Evaluation of a Novel Polymeric Flocculant for Enhanced Water Recovery of Mature Fine Tailings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(PLA4ChMA)
2.3. Flocculation of 5.0 wt.% MFT
2.4. Flocculation of Undiluted MFT
3. Results and Discussion
3.1. Flocculation of Dilute MFT
3.2. Flocculation-Filtration of Undiluted MFT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Masliyah, J.H.; Czarnecki, J.; Xu, Z. Basic Scientific Background. In Handbook on Theory and Practice of Bitumen Recovery from Athabasca Oil Sands, Volume I: Theoretical Basis; Kingsley Knowledge Publishing: Calgary, AB, Canada, 2011; pp. 51–125. [Google Scholar]
- Loerke, R.; Tan, X.; Liu, Q. Dewatering of Oil Sands Mature Fine Tailings by Dual Polymer Flocculation and Pressure Plate Filtration. Energy Fuels 2017, 31, 6986–6995. [Google Scholar] [CrossRef]
- Vedoy, D.R.; Soares, J.B. Water-soluble Polymers for Oil Sands Tailing Treatment: A Review. Can. J. Chem. Eng. 2015, 93, 888–904. [Google Scholar] [CrossRef]
- Alberta Energy Regulator. Fluid Tailings Management for Oil Sands Mining Projects. Directive 085; Alberta Energy Regulator: Calgary, AB, Canada, 2017. [Google Scholar]
- Sedgwick, A.; Kaminksy, H.; Bouffard, J.F. Eureka! I Have the Solution to Oil Sand Tailings-Now What? IOSTC: Edmonton, AB, Canada, 2018. [Google Scholar]
- Botha, L.; Soares, J.B. The Influence of Tailings Composition on Flocculation. Can. J. Chem. Eng. 2015, 93, 1514–1523. [Google Scholar] [CrossRef]
- Kaminsky, H.; Li, Y.; Qureshi, T.; Sedgwick, A.; Moran, K. Influence of Bitumen Content on Flocculation of FFT; Titanium Corporation: Edmonton, AB, Canada, 2018. [Google Scholar]
- Masliyah, J.H.; Czarnecki, J.; Xu, Z. Colloidal Science in Tailings Management. In Handbook on Theory and Practice of Bitumen Recovery from Athabasca Oil Sands Volume I: Theoretical Basis; Kingsley Knowledge Publishing: Calgary, AB, Canada, 2011; pp. 391–448. [Google Scholar]
