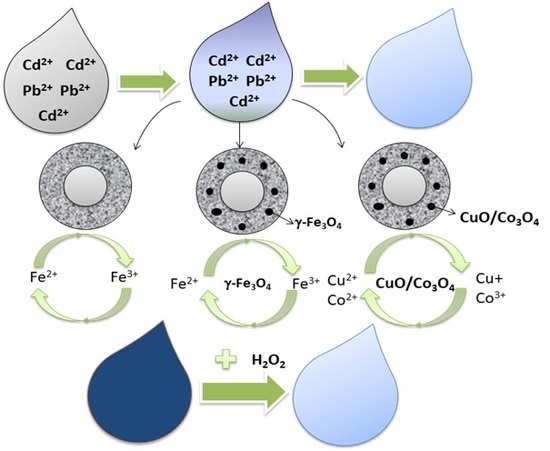
Studies on the Potential of Nonmodified and Metal Oxide-Modified Coal Fly Ash Zeolites For Adsorption of Heavy Metals and Catalytic Degradation of Organics for Waste Water Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Non-Modified and Metal Oxide-Modified CFAZs
2.2. Characterization of Non-Modified and Metal Oxide-Modified CFAZs
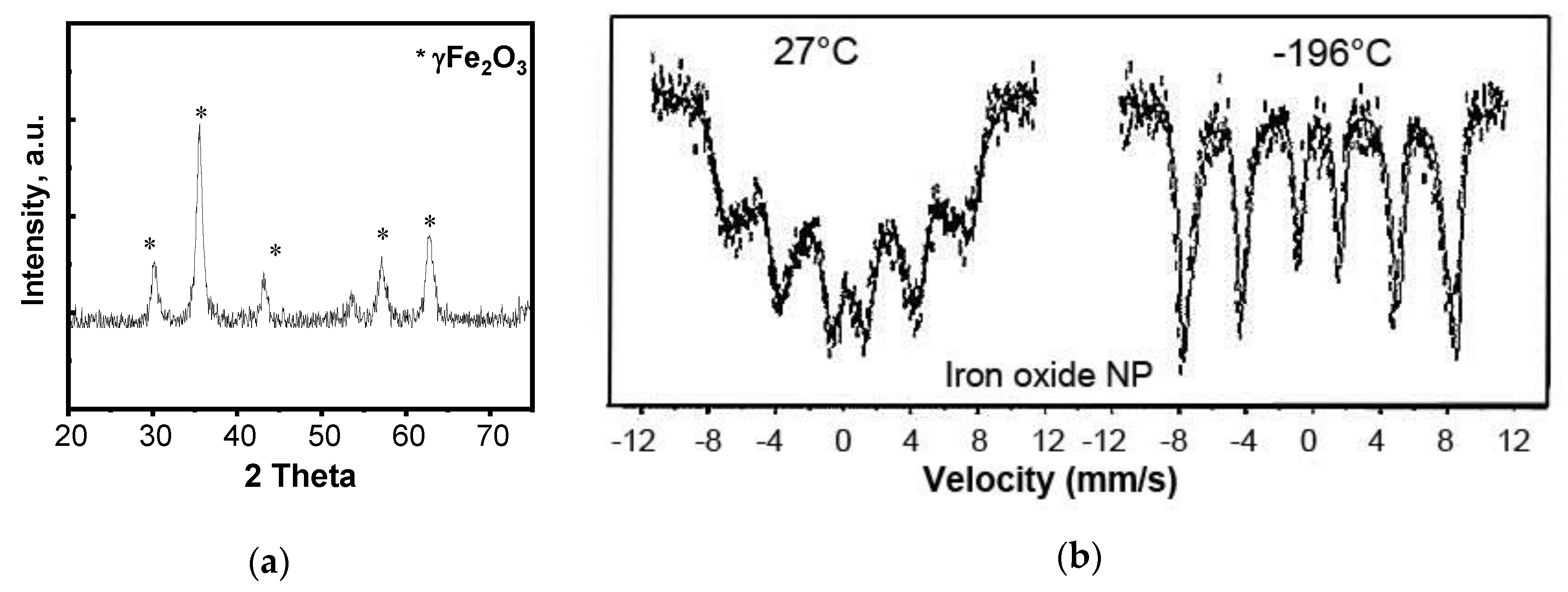
2.3. Magnetite Nanoparticles Characterization
2.4. Heavy Metal Adsorption Tests
2.5. Fenton Oxidation Tests
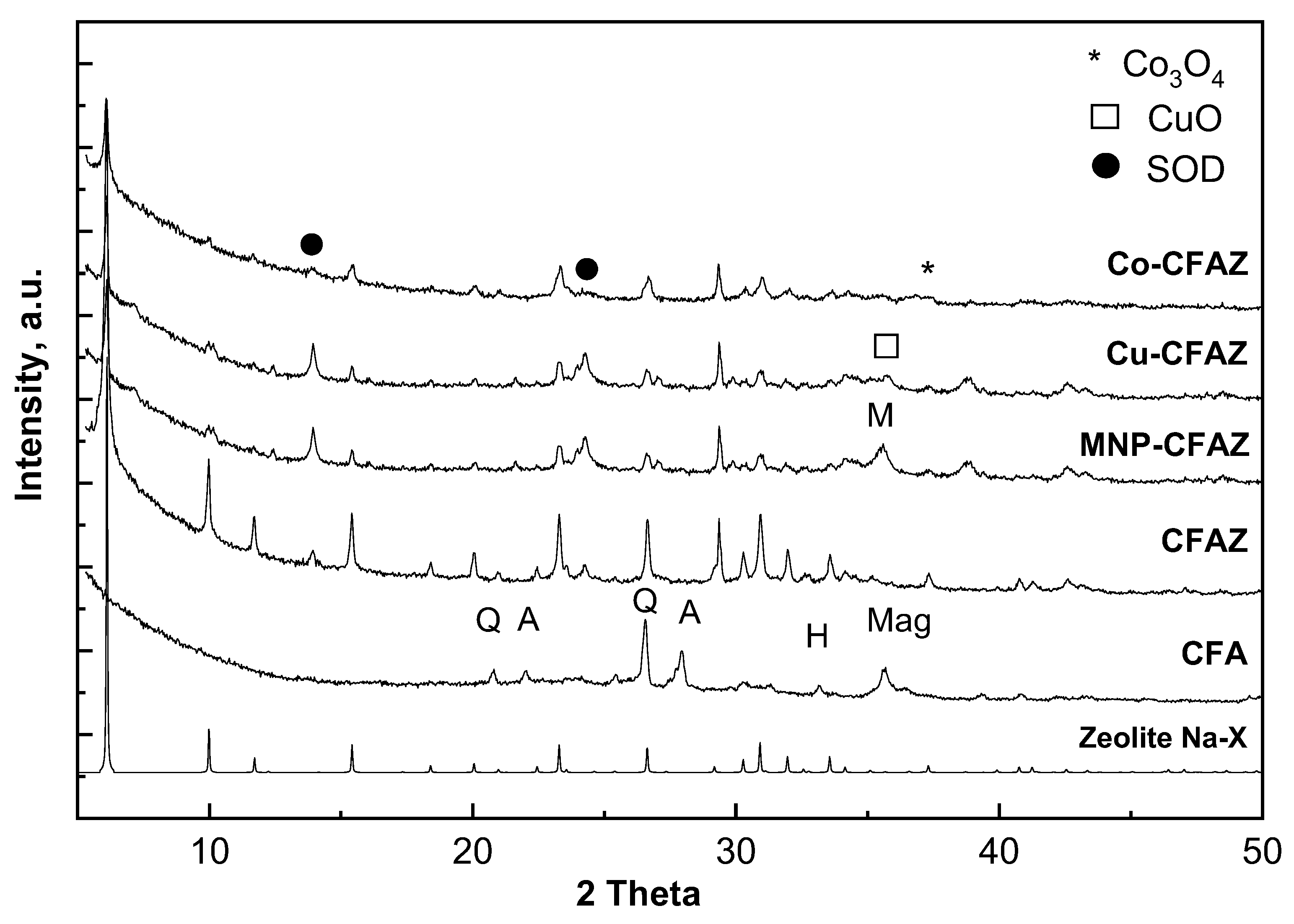
3. Results
3.1. Material Characterization
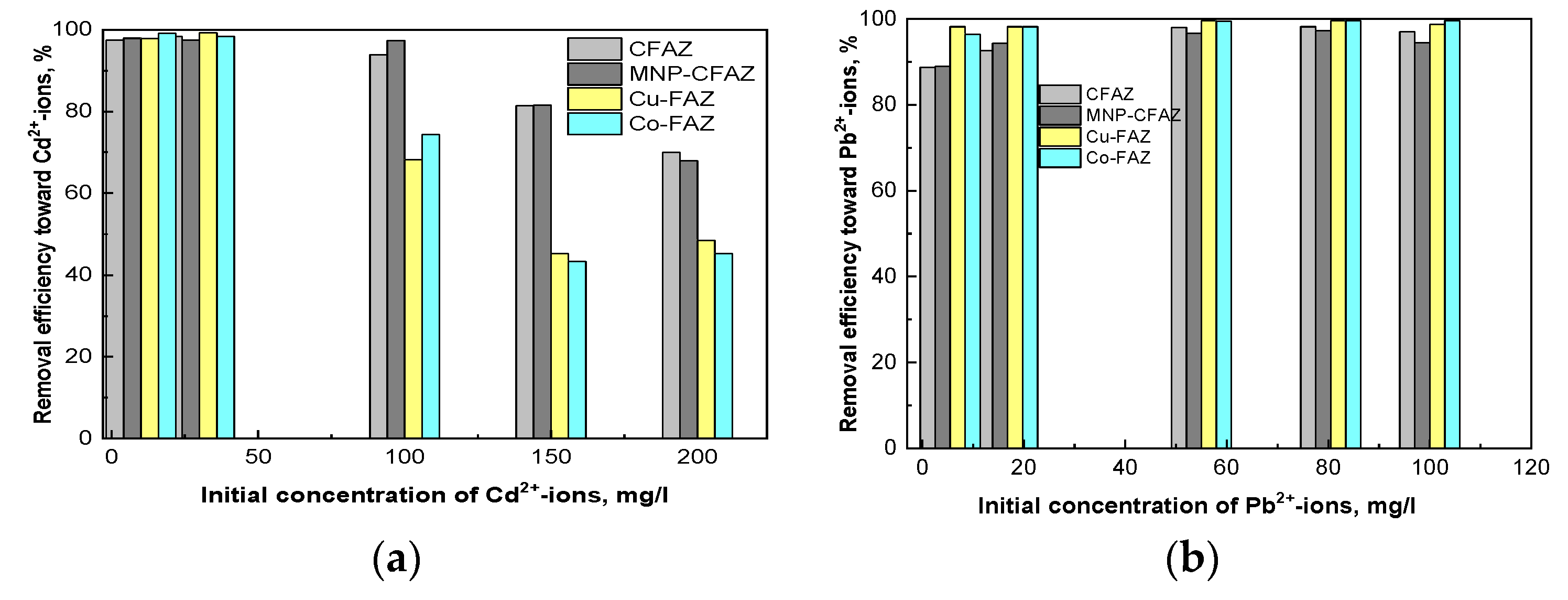
3.2. Adsorption Studies of Cd2+-and Pb2+-ions onto Nonmodified and Modified Coal Fly Ash Zeolites
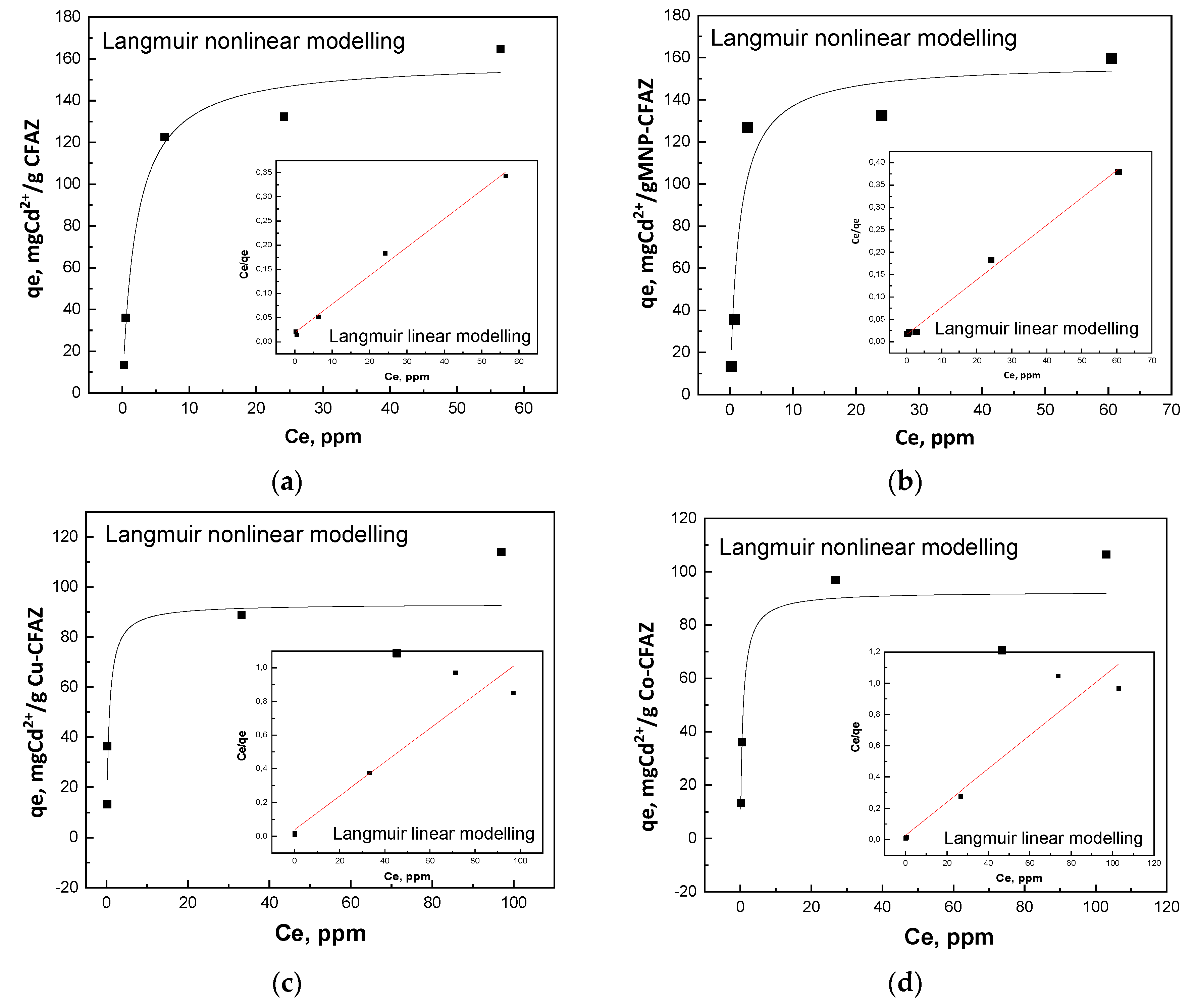
3.2.1. Equilibrium Adsorption Studies
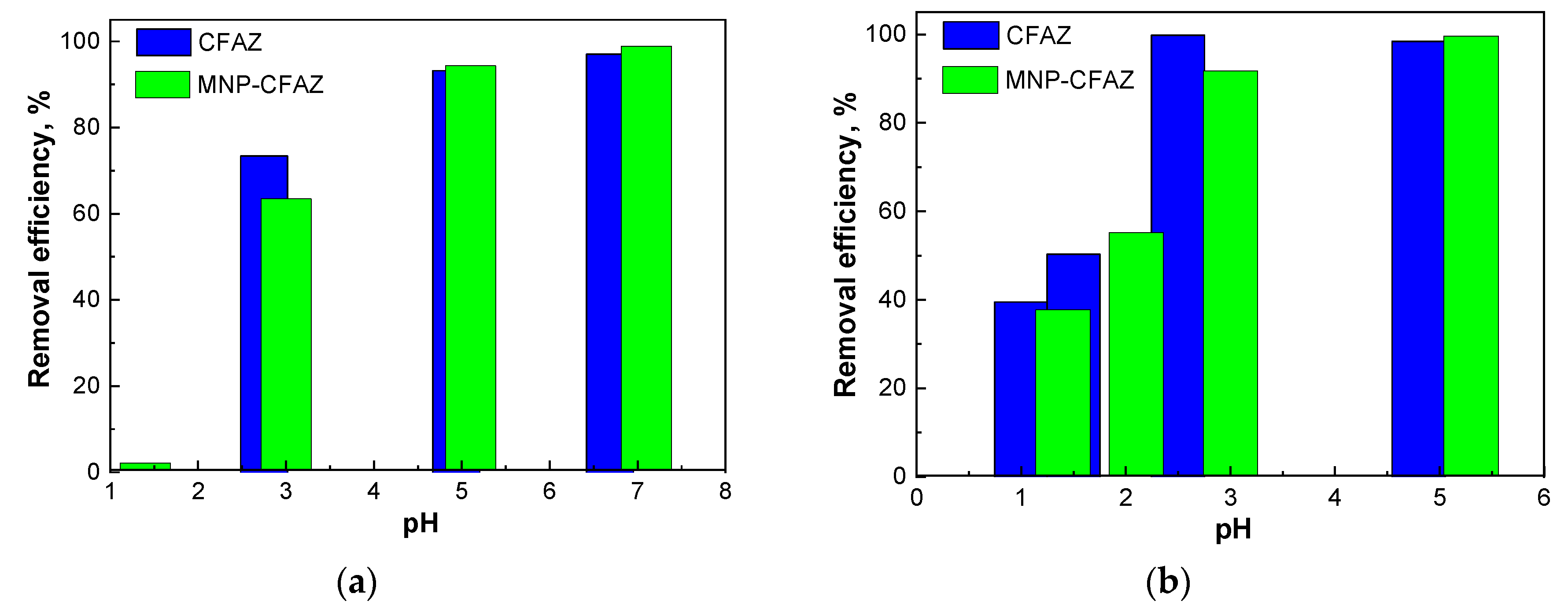
3.2.2. pH-Dependence of Heavy Metal Adsorption onto Nonmodified and Modified CFAZs
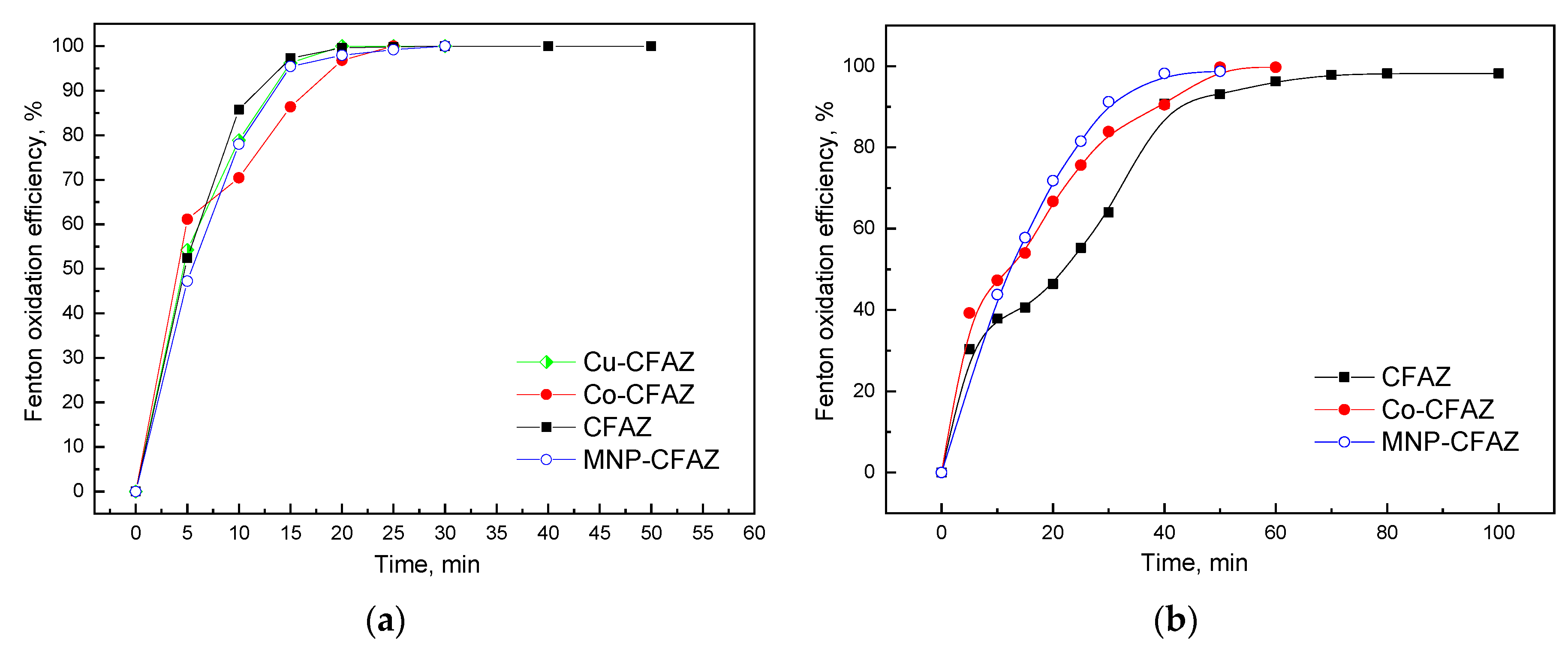
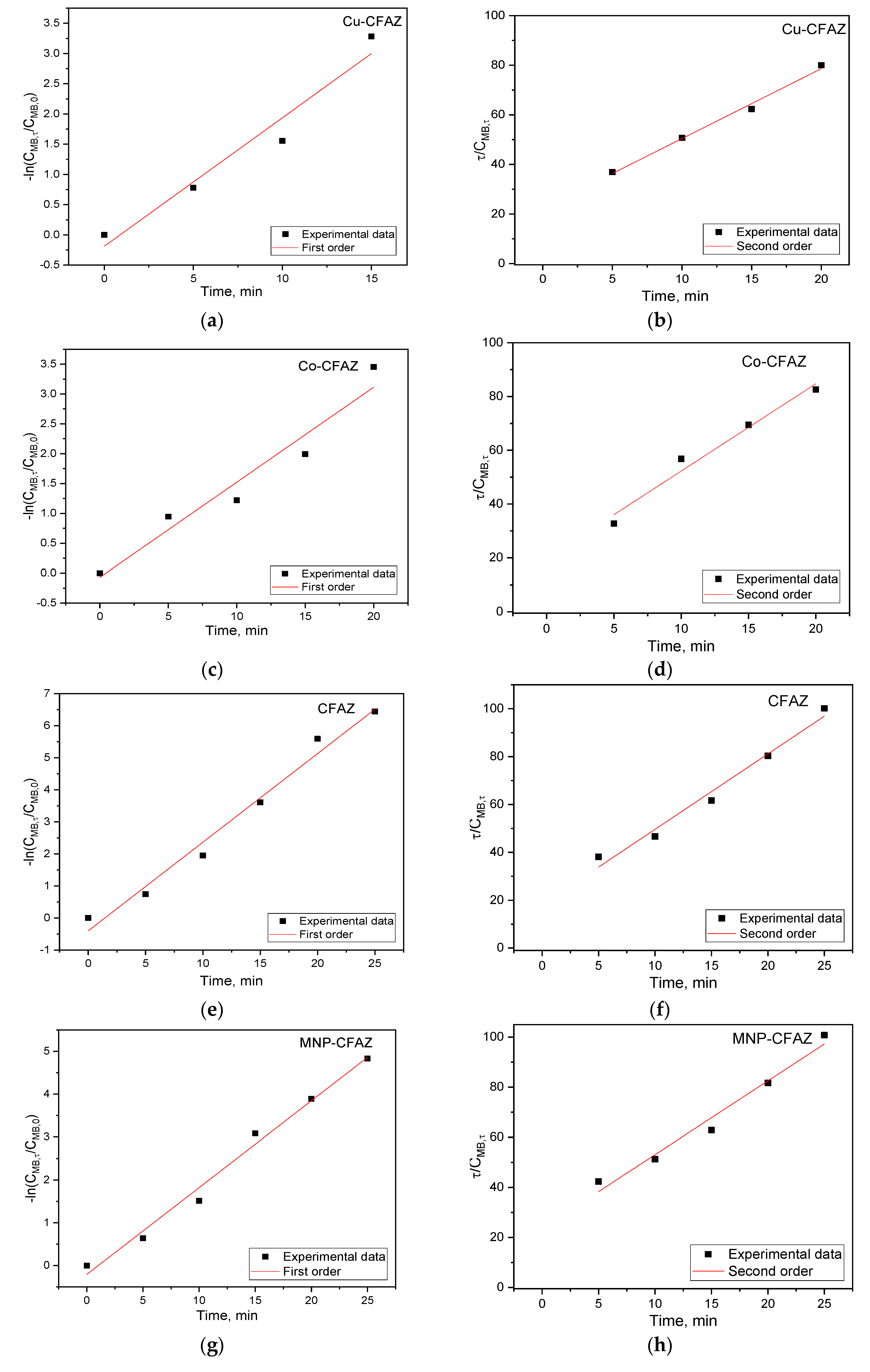
3.2.3. Heterogeneous Fenton Oxidation of Methylene Blue Using Nonmodified and Modified CFAZ
