Environmental Remediation of Antineoplastic Drugs: Present Status, Challenges, and Future Directions
Abstract
:1. Introduction
2. Parameters Determining the Fate and Distribution of ANPs
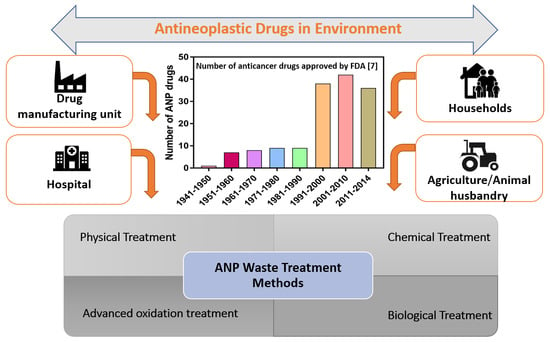
3. Sources of ANPs in the Environment
4. Environmental Risk Assessment (ERA) of ANPs
5. Overview of Existing Methodologies of ANPs Degradation in Environment
5.1. Biological Treatments
5.1.1. Fungal and Bacterial Biodegradation of ANPs
5.1.2. Biodegradation of Antineoplastic Drugs in Sewage Treatment Plants (STPs)
Removal of Alkylating Agents in STP
Removal of Antimetabolites in STP
Removal of Plant Alkaloids in STP
Removal of Antitumor Antibiotics in STP
Removal of Hormonal ANPs in STP
5.1.3. Removal of ANP Drugs Using Membrane Bioreactors
6. Removal of ANPs Using Abiotic Methods
6.1. Adsorption on Abiotic Surfaces
6.2. Membrane Based Filtration Technologies
6.3. Chemical Treatments
6.4. Decontamination Using Oxidation and Advanced Oxidation Methods
6.4.1. Oxidation Methods
6.4.2. Advanced Oxidation Methods
7. Possible Strategies for Improved Remediation of ANP Waste Incorporating Thermophiles
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnson, A.C.; Jürgens, M.D.; Williams, R.J.; Kümmerer, K.; Kortenkamp, A.; Sumpter, J.P. Do cytotoxic chemotherapy drugs discharged into rivers pose a risk to the environment and human health? An overview and UK case study. J. Hydrol. 2008, 348, 167–175. [Google Scholar] [CrossRef]
- Lutterbeck, C.A.; Wilde, M.L.; Baginska, E.; Leder, C.; Machado, Ê.L.; Kümmerer, K. Degradation of cyclophosphamide and 5-fluorouracil by UV and simulated sunlight treatments: Assessment of the enhancement of the biodegradability and toxicity. Environ. Pollut. 2016, 208, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Zamora, P.; Feliu, J.; González Barón, M. Classification of anticancer drugs—A new system based on therapeutic targets. Cancer Treat. Rev. 2003, 29, 515–523. [Google Scholar] [CrossRef]
- Kosjek, T.; Heath, E. Occurrence, fate and determination of cytostatic pharmaceuticals in the environment. Trends Anal. Chem. 2011, 30, 1065–1087. [Google Scholar] [CrossRef]
- Meegan, M.J.; O’Boyle, N.M. Special Issue “Anticancer Drugs”. Pharmaceuticals 2019, 12, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wei, Q.; Zhou, Y.; Wang, J.; Liu, Q.; Xu, H. A systematic analysis of FDA-approved anticancer drugs. BMC Syst. Biol. 2017, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Aitken, M. Global Oncology Trend Report: A Review of 2016 and Outlook to 2020. IMS Inst. Healthc. Inform. 2016, 1, 1–45. [Google Scholar]
- Hoppe-Tichy, T. Current challenges in European oncology pharmacy practice. J. Oncol. Pharm. Pract. 2010, 16, 9–18. [Google Scholar] [CrossRef]
- Salas-Vega, S.; Mossialos, E. Cancer Drugs Provide Positive Value In Nine Countries, But The United States Lags In Health Gains Per Dollar Spent. Health Aff. 2016, 35, 813–823. [Google Scholar] [CrossRef]
- Heath, E.; Isidori, M.; Kosjek, T.; Filipič, M. Fate and Effects of Anticancer Drugs in the Environment, 1st ed.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.; Torre, L.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmarcher, T.; Brådvik, G.; Svedman, C.; Lindgren, P.; Jönsson, B.; Wilking, N. Comparator Report on Cancer in Europe 2019–Disease Burden, Costs and Access to Medicines; IHE: Lund, Sweden, 2019. [Google Scholar]
- EMA Drugs Approved in 2019. Available online: https://pharmaboardroom.com/facts/ema-drugs-approved-in-2019/ (accessed on 17 June 2020).
- 2019 in Review: New Cancer Drug Approvals. Available online: https://www.cancer.org/latest-news/2019-in-review-new-cancer-drug-approvals.html (accessed on 17 June 2020).
- Chen, L.; Zeng, W.-M.; Cai, Y.-D.; Feng, K.-Y.; Chou, K.-C. Predicting Anatomical Therapeutic Chemical (ATC) Classification of Drugs by Integrating Chemical-Chemical Interactions and Similarities. PLoS ONE 2012, 7, e35254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azuma, T. Distribution of Anticancer Drugs in River Waters and Sediments of the Yodo River Basin, Japan. Appl. Sci. 2018, 8, 2043. [Google Scholar] [CrossRef] [Green Version]
- Booker, V.; Halsall, C.; Llewellyn, N.; Johnson, A.; Williams, R. Prioritising anticancer drugs for environmental monitoring and risk assessment purposes. Sci. Total Environ. 2014, 473–474, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Kümmerer, K.; Haiß, A.; Schuster, A.; Hein, A.; Ebert, I. Antineoplastic compounds in the environment—Substances of special concern. Environ. Sci. Pollut. Res. 2016, 23, 14791–14804. [Google Scholar] [CrossRef]
- Heath, E.; Filipič, M.; Kosjek, T.; Isidori, M. Fate and effects of the residues of anticancer drugs in the environment. Environ. Sci. Pollut. Res. 2016, 23, 14687–14691. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, V.W.; Giannis, A.; Wang, J.Y. Removal of cytostatic drugs from aquatic environment: A review. Sci. Total Environ. 2013, 445–446, 281–298. [Google Scholar] [CrossRef]
- Toolaram, A.P.; Kummerer, K.; Schneider, M. Environmental risk assessment of anti-cancer drugs and their transformation products: A focus on their genotoxicity characterization-state of knowledge and short comings. Mutat. Res. Rev. Mutat. Res. 2014, 760, 18–35. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Goncalves, V.M.; Maia, A.S.; Carvalho, M.F.; Castro, P.M.; Tiritan, M.E. Microbial degradation of pharmaceuticals followed by a simple HPLC-DAD method. J. Environ. Sci. Health Part AToxic/Hazard Subst. Environ. Eng. 2012, 47, 2151–2158. [Google Scholar] [CrossRef]
- Murphy, C.D. Microbial degradation of fluorinated drugs: Biochemical pathways, impacts on the environment and potential applications. Appl. Microbiol. Biotechnol. 2016, 100, 2617–2627. [Google Scholar] [CrossRef]
- Steger-Hartmann, T.; Kümmerer, K.; Hartmann, A. Biological Degradation of Cyclophosphamide and Its Occurrence in Sewage Water. Ecotoxicol. Environ. Saf. 1997, 36, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K.; Al-Ahmad, A. Biodegradability of the Anti-tumour Agents 5-Fluorouracil, Cytarabine, and Gemcitabine: Impact of the Chemical Structure and Synergistic Toxicity with Hospital Effluent. Acta Hydrochim. Hydrobiol. 1997, 25, 166–172. [Google Scholar] [CrossRef]