- Motta, F.L.; Gaikwad, R.; Botha, L.; Soares, J.B. Quantifying the effect of polyacrylamide dosage, Na+ and Ca2+ concentrations, and clay particle size on the flocculation of mature fine tailings with robust statistical methods. Chemosphere 2018, 208, 263–272. [Google Scholar] [CrossRef]
- Lu, Q.; Yan, B.; Xie, L.; Huang, J.; Liu, Y.; Zeng, H. A two-step flocculation process on oil sands tailings treatment using oppositely charged polymer flocculants. Sci. Total Environ. 2016, 565, 369–375. [Google Scholar] [CrossRef]
- Bratby, J. Treatment with Polymer in Coagulation and Flocculation in Water and Wastewater Treatment; IWA Publishing: London, UK, 2006; pp. 186–218. [Google Scholar]
- Gumfekar, S.P.; Rooney, T.R.; Hutchinson, R.A.; Soares, J.B. Dewatering Oil Sands Tailings with Degradable Polymer Flocculants. ACS Appl. Mater. Interfaces 2017, 9, 36290–36300. [Google Scholar] [CrossRef]
- Younes, G.; Proper, A.R.; Rooney, T.R.; Hutchinson, R.A.; Gumfekar, S.P.; Soares, J.B. Structure Modifications of Hydrolytically-Degradable Polymer Flocculant for Improved Water Recovery from Mature Fine Tailings. Ind. Eng. Chem. Res. 2018, 57, 10809–10822. [Google Scholar] [CrossRef]
- Lu, Q.; Yan, B.; Xie, L.; Huang, J.; Liu, Y.; Hongbo, Z. A novel flocculant of Al(OH)3–polyacrylamide ionic hybrid. J. Colloid Interface Sci. 2004, 273, 400–405. [Google Scholar]
- Botha, L.; Davey, S.; Nguyen, B.; Swarnakar, A.; Rivard, E.; Soares, J.B. Flocculation of oil sands tailings by hyperbranched functionalized polyethylenes. Miner. Eng. 2017, 108, 71–82. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Chow, R.; Adegoroye, A.; Najafi, A.S. Enhancing Treatment and Geotechnical Stability of Oil Sands Fine Tailings using Thermo-Sensitive Poly(n-Isopropyl Acrylamide). Can. J. Chem. Eng. 2015, 93, 1780–1786. [Google Scholar] [CrossRef]