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gollakota, A.R.; Volli, V.; Shu, C.-M. Progressive utilisation prospects of coal fly ash: A review. Sci. Total Environ. 2019, 672, 951–989. [Google Scholar] [CrossRef]
- Yao, Z.; Ji, X.; Sarker, P.; Tang, J.; Ge, L.; Xia, M.; Xi, Y. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Iyer, R.; Scott, J. Power station fly ash—A review of value-added utilization outside of the construction industry. Resour. Conserv. Recycl. 2001, 31, 217–228. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umaña, J.; Alastuey, A.; Hernández, E.; Lopez-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Murayama, N.; Yamamoto, H.; Shibata, J. Mechanism of zeolite synthesis from coal fly ash by alkali hydrothermal reaction. Int. J. Miner. Process. 2002, 64, 1–17. [Google Scholar] [CrossRef]
- Zgureva, D.; Boycheva, S. Synthesis of Highly Porous Micro- and Nanocrystalline Zeolites from Aluminosilicate By-Products. Count. Chem. Biol. Terror. East Eur. Ctries. 2014, 39, 199–204. [Google Scholar]
- Franus, W.; Wdowin, M.; Franus, M. Synthesis and characterization of zeolites prepared from industrial fly ash. Environ. Monit. Assess. 2014, 186, 5721–5729. [Google Scholar] [CrossRef] [PubMed]
- Belviso, C.; Cavalcante, F.; Fiore, S. Synthesis of zeolite from Italian coal fly ash: Differences in crystallization temperature using seawater instead of distilled water. Waste Manag. 2010, 30, 839–847. [Google Scholar] [CrossRef] [PubMed]
- R., C.A.R.; Williams, C.; Roberts, C.L. A comparative study of two methods for the synthesis of fly ash-based sodium and potassium type zeolites. Fuel 2009, 88, 1403–1416. [Google Scholar] [CrossRef]
- Boycheva, S.V.; Zgureva, D.M. Surface studies of fly ash zeolites via adsorption/desorption isotherms. Bulg. Chem. Commun. 2016, 48, 101–107. [Google Scholar]
- Boycheva, S.; Marinov, I.; Miteva, S.; Zgureva, D. Conversion of coal fly ash into nanozeolite Na-X by applying ultrasound assisted hydrothermal and fusion-hydrothermal alkaline activation. Sustain. Chem. Pharm. 2020, 15, 100217. [Google Scholar] [CrossRef]