- Česen, M.; Kosjek, T.; Laimou-Geraniou, M.; Kompare, B.; Širok, B.; Lambropolou, D.; Heath, E. Occurrence of cyclophosphamide and ifosfamide in aqueous environment and their removal by biological and abiotic wastewater treatment processes. Sci. Total Environ. 2015, 527–528, 465–473. [Google Scholar] [CrossRef]
- Bhalla, A.; Bischoff, K.M.; Sani, R.K. Highly Thermostable Xylanase Production from A Thermophilic Geobacillus sp. Strain WSUCF1 Utilizing Lignocellulosic Biomass. Front. Bioeng. Biotechnol. 2015, 3, 84:1–84:8. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.M.; Chan, K.-G.; Sani, R.K.; Donati, E.R.; Reysenbach, A.-L. Editorial: Genetics, Genomics and –Omics of Thermophiles. Front. Microbiol. 2017, 8, 560:1–560:2. [Google Scholar] [CrossRef]
- Carlson, C.; Singh, N.K.; Bibra, M.; Sani, R.K.; Venkateswaran, K. Pervasiveness of UVC254-resistant Geobacillus strains in extreme environments. Appl. Microbiol. Biotechnol. 2018, 102, 1869–1887. [Google Scholar] [CrossRef]
- Rathinam, N.K.; Tripathi, A.K.; Smirnova, A.; Beyenal, H.; Sani, R.K. Engineering rheology of electrolytes using agar for improving the performance of bioelectrochemical systems. Bioresour. Technol. 2018, 263, 242–249. [Google Scholar] [CrossRef]
- David, A.; Govil, T.; Tripathi, A.; McGeary, J.; Farrar, K.; Sani, R. Thermophilic Anaerobic Digestion: Enhanced and Sustainable Methane Production from Co-Digestion of Food and Lignocellulosic Wastes. Energies 2018, 11, 2058. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, A.K.; Kumari, M.; Kumar, A.; Kumar, S. Generation of Biogas Using Pine Needles as Substrate in Domestic Biogas Plant. Int. J. Renew. Energy. Res. 2015, 5, 6. [Google Scholar]
- David, A.; Tripathi, A.K.; Sani, R.K. Acetate Production from Cafeteria Wastes and Corn Stover Using a Thermophilic Anaerobic Consortium: A Prelude Study for the Use of Acetate for the Production of Value-Added Products. Microorganisms 2020, 8, 353. [Google Scholar] [CrossRef] [Green Version]
- Beek, B.; Böhling, S.; Bruckmann, U.; Franke, C.; Jöhncke, U.; Studinger, G. The Assessment of Bioaccumulation; Springer: Berlin/Heidelberg, Germany, 2001; Volume 2. [Google Scholar]
- Kuntworbe, N.; Alany, R.G.; Brimble, M.; Al-Kassas, R. Determination of pKa and forced degradation of the indoloquinoline antimalarial compound cryptolepine hydrochloride. Pharm. Dev. Technol. 2013, 18, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Ishiuchi, H.; Inoyama, T.; Teranishi, Y.; Yamaoka, M.; Sato, T.; Mino, Y. Occurrence and fate of selected anticancer, antimicrobial, and psychotropic pharmaceuticals in an urban river in a subcatchment of the Yodo River basin, Japan. Environ. Sci. Pollut. Res. Int. 2015, 22, 18676–18686. [Google Scholar] [CrossRef] [PubMed]
- Folens, K.; Abebe, A.; Tang, J.; Ronsse, F.; Du Laing, G. Biosorption of residual cisplatin, carboplatin and oxaliplatin antineoplastic drugs in urine after chemotherapy treatment. Environ. Chem. 2018, 15, 506–512. [Google Scholar] [CrossRef]
- Scheytt, T.; Mersmann, P.; Lindstadt, R.; Heberer, T. Determination of sorption coefficients of pharmaceutically active substances carbamazepine, diclofenac, and ibuprofen, in sandy sediments. Chemosphere 2005, 60, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Besse, J.P.; Latour, J.F.; Garric, J. Anticancer drugs in surface waters: What can we say about the occurrence and environmental significance of cytotoxic, cytostatic and endocrine therapy drugs? Environ. Int. 2012, 39, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Buerge, I.J.; Buser, H.-R.; Poiger, T.; Müller, M.D. Occurrence and Fate of the Cytostatic Drugs Cyclophosphamide and Ifosfamide in Wastewater and Surface Waters. Environ. Sci. Technol. 2006, 40, 7242–7250. [Google Scholar] [CrossRef] [PubMed]
- Aherne, G.W.; Hardcastle, A.; Nield, A.H. Cytotoxic drugs and the aquatic environment: Estimation of bleomycin in river and water samples. J. Pharm. Pharmacol. 1990, 42, 741–742. [Google Scholar] [CrossRef]
- Jureczko, M.; Kalka, J. Cytostatic pharmaceuticals as water contaminants. Eur. J. Pharmacol. 2020, 866, 172816. [Google Scholar] [CrossRef]
- Ghafuria, Y. Environmental risk assessment of platinum cytotoxic drugs: A focus on toxicity characterization of hospital effluents. Int. J. Environ. Sci. Technol. 2018, 15, 1983–1990. [Google Scholar] [CrossRef]
- Richardson, M.L.; Bowron, J.M. The fate of pharmaceutical chemicals in the aquatic environment. J. Pharm. Pharm. 1985, 37, 1–12. [Google Scholar] [CrossRef]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use–present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Ort, C.; Lawrence, M.G.; Reungoat, J.; Eaglesham, G.; Carter, S.; Keller, J. Determining the fraction of pharmaceutical residues in wastewater originating from a hospital. Water Res. 2010, 44, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Mahnik, S.N.; Lenz, K.; Weissenbacher, N.; Mader, R.M.; Fuerhacker, M. Fate of 5-fluorouracil, doxorubicin, epirubicin, and daunorubicin in hospital wastewater and their elimination by activated sludge and treatment in a membrane-bio-reactor system. Chemosphere 2007, 66, 30–37. [Google Scholar] [CrossRef]
- Weissbrodt, D.; Kovalova, L.; Ort, C.; Pazhepurackel, V.; Moser, R.; Hollender, J.; Siegrist, H.; McArdell, C.S. Mass Flows of X-ray Contrast Media and Cytostatics in Hospital Wastewater. Environ. Sci. Technol. 2009, 43, 4810–4817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Judson, I.R.; Beale, P.J.; Trigo, J.M.; Aherne, W.; Crompton, T.; Jones, D.; Bush, E.; Reigner, B. A human capecitabine excretion balance and pharmacokinetic study after administration of a single oral dose of 14C-labelled drug. Investig. New Drugs 1999, 17, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Souza, D.M.; Reichert, J.F.; Martins, A.F. A simultaneous determination of anti-cancer drugs in hospital effluent by DLLME HPLC-FLD, together with a risk assessment. Chemosphere 2018, 201, 178–188. [Google Scholar] [CrossRef]