- Reis, L.G.; Oliveira, R.S.; Palhares, T.N.; Spinelli, L.S.; Lucas, E.F.; Vedoy, D.R.; Asare, E.; Soares, J.B. Using acrylamide-polypropylene oxide copolymers to dewater and densify mature fine tailings. Miner. Eng. 2016, 95, 29–39. [Google Scholar] [CrossRef]
- Long, J.; Li, H.; Xu, Z.; Masliyah, J. Role of Colloidal Interactions in Oil Sand Tailings Treatment. AIChE J. 2006, 52, 371–383. [Google Scholar] [CrossRef]
- Younes, G. Hydrolytically Degradable Cationic Flocculants for Improved Water Recovery from Mature Fine Tailings. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2018. [Google Scholar]
- Gumfekar, S.P. Multifunctional Water-Soluble Polymers for Dewatering of Oil Sands Tailings. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2018. [Google Scholar]
- Gumfekar, S.P.; Soares, J.B. A novel hydrophobically-modified polyelectrolyte for enhanced dewatering of clay suspension. Chemosphere 2018, 194, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Nittala, A.; Gumfekar, S.P.; Soares, J.B. Multifunctional CO2-switchable polymers for the flocculation of oil sands tailings. J. Appl. Polym. Sci. 2019, 136, 47578. [Google Scholar] [CrossRef]
- Yu, Y.; Ferrari, R.; Lattuade, M.; Storti, G.; Morbidelli, M.; Moscatelli, D. PLA-Based Nanoparticles with Tunable Hydrophobicity and degradation kinetics. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 5191–5200. [Google Scholar] [CrossRef]
- Colombo, C.; Dragoni, L.; Gatti, S.; Pesce, R.; Rooney, T.R.; Mavroudakis, E.; Ferrari, R.; Moscatelli, D. Tunable Degradation Behavior of PEGylated Polyester-Based Nanoparticles Obtained Through Emulsion Radical Polymerization. Ind. Eng. Chem. Res. 2014, 53, 9128–9135. [Google Scholar] [CrossRef]