- Sivalingam, S.; Sen, S. Rapid ultrasound assisted hydrothermal synthesis of highly pure nanozeolite X from fly ash for efficient treatment of industrial effluent. Chemosphere 2018, 210, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Boycheva, S.; Lazarova, H.; Zgureva, D.; Lázár, K.; Szegedi, Á. VOC oxidation and CO2 adsorption on dual adsorption/catalytic system based on fly ash zeolites. Catal. Today 2019. [Google Scholar] [CrossRef]
- Supelano, G.; Cuaspud, J.G.; Moreno-Aldana, L.C.; Ortiz, C.; Trujillo, C.; Palacio, C.; Vargas, C.P.; Gómez, J.M. Synthesis of magnetic zeolites from recycled fly ash for adsorption of methylene blue. Fuel 2020, 263, 116800. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva, D.; Vassilev, V. Kinetic and thermodynamic studies on the thermal behaviour of fly ash from lignite coals. Fuel 2013, 108, 639–646. [Google Scholar] [CrossRef]
- Feng, D.; Aldrich, C.; Tan, H. Removal of heavy metal ions by carrier magnetic separation of adsorptive particulates. Hydrometallurgy 2000, 56, 359–368. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Townsend, R.P.; Coker, E.N. Chapter 11 Ion exchange in zeolites. Adv. Pharmacol. 