- Johnson Andrew, C.; Oldenkamp, R.; Dumont, E.; Sumpter John, P. Predicting concentrations of the cytostatic drugs cyclophosphamide, carboplatin, 5-fluorouracil, and capecitabine throughout the sewage effluents and surface waters of europe. Environ. Toxicol. Chem. 2013, 32, 1954–1961. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Choi, K. Comparison of regulatory frameworks of environmental risk assessments for human pharmaceuticals in EU, USA, and Canada. Sci. Total Environ. 2019, 671, 1026–1035. [Google Scholar] [CrossRef]
- Dundas, C.M.; Graham, A.J.; Romanovicz, D.K.; Keitz, B.K. Extracellular Electron Transfer by Shewanella oneidensis Controls Palladium Nanoparticle Phenotype. ACS Synth. Biol. 2018, 7, 2726–2736. [Google Scholar] [CrossRef] [PubMed]
- Rowney, N.C.; Johnson, A.C.; Williams, R.J. Erratum: Cytotoxic drugs in drinking water: A prediction and risk assessment exercise for the Thames catchment in the United Kingdom. Environ. Toxicol. Chem. 2011, 30, 1729. [Google Scholar] [CrossRef]
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristovao, M.B.; Janssens, R.; Yadav, A.; Pandey, S.; Luis, P.; Van der Bruggen, B.; Dubey, K.K.; Mandal, M.K.; Crespo, J.G.; Pereira, V.J. Predicted concentrations of anticancer drugs in the aquatic environment: What should we monitor and where should we treat? J. Hazard. Mater. 2020, 392, 122330. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.A.; Scheringer, M.; Fenner, K.; Hungerbuhler, K. A framework for evaluating the contribution of transformation products to chemical persistence in the environment. Environ. Sci. Technol. 2011, 45, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Escher, B.I.; Fenner, K. Recent Advances in Environmental Risk Assessment of Transformation Products. Environ. Sci. Technol. 2011, 45, 3835–3847. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [Green Version]
- Seruga Music, M.; Hrenovic, J.; Goic-Barisic, I.; Hunjak, B.; Skoric, D.; Ivankovic, T. Emission of extensively-drug-resistant Acinetobacter baumannii from hospital settings to the natural environment. J. Hosp. Infect. 2017, 96, 323–327. [Google Scholar] [CrossRef]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist 2015, 8, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Mustafa, B.-E.; Rehman, S.-U.; Bashir, M.K.; Ashraf, M.A. Emergence of Antimicrobial Resistance, Causes, Molecular Mechanisms, and Prevention Strategies: A Bovine Perspective. In Bovine Science—A Key to Sustainable Development; InTech Open: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Amadio, J.; Murphy, C.D. Production of human metabolites of the anti-cancer drug flutamide via biotransformation in Cunninghamella species. Biotechnol. Lett. 2011, 33, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busi, S.; Swaraj Pattnaik, S. Chapter 9—Current Status and Applications of Actinobacteria in the Production of Anticancerous Compounds. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, B.P., Gupta, V.K., Passari, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 137–153. [Google Scholar] [CrossRef]
- Cruz-Morato, C.; Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barcelo, D.; Marco-Urrea, E.; Vicent, T.; Sarra, M. Degradation of pharmaceuticals in non-sterile urban wastewater by Trametes versicolor in a fluidized bed bioreactor. Water Res. 2013, 47, 5200–5210. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Climent, L.; Cruz-Morato, C.; Marco-Urrea, E.; Vicent, T.; Sarra, M.; Rodriguez-Mozaz, S.; Barcelo, D. Non conventional biological treatment based on Trametes versicolor for the elimination of recalcitrant anticancer drugs in hospital wastewater. Chemosphere 2015, 136, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Tadkaew, N.; Hai, F.I.; McDonald, J.A.; Khan, S.J.; Nghiem, L.D. Removal of trace organics by MBR treatment: The role of molecular properties. Water Res. 2011, 45, 2439–2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Rodríguez, C.E.; Jesús García-Galán, M.; Blánquez, P.; Díaz-Cruz, M.S.; Barceló, D.; Caminal, G.; Vicent, T. Continuous degradation of a mixture of sulfonamides by Trametes versicolor and identification of metabolites from sulfapyridine and sulfathiazole. J. Hazard. Mater. 2012, 213, 347–354. [Google Scholar] [CrossRef]
- Casini, A.; Scozzafava, A.; Mastrolorenzo, A.; Supuran, L.T. Sulfonamides and sulfonylated derivatives as anticancer agents. Curr. Cancer.Drug Targets 2002, 2, 55–75. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Leech, D.; Paice, M.G. Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochim. Biophys. Acta. Gen. Subj. 1998, 1379, 381–390. [Google Scholar] [CrossRef]
- Schwarz, J.; Aust, M.-O.; Thiele-Bruhn, S. Metabolites from fungal laccase-catalysed transformation of sulfonamides. Chemosphere 2010, 81, 1469–1476. [Google Scholar] [CrossRef]
- Marco-Urrea, E.; Perez-Trujillo, M.; Blanquez, P.; Vicent, T.; Caminal, G. Biodegradation of the analgesic naproxen by Trametes versicolor and identification of intermediates using HPLC-DAD-MS and NMR. Bioresour. Technol. 2010, 101, 2159–2166. [Google Scholar] [CrossRef]
- Thun, M.J.; Henley, S.J.; Patrono, C. Nonsteroidal Anti-inflammatory Drugs as Anticancer Agents: Mechanistic, Pharmacologic, and Clinical Issues. J. Natl. Cancer. Inst. 2002, 94, 252–266. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.D.; Palmer-Brown, W.; Quinn, L.; Saccomanno, M. 7-Microbial metabolism of fluorinated drugs. In Fluorine in Life Sciences: Pharmaceuticals, Medicinal Diagnostics, and Agrochemicals; Haufe, G., Leroux, F.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 281–299. [Google Scholar] [CrossRef]
- Hidde Boersma, F.G.; Colin McRoberts, W.; Cobb, S.L.; Murphy, C.D. A 19F NMR study of fluorobenzoate biodegradation by Sphingomonas sp. HB-1. Fems. Microbiol. Lett. 2004, 237, 355–361. [Google Scholar] [CrossRef]