- Rooney, T.R.; Chovancova, A.; Lacik, I.; Hutchinson, R.A. Pulsed laser studies of cationic reactive surfactant radical propagation. Polymer 2017, 130, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Rooney, T.R.; Moscatelli, D.; Hutchinson, R.A. Polylactic acid macromonomer radical propagation kinetics and degradation behaviour. React. Chem. Eng. 2017, 2, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Russell, D.; Meunier, L.; Hutchinson, R.A. Characterization of degradation products from a hydrolytically degradable cationic flocculant. Polym. Degrad. Stabil. 2020, 174, 109097. [Google Scholar] [CrossRef]
- MacKinnon, M.; Matthews, J.; Shaw, W.; Cuddy, R. Water Quality Issues Associated with Composite Tailings (CT) Technology for Managing Oil Sands Tailings. Int. J. Min. Reclam. Environ. 2001, 15, 235–256. [Google Scholar] [CrossRef]
- Xu, Y.; Dabros, T.; Kan, J. Filterability of oil sands tailings. Process Saf. Environ. 2008, 86, 268–276. [Google Scholar] [CrossRef]
- Gumfekar, S.P.; Vajihinejad, V.; Soares, J.B. Advanced Polymer Flocculants for Solid–Liquid Separation in Oil Sands Tailings. Macromol. Rapid Commun. 2019, 40, 1800644. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tan, X.; Liu, Q. Dual Polymer Flocculants for Mature Fine Tailings Dewatering. Can. J. Chem. Eng. 2017, 95, 3–10. [Google Scholar] [CrossRef]
- Alamgir, A.; Masliyah, J.; Xu, Z. Al-PAM Assisted Filtration of Mature Fine Tailing; IOSTC: Edmonton, AB, Canada, 2010. [Google Scholar]
- Pennetta de Oliveira, L.; Gumfekar, S.P.; Motta, F.; Soares, J.B. Dewatering of Oil Sands Tailings with Novel Chitosan-Based Flocculants. Energy Fuel 2018, 32, 5271–5278. [Google Scholar] [CrossRef]
- Li, Y.; Kaminsky, H.; Romaniuk, N.; Tate, M. Filter Press Modification to Assess Dewatering Performance of Fluid Fine Tailings; IOSTC: Edmonton, AB, Canada, 2018. [Google Scholar]
- Ezenwajiaku, I.H.; Chovancová, A.; Lister, K.C.; Lacík, I.; Hutchinson, R.A. Experimental and Modeling Investigation of Radical Homopolymerization of 2-(Methacryloyloxyethyl) Trimethylammonium Chloride in Aqueous Solution. Macromol. React. Eng. 2020, 14, 1900033. [Google Scholar] [CrossRef]