2001, 137, 467–524. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umaña, J.C.; Juan, R.; Hernandez, S.; Fernandez-Pereira, C.; Ayora, C.; Janssen, M.; García-Martínez, J.; Linares-Solano, A.; et al. Application of zeolitic material synthesised from fly ash to the decontamination of waste water and flue gas. J. Chem. Technol. Biotechnol. 2002, 77, 292–298. [Google Scholar] [CrossRef]
- Visa, M. Synthesis and characterization of new zeolite materials from fly ash for heavy metal removal in advanced water treatment. Powder Technol. 2016, 204, 338–347. [Google Scholar] [CrossRef]
- He, X.; Yao, B.; Xia, Y.; Huang, H.; Gan, Y.; Zhang, W. Coal fly ash derived zeolite for highly efficient removal of Ni2+ inwaste water. Powder Technol. 2020, 367, 40–46. [Google Scholar] [CrossRef]
- Joseph, I.; Tosheva, L.; Doyle, A. Simultaneous removal of Cd(II), Co(II), Cu(II), Pb(II), and Zn(II) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly ash. J. Environ. Chem. Eng. 2020, 8, 103895. [Google Scholar] [CrossRef]
- Koshy, N.; Singh, D. Fly ash zeolites for water treatment applications. J. Environ. Chem. Eng. 2016, 4, 1460–1472. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Wang, L.; Sun, X.; Huang, J. Adsorption of Dye from Wastewater by Zeolites Synthesized from Fly Ash: Kinetic and Equilibrium Studies. Chin. J. Chem. Eng. 2009, 17, 513–521. [Google Scholar] [CrossRef]
- Cunico, P.; Kumar, A.; Fungaro, D. Adsorption of dyes from simulated textile wastewater onto modified nanozeolite from coal fly ash. J. Nanosci. Nanoeng. 2015, 1, 148–161. [Google Scholar]
- Olmos, R.G.; Roland, U.; Toufar, H.; Kopinke, F.-D.; Georgi, A. Fe-zeolites as catalysts for chemical oxidation of MTBE in water with H2O2. Appl. Catal. B 2009, 89, 356–364. [Google Scholar] [CrossRef]
- Arimi, M.M. Modified natural zeolite as heterogeneous Fenton catalyst in treatment of recalcitrants in industrial effluent. Prog. Nat. Sci. 2017, 27, 275–282. [Google Scholar] [CrossRef]
- Olmos, R.G.; Martín, M.J.; Georgi, A.; Kopinke, F.-D.; Oller, I.; Malato, S. Fe-zeolites as heterogeneous catalysts in solar Fenton-like reactions at neutral pH. Appl. Catal. B 2012, 125, 51–58. [Google Scholar] [CrossRef]