- Kim, E.J.; Jeon, J.R.; Kim, Y.M.; Murugesan, K.; Chang, Y.S. Mineralization and transformation of monofluorophenols by Pseudonocardia benzenivorans. Appl. Microbiol. Biotechnol. 2010, 87, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Lutterbeck, C.A.; Wilde, M.L.; Baginska, E.; Leder, C.; Machado, E.L.; Kummerer, K. Degradation of 5-FU by means of advanced (photo)oxidation processes: UV/H2O2, UV/Fe2+/H2O2 and UV/TiO2—Comparison of transformation products, ready biodegradability and toxicity. Sci. Total Environ. 2015, 527–528, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Engesser, K.-H.; Rubio, M.A.; Knackmuss, H.-J. Bacterial metabolism of side-chain-fluorinated aromatics: Unproductive meta-cleavage of 3-trifluoromethylcatechol. Appl. Microbiol. Biotechnol. 1990, 32, 600–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westman, E.L.; Canova, M.J.; Radhi, I.J.; Koteva, K.; Kireeva, I.; Waglechner, N.; Wright, G.D. Bacterial Inactivation of the Anticancer Drug Doxorubicin. Chem. Biol. 2012, 19, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Pepper, I.L.; Gentry, T.J. Chapter 4—Earth Environments. In Environmental. Microbiology, 3rd ed.; Pepper, I.L., Gerba, C.P., Gentry, T.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 59–88. [Google Scholar] [CrossRef]
- Friedrich, T.; Scheide, D. The respiratory complex I of bacteria, archaea and eukarya and its module common with membrane-bound multisubunit hydrogenases1. FEBS Lett. 2000, 479, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [Green Version]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef]
- Chang, H.; Wan, Y.; Wu, S.; Fan, Z.; Hu, J. Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters: Comparison to estrogens. Water Res. 2011, 45, 732–740. [Google Scholar] [CrossRef]
- Fan, Z.; Wu, S.; Chang, H.; Hu, J. Behaviors of glucocorticoids, androgens and progestogens in a municipal sewage treatment plant: Comparison to estrogens. Environ. Sci. Technol. 2011, 45, 2725–2733. [Google Scholar] [CrossRef]
- Ortiz de García, S.; Pinto Pinto, G.; García Encina, P.; Irusta Mata, R. Consumption and occurrence of pharmaceutical and personal care products in the aquatic environment in Spain. Sci. Total Environ. 2013, 444, 451–465. [Google Scholar] [CrossRef]
- Gómez-Canela, C.; Santos, M.S.F.; Franquet-Griell, H.; Alves, A.; Ventura, F.; Lacorte, S. Predicted Environmental Concentrations: A Useful Tool to Evaluate the Presence of Cytostatics in Surface Waters. In Fate and Effects of Anticancer Drugs in the Environment; Heath, E., Isidori, M., Kosjek, T., Filipič, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 27–54. [Google Scholar] [CrossRef]
- Aukema, K.G.; Escalante, D.E.; Maltby, M.M.; Bera, A.K.; Aksan, A.; Wackett, L.P. In Silico Identification of Bioremediation Potential: Carbamazepine and Other Recalcitrant Personal Care Products. Environ. Sci. Technol. 2017, 51, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Ioannou-Ttofa, L.; Fatta-Kassinos, D. Cytostatic Drug Residues in Wastewater Treatment Plants: Sources, Removal Efficiencies and Current Challenges. In Fate and Effects of. Anticancer Drugs in the Environment; Heath, E., Isidori, M., Kosjek, T., Filipič, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 103–138. [Google Scholar] [CrossRef]
- Chu, C.S.; Rubin, S.C. 17—Basic Principles of Chemotherapy. In Clinical Gynecologic Oncology, 9th ed.; DiSaia, P.J., Creasman, W.T., Mannel, R.S., McMeekin, D.S., Mutch, D.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 449–469. [Google Scholar] [CrossRef]
- Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barcelo, D. Development of a UPLC-MS/MS method for the determination of ten anticancer drugs in hospital and urban wastewaters, and its application for the screening of human metabolites assisted by information-dependent acquisition tool (IDA) in sewage samples. Anal. Bioanal. Chem. 2013, 405, 5937–5952. [Google Scholar] [CrossRef] [Green Version]
- Negreira, N.; de Alda, M.L.; Barcelo, D. Cytostatic drugs and metabolites in municipal and hospital wastewaters in Spain: Filtration, occurrence, and environmental risk. Sci. Total Environ. 2014, 497–498, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Rabii, F.W.; Segura, P.A.; Fayad, P.B.; Sauve, S. Determination of six chemotherapeutic agents in municipal wastewater using online solid-phase extraction coupled to liquid chromatography-tandem mass spectrometry. Sci. Total Environ. 2014, 487, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Isidori, M.; Lavorgna, M.; Russo, C.; Kundi, M.; Zegura, B.; Novak, M.; Filipic, M.; Misik, M.; Knasmueller, S.; de Alda, M.L.; et al. Chemical and toxicological characterisation of anticancer drugs in hospital and municipal wastewaters from Slovenia and Spain. Environ. Pollut. 2016, 219, 275–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Shao, B.; Zhang, J.; Li, K. A preliminary study on the occurrence of cytostatic drugs in hospital effluents in Beijing, China. Bull. Environ. Contam. Toxicol. 2010, 84, 39–45. [Google Scholar] [CrossRef]
- Thomas, K.V.; Dye, C.; Schlabach, M.; Langford, K.H. Source to sink tracking of selected human pharmaceuticals from two Oslo city hospitals and a wastewater treatment works. J. Environ. Monit. 2007, 9, 1410–1418. [Google Scholar] [CrossRef]
- Michael, C.; Bayona, J.M.; Lambropoulou, D.; Aguera, A.; Fatta-Kassinos, D. Two important limitations relating to the spiking of environmental samples with contaminants of emerging concern: How close to the real analyte concentrations are the reported recovered values? Environ. Sci. Pollut. Res. Int. 2017, 24, 15202–15205. [Google Scholar] [CrossRef]
- Freres, P.; Jerusalem, G.; Moonen, M. Chapter 2—Categories of Anticancer Treatments. In Anti-Cancer Treatments and Cardiotoxicity; Lancellotti, P., Zamorano Gómez, J.L., Galderisi, M., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 7–11. [Google Scholar] [CrossRef]
- Kiffmeyer, T.; Götze, H.-J.; Jursch, M.; Lüders, U. Trace enrichment, chromatographic separation and biodegradation of cytostatic compounds in surface water. Fresenius J. Anal. Chem. 1998, 361, 185–191. [Google Scholar] [CrossRef]
- Rosano, T.G. Ellenhorn’s Medical Toxicology: Diagnosis and Treatment of Human Poisoning. Clin. Chem. 1998, 44, 366. [Google Scholar] [CrossRef]
- Kosjek, T.; Perko, S.; Zigon, D.; Heath, E. Fluorouracil in the environment: Analysis, occurrence, degradation and transformation. J. Chromatogr. A 2013, 1290, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.T.; Bouwer, E.J.; Coelhan, M. Occurrence and biodegradability studies of selected pharmaceuticals and personal care products in sewage effluent. Agric. Water. Manag. 2006, 86, 72–80. [Google Scholar] [CrossRef]
