a | 7.0 ± 1.4 a | 3.6 ± 0.6 a |
| 2 | 2.6 ± 0.3 a | 47.0 ± 2.1 b | 45.1 ± 3.2 b | 0.8 ± 0.1 a | 4.9 ± 1.6 a | 5.2 ± 1.1 a | 2.1 ± 1.1 a | 4.2 ± 0.5 a | |
| 3 | 6 ± 1 a | 76.2 ± 3.8 c | 48.4 ± 1.9.b | 0.7 ± 0.1 a | 10.5 ± 0.9 a | 10 ± 1 a | 5.6 ± 0.8 a | 7.3 ± 1.4 a | |
| 4 | 2.2 ± 0.4 a | 39.4 ± 1.3 b | 43.6 ± 2.8 b | 0.4 ± 0.1 a | 14.6 ± 1.7 a | 12.1 ± 1.2 a | 5.6 ± 2.5 a | 5.1 ± 0.8 a |
| Proposed Compound | M-H]- | MS/MS Fragments | Sample and Percentage | References |
|---|---|---|---|---|
| Galloyl-HHDP-hexoside | 663 | - | 3 (5.27%) | [23] |
| Hydroxytyrosol hexoside dimer | 631 | 153; 315 | 2 (15.00%), 3 (11.09%), 6 (17.48%), 7 (13.85%) | [24] |
| Stachyose | 665 | 4 (2.64%), 7 (13.85%) | [25] | |
| Secoirioid derivative | 815 | 407; 375; 313 | 2 (63.66%), 3 (39.98%), 5 (50.49%), 6 (35.20%), 7 (37.85%) | [26] |
| Acyclodihydroelenolic acid hexoside | 407 | 389; 165 | 4 (12.75), 7 (34.91%) | [25] |
| Isonuezhenide | 685 | 523; 453; 421; 299; 223 | 3 (5.24%), 4 (3.12%) | [27] |
| Caffeoyl-6-oleoside | 551 | - | 2 (6.61%), 3 (6.75%), 6 (6.56%) | [24] |
| p-Coumaroyl-6-oleoside | 535 | 491 | 2 (6.70%), 3 (14.91%) | [24] |
| (+)−1-Hydroxypinoresinol 1-O-β-D-glucopyranoside | 535 | - | 6 (9.29%) | [25] |
| Elenolic acid dialdehyde linked to hydroxytyrosol | 319 | 195 | 1 (10.15%) | [28] |
| G13 | 1071 | 909; 837; 771; 685; 523; 385 | 2 (8.03%), 3 (7.99%), 4 (33.87%), 5 (22.95%) | [27] |
| oleuropein aglycone | 377 | 275; 149 | 1 (54.61%), 8 (33.60%), 9 (17.76%), 10 (59.86%), 11 (37.99%) | [25] |
| Forsythoside B | 755 | 447 | 8 (12.30%) | [29] |
| Leucosceptoside B | 781 | - | 6 (9.63%) | [29] |
| Secoisolariciresinol | 361 | - | 11 (4.53%) | [30] |
| Oleuropein hexoside | 701 | 539 | 1 (6.06%) | [27] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jebabli, H.; Nsir, H.; Taamalli, A.; Abu-Reidah, I.; Álvarez-Martínez, F.J.; Losada-Echeberria, M.; Barrajón Catalán, E.; Mhamdi, R. Industrial-Scale Study of the Chemical Composition of Olive Oil Process-Derived Matrices. Processes 2020, 8, 701. https://doi.org/10.3390/pr8060701
Jebabli H, Nsir H, Taamalli A, Abu-Reidah I, Álvarez-Martínez FJ, Losada-Echeberria M, Barrajón Catalán E, Mhamdi R. Industrial-Scale Study of the Chemical Composition of Olive Oil Process-Derived Matrices. Processes. 2020; 8(6):701. https://doi.org/10.3390/pr8060701
Chicago/Turabian StyleJebabli, Haifa, Houda Nsir, Amani Taamalli, Ibrahim Abu-Reidah, Francisco Javier Álvarez-Martínez, Maria Losada-Echeberria, Enrique Barrajón Catalán, and Ridha Mhamdi. 2020. "Industrial-Scale Study of the Chemical Composition of Olive Oil Process-Derived Matrices" Processes 8, no. 6: 701. https://doi.org/10.3390/pr8060701
APA StyleJebabli, H., Nsir, H., Taamalli, A., Abu-Reidah, I., Álvarez-Martínez, F. J., Losada-Echeberria, M., Barrajón Catalán, E., & Mhamdi, R. (2020). Industrial-Scale Study of the Chemical Composition of Olive Oil Process-Derived Matrices. Processes, 8(6), 701. https://doi.org/10.3390/pr8060701