- Lister, K.C. Evaluation of a Novel Polymeric Flocculant for Enhanced Water Recovery of Mature Fine Tailings. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2020. [Google Scholar]
- Sadighian, A.; Revington, A.; Kaminsky, H.; Moyls, B.; Li, Y.; Omotoso, O. A New Protocol to Assess the Quality of Tailings Flocculation/Coagulation: A Collaboration to Improve Tailings Treatment at Suncor Energy; IOSTC: Edmonton, AB, Canada, 2018. [Google Scholar]
- Boxill, L. The Impact of Fabric and Surface Characteristics on the Engineering Behaviour of Polymer-Amended Mature Fine Tailings. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2016. [Google Scholar]
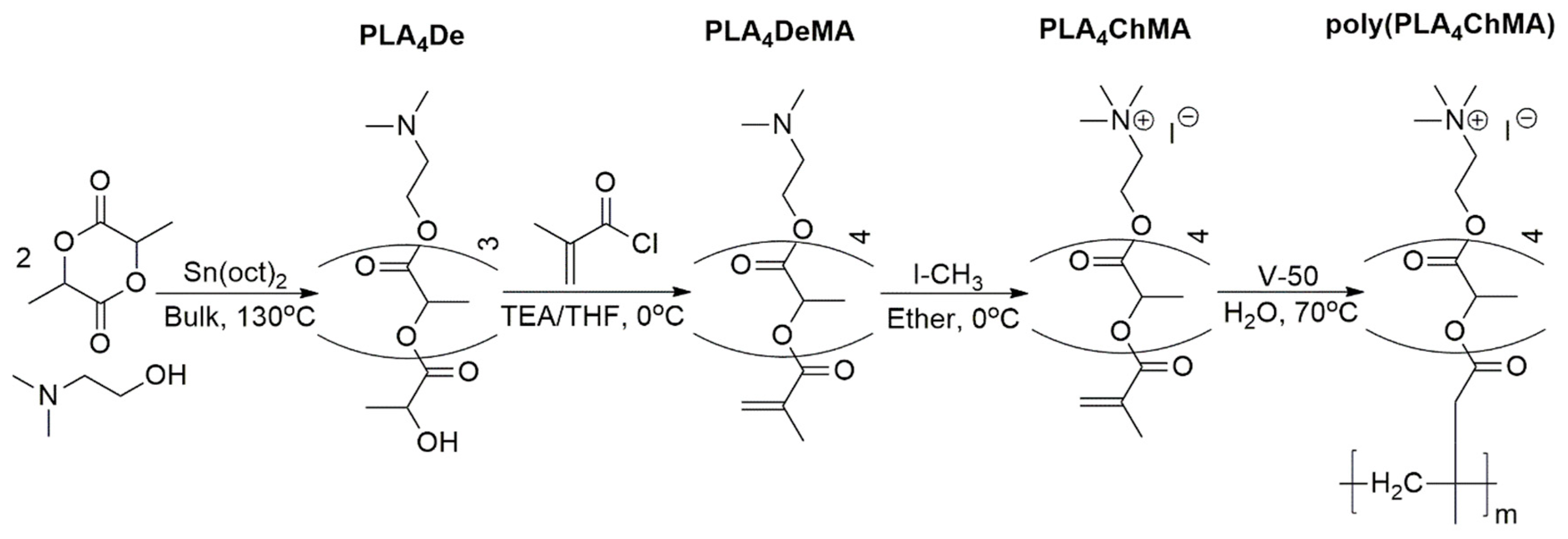




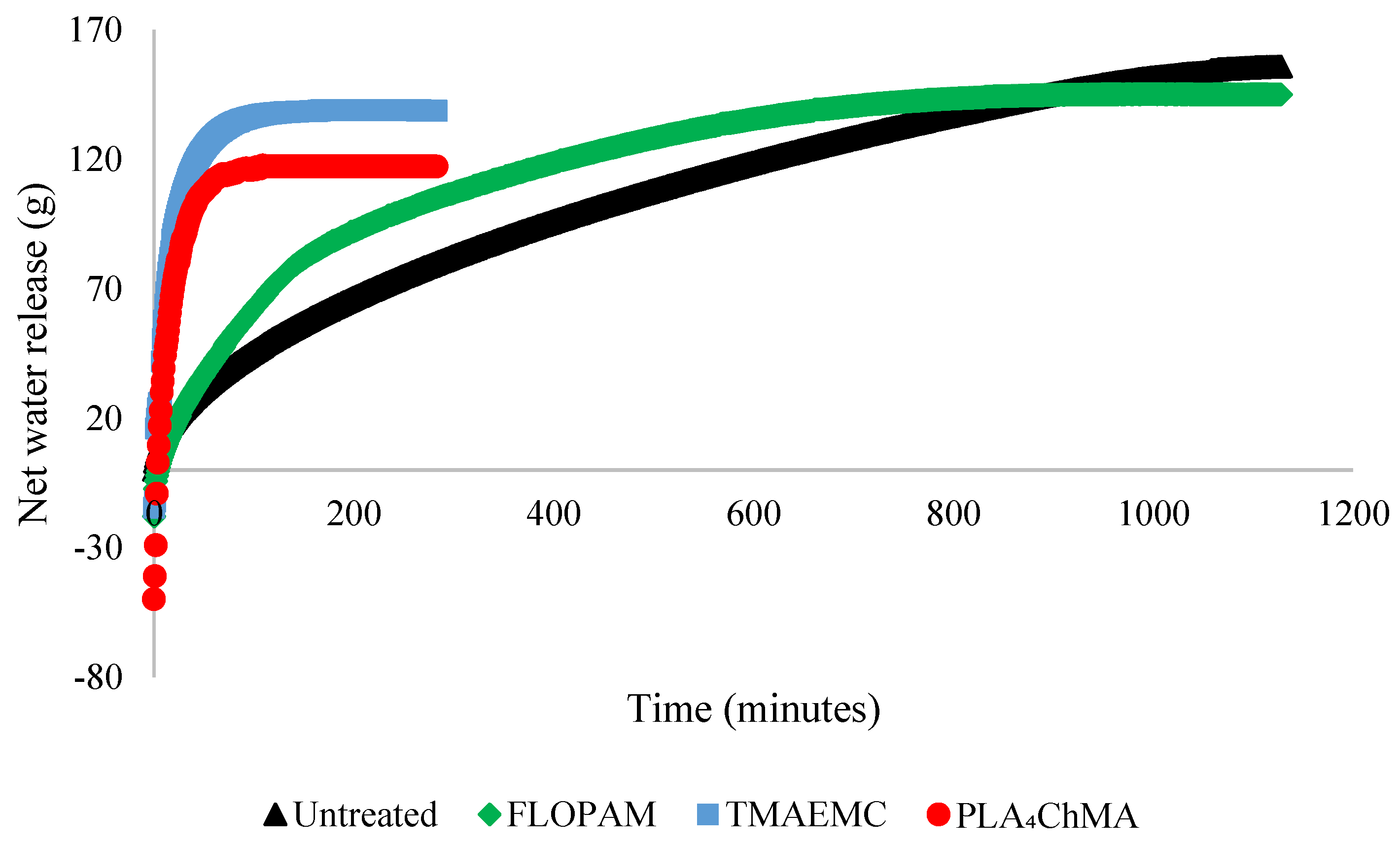
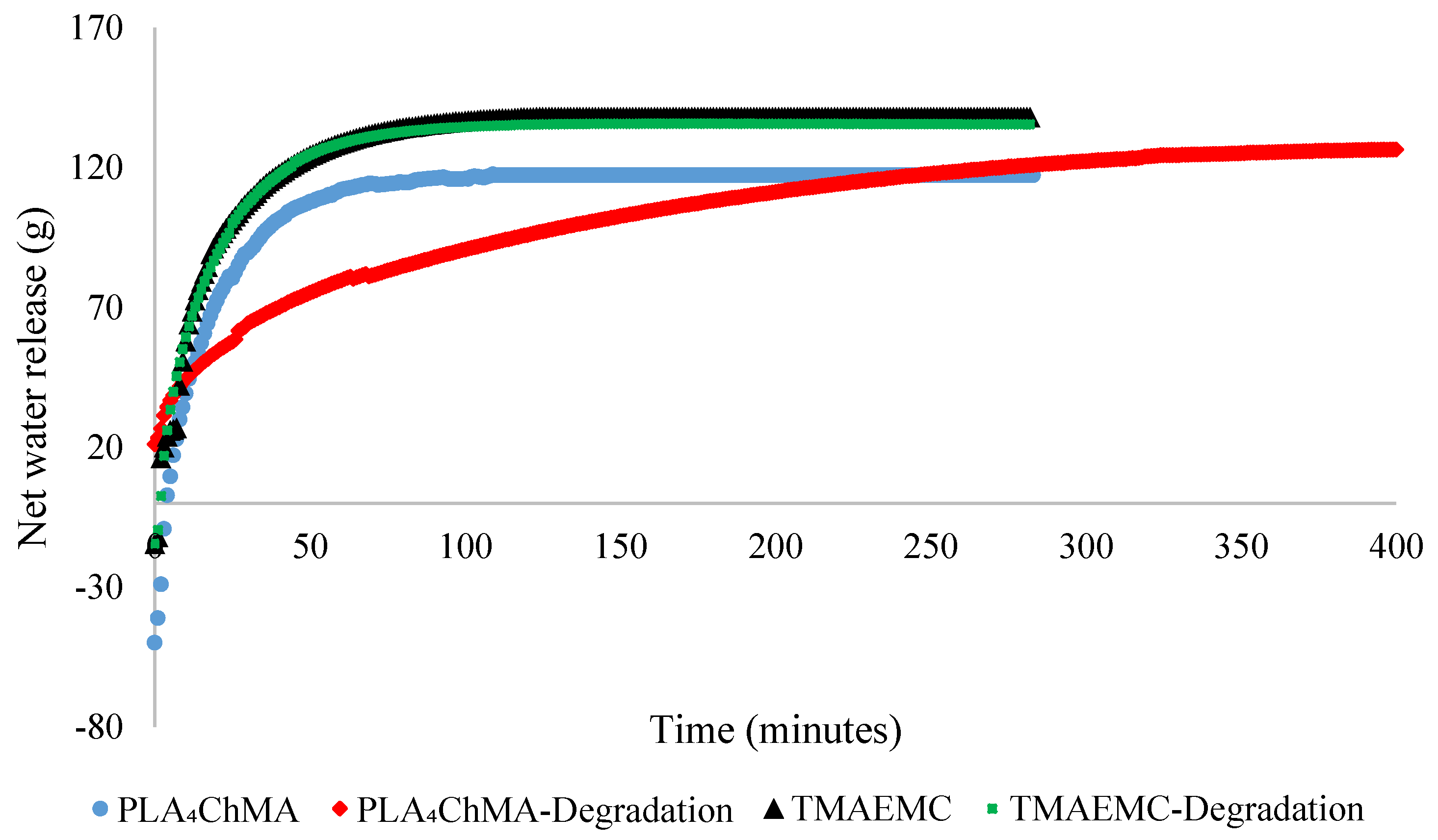

| Flocculant | Time to 60% Compaction (min) | Total Net Water Release (g) | Solids Content after Filtration (wt.%) | Filtration after Degradation? (Yes/No) |
|---|---|---|---|---|
| Untreated | 662 | 155.3 | 73.5 ± 0.1 | N |
| FLOPAM A3338 | 463 | 144.5 | 69.0 ± 9.0 | N |
| Poly(PLA4ChMA) | 106 | 117.1 | 65.4 ± 0.2 | N |
| Poly(TMAEMC) | 52 | 139.0 | 68.1 ± 5.1 | N |
| Poly(PLA4ChMA) | 245 | 127.0 | 66.8 ± 10.3 | Y |
| Poly(TMAEMC) | 52 | 136.0 | 64.9 ± 0.8 | Y |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lister, K.C.; Kaminsky, H.; Hutchinson, R.A. Evaluation of a Novel Polymeric Flocculant for Enhanced Water Recovery of Mature Fine Tailings. Processes 2020, 8, 735. https://doi.org/10.3390/pr8060735
Lister KC, Kaminsky H, Hutchinson RA. Evaluation of a Novel Polymeric Flocculant for Enhanced Water Recovery of Mature Fine Tailings. Processes. 2020; 8(6):735. https://doi.org/10.3390/pr8060735
Chicago/Turabian StyleLister, Kyle C., Heather Kaminsky, and Robin A. Hutchinson. 2020. "Evaluation of a Novel Polymeric Flocculant for Enhanced Water Recovery of Mature Fine Tailings" Processes 8, no. 6: 735. https://doi.org/10.3390/pr8060735