- Straub, J.O. Combined environmental risk assessment for 5-fluorouracil and capecitabine in Europe. Integr. Environ. Assess Manag. 2010, 6, 540–566. [Google Scholar] [CrossRef] [PubMed]
- Lutterbeck, C.A.; Baginska, E.; Machado, E.L.; Kummerer, K. Removal of the anti-cancer drug methotrexate from water by advanced oxidation processes: Aerobic biodegradation and toxicity studies after treatment. Chemosphere 2015, 141, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Camacho-Munoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Simultaneous determination of a selected group of cytostatic drugs in water using high-performance liquid chromatography-triple-quadrupole mass spectrometry. J. Sep. Sci. 2011, 34, 3166–3177. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence and Ecotoxicological Risk Assessment of 14 Cytostatic Drugs in Wastewater. Water Air Soil Pollut. 2014, 225, 1896. [Google Scholar] [CrossRef]
- Kosjek, T.; Negreira, N.; de Alda, M.L.; Barcelo, D. Aerobic activated sludge transformation of methotrexate: Identification of biotransformation products. Chemosphere 2015, 119, S42–S50. [Google Scholar] [CrossRef] [Green Version]
- Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barcelo, D. Incidence of anticancer drugs in an aquatic urban system: From hospital effluents through urban wastewater to natural environment. Environ. Pollut. 2014, 193, 216–223. [Google Scholar] [CrossRef]
- Pietsch, J.; Gunther, J.; Henle, T.; Dressler, J. Simultaneous determination of thirteen plant alkaloids in a human specimen by SPE and HPLC. J. Sep. Sci. 2008, 31, 2410–2416. [Google Scholar] [CrossRef]
- Kosjek, T.; Negreira, N.; Heath, E.; López de Alda, M.; Barceló, D. Aerobic activated sludge transformation of vincristine and identification of the transformation products. Sci. Total Environ. 2018, 610–611, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Jeswani, G.; Paul, S.D. Chapter 15—Recent Advances in the Delivery of Chemotherapeutic Agents. In Nano-and Microscale Drug Delivery Systems; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 281–298. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Xin, C.; Zheng, Y.; Cheng, Y.; Sun, S.; Li, R.; Zhu, X.-G.; Dai, S.Y.; Rentzepis, P.M.; et al. Enhanced limonene production in cyanobacteria reveals photosynthesis limitations. Proc. Natl. Acad. Sci. USA 2016, 113, 14225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franquet-Griell, H.; Medina, A.; Sans, C.; Lacorte, S. Biological and photochemical degradation of cytostatic drugs under laboratory conditions. J. Hazard. Mater. 2017, 323, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.H.; Thomas, K.V. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci. Total Environ. 2006, 356, 143–153. [Google Scholar] [CrossRef]
- Gómez-Canela, C.; Ventura, F.; Caixach, J.; Lacorte, S. Occurrence of cytostatic compounds in hospital effluents and wastewaters, determined by liquid chromatography coupled to high-resolution mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 3801–3814. [Google Scholar] [CrossRef] [PubMed]
- Melin, T.; Jefferson, B.; Bixio, D.; Thoeye, C.; De Wilde, W.; De Koning, J.; van der Graaf, J.; Wintgens, T. Membrane bioreactor technology for wastewater treatment and reuse. Desalination 2006, 187, 271–282. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, J.; Zeng, G.; Shi, L.; Shi, Y.; Yi, K. Fate of pharmaceuticals during membrane bioreactor treatment: Status and perspectives. Bioresour. Technol. 2018, 268, 733–748. [Google Scholar] [CrossRef]
- Lenz, K.; Mahnik, S.N.; Weissenbacher, N.; Mader, R.M.; Krenn, P.; Hann, S.; Koellensperger, G.; Uhl, M.; Knasmuller, S.; Ferk, F.; et al. Monitoring, removal and risk assessment of cytostatic drugs in hospital wastewater. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2007, 56, 141–149. [Google Scholar] [CrossRef]
- Delgado, L.F.; Dorandeu, C.; Marion, B.; Gonzalez, C.; Faucet-Marquis, V.; Schetrite, S.; Albasi, C. Removal of a cytostatic drug by a membrane bioreactor. Desalin. Water Treat. 2009, 9, 112–118. [Google Scholar] [CrossRef]
- Delgado, L.F.; Faucet-Marquis, V.; Pfohl-Leszkowicz, A.; Dorandeu, C.; Marion, B.; Schetrite, S.; Albasi, C. Cytotoxicity micropollutant removal in a crossflow membrane bioreactor. Bioresour. Technol. 2011, 102, 4395–4401. [Google Scholar] [CrossRef] [Green Version]
- Kovalova, L.; Siegrist, H.; Singer, H.; Wittmer, A.; McArdell, C.S. Hospital wastewater treatment by membrane bioreactor: Performance and efficiency for organic micropollutant elimination. Environ. Sci. Technol. 2012, 46, 1536–1545. [Google Scholar] [CrossRef] [Green Version]
- Avella, A.C.; Delgado, L.F.; Görner, T.; Albasi, C.; Galmiche, M.; de Donato, P. Effect of cytostatic drug presence on extracellular polymeric substances formation in municipal wastewater treated by membrane bioreactor. Bioresour. Technol. 2010, 101, 518–526. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Chang, V.W.C.; She, Q.; Tang, C.Y. Removal of cytostatic drugs from wastewater by an anaerobic osmotic membrane bioreactor. Chem. Eng. J. 2018, 339, 153–161. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, L.; Ng, J.-W.; Lee, C.; Chang, V.W.C.; Tang, C.Y. Development of anaerobic osmotic membrane bioreactor for low-strength wastewater treatment at mesophilic condition. J. Membr. Sci. 2015, 490, 197–208. [Google Scholar] [CrossRef]
- Chang, H.-M.; Sun, Y.-C.; Chien, I.C.; Chang, W.-S.; Ray, S.S.; Cao, D.T.N.; Cong Duong, C.; Chen, S.-S. Innovative upflow anaerobic sludge osmotic membrane bioreactor for wastewater treatment. Bioresour. Technol. 2019, 287, 121466. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, X.; Tay, M.Q.X.; Oh, S.; Yang, L.; Tang, C.; Cao, B. Metagenomic insights into the influence of salinity and cytostatic drugs on the composition and functional genes of microbial community in forward osmosis anaerobic membrane bioreactors. Chem. Eng. J. 2017, 326, 462–469. [Google Scholar] [CrossRef]
- Kovalova, L.; McArdell, C.S.; Hollender, J. Challenge of high polarity and low concentrations in analysis of cytostatics and metabolites in wastewater by hydrophilic interaction chromatography/tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sorensen, B.; Nors Nielsen, S.; Lanzky, P.F.; Ingerslev, F.; Holten Lutzhoft, H.C.; Jorgensen, S.E. Occurrence, fate and effects of pharmaceutical substances in the environment-a review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Hamon, P.; Moulin, P.; Ercolei, L.; Marrot, B. Oncological ward wastewater treatment by membrane bioreactor: Acclimation feasibility and pharmaceuticals removal performances. J. Water Process Eng. 2018, 21, 9–26. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-H.; Lin, A.Y.-C. Is the phototransformation of pharmaceuticals a natural purification process that decreases ecological and human health risks? Environ. Pollut. 2014, 186, 203–215. [Google Scholar] [CrossRef]
- Badia-Fabregat, M.; Oller, I.; Malato, S. Overview on Pilot-Scale Treatments and New and Innovative Technologies for Hospital Effluent. In Hospital Wastewaters: Characteristics, Management, Treatment and Environmental Risks; Verlicchi, P., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 209–230. [Google Scholar] [CrossRef]
- Seira, J.; Sablayrolles, C.; Montréjaud-Vignoles, M.; Albasi, C.; Joannis-Cassan, C. Elimination of an anticancer drug (cyclophosphamide) by a membrane bioreactor: Comprehensive study of mechanisms. Biochem. Eng. J. 2016, 114, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Park, G.; Herckes, P.; Westerhoff, P. Physicochemical Treatment of Three Chemotherapy Drugs: Irinotecan, Tamoxifen, and Cyclophosphamide. J. Adv. Oxid. Technol. 2008, 11, 254–260. [Google Scholar] [CrossRef]
- Paci, A.; Martens, T.; Royer, J. Anodic oxidation of ifosfamide and cyclophosphamide: A biomimetic metabolism model of the oxazaphosphorinane anticancer drugs. Bioorg. Med. Chem. Lett. 2001, 11, 1347–1349. [Google Scholar] [CrossRef]
- Oh, B.S.; Oh, S.; Kim, S.-J.; Choi, Y.; Hwang, T.-M. Optimization of wastewater reclamation and reuse system using membrane filtration and oxidation processes: Removal of pharmaceuticals. Desalin. Water Treat. 2016, 57, 10146–10151. [Google Scholar] [CrossRef]
- Shojaee Nasirabadi, P.; Saljoughi, E.; Mousavi, S.M. Membrane processes used for removal of pharmaceuticals, hormones, endocrine disruptors and their metabolites from wastewaters: A review. Desalin. Water Treat. 2016, 57, 24146–24175. [Google Scholar] [CrossRef]
- Dolar, D.; Košutić, K. Chapter 10—Removal of Pharmaceuticals by Ultrafiltration (UF), Nanofiltration (NF), and Reverse Osmosis (RO). In Comprehensive Analytical Chemistry; Petrovic, M., Barcelo, D., Pérez, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 62, pp. 319–344. [Google Scholar]
- Kimura, K.; Amy, G.; Drewes, J.E.; Heberer, T.; Kim, T.-U.; Watanabe, Y. Rejection of organic micropollutants (disinfection by-products, endocrine disrupting compounds, and pharmaceutically active compounds) by NF/RO membranes. J. Membr. Sci. 2003, 227, 113–121. [Google Scholar] [CrossRef]
- Pronk, W.; Biebow, M.; Boller, M. Electrodialysis for Recovering Salts from a Urine Solution Containing Micropollutants. Environ. Sci. Technol. 2006, 40, 2414–2420. [Google Scholar] [CrossRef]
- Wang, L.; Albasi, C.; Faucet-Marquis, V.; Pfohl-Leszkowicz, A.; Dorandeu, C.; Marion, B.; Causserand, C. Cyclophosphamide removal from water by nanofiltration and reverse osmosis membrane. Water Res. 2009, 43, 4115–4122. [Google Scholar] [CrossRef] [Green Version]
- Escher, B.I.; Pronk, W.; Suter, M.J.F.; Maurer, M. Monitoring the Removal Efficiency of Pharmaceuticals and Hormones in Different Treatment Processes of Source-Separated Urine with Bioassays. Environ. Sci. Technol. 2006, 40, 5095–5101. [Google Scholar] [CrossRef]
- Benvenuto, J.A.; Connor, T.H.; Monteith, D.K.; Laidlaw, J.L.; Adams, S.C.; Matney, T.S.; Theiss, J.C. Degradation and Inactivation of Antitumor Drugs. J. Pharm. Sci. 1993, 82, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Castegnaro, M.; Sportouch, M.H.; De Meo, M.; Laget, M.; Michelon, J.; Garren, L.; Hansel, S. Chemical degradation of wastes of antineoplastic agents 2-Six Anthracyclines: Idarubicin, doxorubicin, epirubicin, pirarubicin, aclarubicin, and daunorubicin. Int. Arch. Occup. Environ. Health 1997, 70, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Hansel, S.; Castegnaro, M.; Sportouch, M.H.; De Meo, M.; Milhavet, J.C.; Laget, M.; Dumenil, G. Chemical degradation of wastes of antineoplastic agents: Cyclophosphamide, ifosfamide, and melphalan. Int. Arch. Occup. Environ. Health. 1997, 69, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Lunn, G.; Sansone, E.B.; Andrews, A.W.; Hellwig, L.C. Degradation and disposal of some antineoplastic drugs. J. Pharm. Sci. 1989, 78, 652–659. [Google Scholar] [CrossRef]
- Macholz, R. Laboratory decontamination and destruction of carcinogens in laboratory wastes: Some antineoplastic agents. International Agency for Research on Cancer. IARC Sci. Publ. 1985, 32, 1–634. [Google Scholar]
- Martignac, M.; Balayssac, S.; Gilard, V.; Benoit-Marquié, F. Photochemical Degradation of the Anticancer Drug Bortezomib by V–UV/UV (185/254 nm) Investigated by 1H NMR Fingerprinting: A Way to Follow Aromaticity Evolution. J. Phys. Chem. A 2015, 119, 6215–6222. [Google Scholar] [CrossRef]
- Lin, A.Y.-C.; Wang, X.-H.; Lee, W.-N. Phototransformation Determines the Fate of 5-Fluorouracil and Cyclophosphamide in Natural Surface Waters. Environ. Sci. Technol. 2013, 47, 4104–4112. [Google Scholar] [CrossRef]
- Trawiński, J.; Skibiński, R. Studies on photodegradation process of psychotropic drugs: A review. Environ. Sci. Pollut. Res. Int. 2017, 24, 1152–1199. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Canela, C.; Bolivar-Subirats, G.; Tauler, R.; Lacorte, S. Powerful combination of analytical and chemometric methods for the photodegradation of 5-Fluorouracil. J. Pharm. Biomed. Anal. 2017, 137, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ganzenko, O.; Oturan, N.; Sirés, I.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Fast and complete removal of the 5-fluorouracil drug from water by electro-Fenton oxidation. Environ. Chem. Lett. 2018, 16, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Koltsakidou, A.; Antonopoulou, M.; Sykiotou, M.; Εvgenidou, Ε.; Konstantinou, I.; Lambropoulou, D.A. Photo-Fenton and Fenton-like processes for the treatment of the antineoplastic drug 5-fluorouracil under simulated solar radiation. Environ. Sci. Pollut. Res. 2017, 24, 4791–4800. [Google Scholar] [CrossRef] [PubMed]
- Anheden, M.; Goswami, D.Y.; Svedberg, G. Photocatalytic Treatment of Wastewater From 5–Fluorouracil Manufacturing. J. Solar Energy Eng. 1996, 118, 2–8. [Google Scholar] [CrossRef]
- Burleson, G.R.; Chambers, T.M. Effect of ozonation on the mutagenicity of carcinogens in aqueous solution. Environ. Mutagenesis 1982, 4, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Gouider, M.; Mlaik, N.; Feki, M.; Sayadi, S. Integrated physicochemical and biological treatment process for fluoride and phosphorus removal from fertilizer plant wastewater. Water Environ. Res. 2011, 83, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Xu, Z.; Li, X.; You, W.; Zhen, Y. Advanced treatment of dyeing wastewater towards reuse by the combined Fenton oxidation and membrane bioreactor process. J. Environ. Sci. 2010, 22, 1657–1665. [Google Scholar] [CrossRef]
- Calza, P.; Medana, C.; Sarro, M.; Rosato, V.; Aigotti, R.; Baiocchi, C.; Minero, C. Photocatalytic degradation of selected anticancer drugs and identification of their transformation products in water by liquid chromatography–high resolution mass spectrometry. J. Chromatogr. A 2014, 1362, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.; Hsueh, J.H.; Hong, P.K. Removal of antineoplastic drugs cyclophosphamide, ifosfamide, and 5-fluorouracil and a vasodilator drug pentoxifylline from wastewaters by ozonation. Environ. Sci. Pollut. Res. Int. 2015, 22, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Negreira, N.; Regueiro, J.; Lopez de Alda, M.; Barcelo, D. Degradation of the anticancer drug erlotinib during water chlorination: Non-targeted approach for the identi fi cation of transformation products. Water Res. 2015, 85, 103–113. [Google Scholar] [CrossRef]
- Huo, Z.; Wang, S.; Shao, H.; Wang, H.; Xu, G. Radiolytic degradation of anticancer drug capecitabine in aqueous solution: Kinetics, reaction mechanism, and toxicity evaluation. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Barışçı, S.; Turkay, O.; Ulusoy, E.; Şeker, M.G.; Yüksel, E.; Dimoglo, A. Electro-oxidation of cytostatic drugs: Experimental and theoretical identification of by-products and evaluation of ecotoxicological effects. Chem. Eng. J. 2018, 334, 1820–1827. [Google Scholar] [CrossRef]
- Governo, M.; Santos, M.S.F.; Alves, A.; Madeira, L.M. Degradation of the cytostatic 5-Fluorouracil in water by Fenton and photo-assisted oxidation processes. Environ. Sci. Pollut. Res. 2017, 24, 844–854. [Google Scholar] [CrossRef]
- Koltsakidou, A.; Antonopoulou, M.; Evgenidou, E.; Konstantinou, I.; Lambropoulou, D.A. Cytarabine degradation by simulated solar assisted photocatalysis using TiO2. Chem. Eng. J. 2017, 316, 823–831. [Google Scholar] [CrossRef]
- Janssens, R.; Cristóvão, B.M.; Bronze, M.R.; Crespo, J.G.; Pereira, V.J.; Luis, P. Photocatalysis Using UV-A and UV-C Light Sources for Advanced Oxidation of Anti-Cancer Drugs Spiked in Laboratory-Grade Water and Synthetic Urine. Ind. Eng. Chem. Res. 2020, 59, 647–653. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Bertanza, G.; Setti, M.; Barbieri, G.; Frattarola, A. Integrating novel (thermophilic aerobic membrane reactor-TAMR) and conventional (conventional activated sludge-CAS) biological processes for the treatment of high strength aqueous wastes. Bioresour. Technol. 2018, 255, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kårelid, V.; Larsson, G.; Björlenius, B. Pilot-scale removal of pharmaceuticals in municipal wastewater: Comparison of granular and powdered activated carbon treatment at three wastewater treatment plants. J. Environ. Manag. 2017, 193, 491–502. [Google Scholar] [CrossRef]
- Lienert, J.B.; Bürki, T.; Escher, B.I. Reducing micropollutants with source control: Substance flow analysis of 212 pharmaceuticals in faeces and urine. Water Sci. Technol. 2007, 56, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Wilsenach, J.A.; Van Loosdrecht, M.C.M. Effects of Separate Urine Collection on Advanced Nutrient Removal Processes. Environ. Sci. Technol. 2004, 38, 1208–1215. [Google Scholar] [CrossRef]
- Wilsenach, J.; van Loosdrecht, M. Impact of separate urine collection on wastewater treatment systems. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2003, 48, 103–110. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abba, A.; Bertanza, G. Treatment of high strength pharmaceutical wastewaters in a Thermophilic Aerobic Membrane Reactor (TAMR). Water Res. 2014, 63, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.; Bokhary, A.; Fatehi, P.; Kong, F.; Lin, H.; Liao, B. Thermophilic membrane bioreactors: A review. Bioresour. Technol. 2017, 243, 1180–1193. [Google Scholar] [CrossRef]
- Barisci, S.; Turkay, O.; Ulusoy, E.; Soydemir, G.; Seker, M.G.; Dimoglo, A. Electrochemical treatment of anti-cancer drug carboplatin on mixed-metal oxides and boron doped diamond electrodes: Density functional theory modelling and toxicity evaluation. J. Hazard. Mater. 2017, 344, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, T.; Han, W.; Sun, X.; Li, J.; Shen, J.; Wang, L. Electrochemical treatment of anticancer drugs wastewater containing 5-Fluoro-2-Methoxypyrimidine using a tubular porous electrode electrocatalytic reactor. Electrochim. Acta. 2016, 220, 211–221. [Google Scholar] [CrossRef]
- Manson, M.M. Epoxides—Is there a human health problem? Br. J. Ind. Med. 1980, 37, 317–336. [Google Scholar] [CrossRef]




| ANP Drug | Chemical Structure | log Kow | pKa f | Solubility in Water (at 25 °C) | Toxicity (LD50 d Value, Oral) | Biological Half-Life e | BCF c |
|---|---|---|---|---|---|---|---|
| 5-FU | 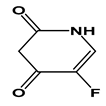 | −0.89 | 8.02 | 11.1 g/L | 230 mg/kg (mice) | 10–20 min | 3 |
| CP | 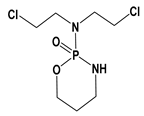 | 0.63 | 2.84 | 10–50 g/L | 275 to >400 mg/kg (Rats) | 3–12 h | 2.1 |
| DOX |  | 1.27 | 7.34 (pKa1) 8.46 (pKa2) 9.46 (pKa3) | 2.6 g/L | 16 mg/kg (rats) | 20–48 h | 1 |
| TAM | 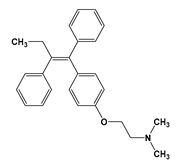 | 6.30 | 8.87 | Insoluble | 4100 mg/kg (rats) a | 5–7 days | 827 |
| CPC |  | 0.56 | 1.9 | 26 g/L | >2120 mg/kg (women) | 45–60 min | NA |
| MTX | 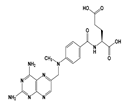 | 1.85 | 4.70 | 2.6 g/L | 135 mg/kg (rats) | Low doses: 3–10 h High doses: 8–15 h | 3.2 |
| IF |  | 0.86 | 1.45 | 3.8 g/L | 150–190 mg/kg (rats) | 7–15 h | 3 |
| CPT | 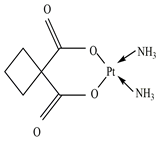 | −0.46 | 6.6 | 15 g/L | 61 mg/kg (rats) | 1.1–2 h | NA |
| PEM | 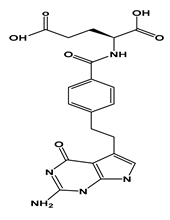 | 0.16 | 3.6 (pKa1) 4.4 (pKa2) | Insoluble | 1754 mg/kg (rats) | 3.5 h | 3.2 |
| GEM | 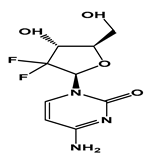 | −2.01 | 3.6 | 51.3 g/L | 500 mg/kg (mice and rats) | Short infusions: 42–94 min Long infusions: 245–638 min | 1 |
| CTB | 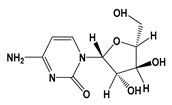 | −2.46 | 4.22 | 17.6 g/L | 3150 mg/kg (mice) | 10 min | 1 |
| AZT |  | 0.10 | 7.87 | Insoluble | 400 mg/kg (rats) | 12–15 min | NA |
| ETS |  | 0.60 | 9.8 | Sparingly soluble (0.08 g/L) | 118 mg/kg (mice) | 4–11 h | 3 |
| SFL |  | −0.62 | 10.6 | 7.5 g/L | 3700 mg/kg (mice) | Dependent on renal function | 0.2 |
| DAU | 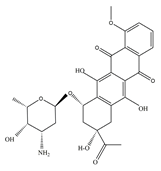 | 1.83 | 7.85 | 30 g/L | 1737 mg/kg (mice) | 36 ± 13 h | 110 |
| USA | EU | Canada | |
|---|---|---|---|
| Implementing organization | Food and Drug Administration (FDA) | European Medical Agency (EMA) | Health Canada and Environment and Climate Change Canada |
| Regulated product | All drugs manufactured for sale in EU member states | New Drugs | New Substances |
| Timing of ERA | When applying for marketing approval | New Drug applications [56] | Before notification |
| ERA Methodology | Phase-tiered based approach (Phase I; Phase II-Tier A and Tier B) | Tiered based approach (Tier 1, Tier 2, Tier 3) | Classification based approach (polymers, living organisms, chemicals) |
| Drug exclusion criteria | PEC a < 10 ng/L | EIC b < 1000 ng/L | PEC < 100 ng/L |
| Risk Assessment Criteria | PEC/PNEC c ≥ 1 | EC d50/MEEC e < 10 | PEC/PNEC ≥ 1 |
| Strengths | Responsibility lies with government Tiered approach | Responsibility lies with government Tiered approach | Responsibility lies with government Analyzes substances that are presumed to be non-toxic. |
| Weaknesses | No ERA for existing drugs. Non-consistent ERA procedure between member states | No ERA for existing drugs | Non-tiered approach |
| ANP Drug | Type of Biological Treatment | Mechanism of Bioremediation | Initial Concentration (ng/L) | % Elimination of the Parent Drug | Reference |
|---|---|---|---|---|---|
| TAM | Fluidized bed bioreactor (with Trametes versicolor) | Intracellular transformation by cytochrome P-450 system | 970 | 91% | [70] |
| IF | CAS-STP a | N.D. | 16.4 | 45% | [99] |
| FLT | Biotransformation by Caenorhabditis elegans | Cytochrome P450 mediated oxidation | 9 × 107 b | 50% | [67] |
| CP | LSSTP c | Biodegradation | 6–143 | >80% | [25] |
| CPC | STP | Biodegradation | 158 | 100% | [98] |
| CTB | CAS-STP | N.D. | N.D. | 24% | [19] |
| DOC | CAS-STP | Biodegradation and sorption | 65–219 | ≈100% | [112] |
| ETS | CAS-STP | N.D. | 15 ng/L | 77% | [109] |
| VNB | CAS-STP | Non-biodegradable | <LOD d | 0% e | [110] |
| PAC | CAS-STP | Biodegradation and sorption | <LOD-18 | 100% | [112] |
| GEM | CAS-STP | Adsorption to sewage sludge | 840 | 40–79% | [132] |
| BLC | CAS-STP | N.D. | 11–19 | - | [133] |
| MTN | AnOMBR f | Adsorption and biodegradation | 100 | 100% | [128] |
| 5-FU | eMBR g | Biotransformation and sorption | 0–1.2 × 106 h | >90% | [134] |
| DOX | CAS-STP | Biodegradation | 2.5–2.7 | 100% | [96] |
| Treatment Process | Target ANP Drug | % Elimination | No. of TPs Formed | Biodegradability Treated Drug and Its TPs | Change in Toxicity (Drug vs. TPs) | Reference |
|---|---|---|---|---|---|---|
| Advanced (Photo)Oxidation (UV/TiO2) | DOX | 100% in 30 min | 17 TPs | N.D. | Decrease in toxicity in 2 h (3% toxicity reduction) | [162] |
| Advanced (Photo)Oxidation (UV/H2O2) | MTX | >99% in 16 min | 6 TPs | Very low biodegradability | 40% decrease in toxicity | [108] |
| UV Photolysis | 5-FU | 100% elimination in 32 min | 3 TPs | Increased biodegradability | 45% decrease in toxicity | [2] |
| Ozonation (O3) | CP | 69.8% at pH 9 61.2% at pH 5 | N.D. | N.D. | Increase in acute toxicity | [163] |
| Chlorination | ERL | 95% in 1 h | 16 TPs | N.D. | Increase in toxicity of TPs | [164] |
| Electron beam irradiation | CPC | 73% for 50 mg/L CPC and 36% for 150 mg/L CPC | 5 TPs | N.D. | TPs had lower toxicity than parent compound | [165] |
| Electro-oxidation | MTX | 100% in 30 min at CD b of 30mA/cm2 | 4 TPs | N.D. | Increase in toxicity | [166] |
| Photo Fenton oxidation (UV/Vis/H2O2/Fe2+) | 5-FU | 100% in 1 h | N.D. | N.D. | Decrease in toxicity | [167] |
| TiO2/H2O2/SSL c | CTB | 100% in <45 min | 4 TPs | N.D. | 11% decrease in toxicity | [168] |
| Advanced (Photo)Oxidation (UV-C/TiO2) | ETS | 100% elimination | N.D. | N.D. | Not tested for toxicity | [169] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, A.K.; David, A.; Govil, T.; Rauniyar, S.; Rathinam, N.K.; Goh, K.M.; Sani, R.K. Environmental Remediation of Antineoplastic Drugs: Present Status, Challenges, and Future Directions. Processes 2020, 8, 747. https://doi.org/10.3390/pr8070747
Tripathi AK, David A, Govil T, Rauniyar S, Rathinam NK, Goh KM, Sani RK. Environmental Remediation of Antineoplastic Drugs: Present Status, Challenges, and Future Directions. Processes. 2020; 8(7):747. https://doi.org/10.3390/pr8070747
Chicago/Turabian StyleTripathi, Abhilash Kumar, Aditi David, Tanvi Govil, Shailabh Rauniyar, Navanietha Krishnaraj Rathinam, Kian Mau Goh, and Rajesh Kumar Sani. 2020. "Environmental Remediation of Antineoplastic Drugs: Present Status, Challenges, and Future Directions" Processes 8, no. 7: 747. https://doi.org/10.3390/pr8070747
APA StyleTripathi, A. K., David, A., Govil, T., Rauniyar, S., Rathinam, N. K., Goh, K. M., & Sani, R. K. (2020). Environmental Remediation of Antineoplastic Drugs: Present Status, Challenges, and Future Directions. Processes, 8(7), 747. https://doi.org/10.3390/pr8070747