- Singh, L.; Rekha, P.; Chand, S. Cu-impregnated zeolite Y as highly active and stable heterogeneous Fenton-like catalyst for degradation of Congo red dye. Sep. Purif. Technol. 2016, 170, 321–336. [Google Scholar] [CrossRef]
- Wang, N.; Xu, H.; Li, S. A microwave-activated coal fly ash catalyst for the oxidative elimination of organic pollutants in a Fenton-like process. RSC Adv. 2019, 9, 7747–7756. [Google Scholar] [CrossRef]
- Ramírez, H.; Núñez, M.M.G.; Bogoya, A.B.; Gomez, D.F.B.; Ramos, C.; Di Luca, C.; Inchaurrondo, N.; Haure, P. Synthesis of coal fly ash zeolite for the catalytic wet peroxide oxidation of Orange II. Environ. Sci. Pollut. Res. 2018, 26, 4277–4287. [Google Scholar] [CrossRef]
- Subbulekshmi, N.; Subramanian, E. Nano CuO immobilized fly ash zeolite Fenton-like catalyst for oxidative degradation of p -nitrophenol and p -nitroaniline. J. Environ. Chem. Eng. 2017, 5, 1360–1371. [Google Scholar] [CrossRef]
- Boycheva, S.; Marinov, I.; Lazarova, H.; Zgureva, D.; Václavíková, M.; Popova, M. Cobalt- and copper-modified fly ash nanozeolites for environmental protection systems. In Proceedings of the CEST 2019, Rhodes, Greece, 4–7 September 2019; p. 563. [Google Scholar]
- Simate, G.S.; Maledi, N.; Ochieng, A.; Ndlovu, S.; Zhang, J.; Walubita, L.F. Coal-based adsorbents for water and wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 2291–2312. [Google Scholar] [CrossRef]
- Sivalingam, S.; Kella, T.; Maharana, M.; Sen, S. Efficient sono-sorptive elimination of methylene blue by fly ash-derived nano-zeolite X: Process optimization, isotherm and kinetic studies. J. Clean. Prod. 2019, 208, 1241–1254. [Google Scholar] [CrossRef]
- Tauanov, Z.; Tsakiridis, P.; Mikhalovsky, S.; Inglezakis, V.J. Synthetic coal fly ash-derived zeolites doped with silver nanoparticles for mercury (II) removal from water. J. Environ. Manag. 2018, 224, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wan, Z.; Daniels, J.; Li, Z.; Xiao, G.; Yu, J.; Xu, D.; Guo, H.; Zhang, D.; May, E.F.; et al. Synthesis of high quality zeolites from coal fly ash: Mobility of hazardous elements and environmental applications. J. Clean. Prod. 2018, 202, 390–400. [Google Scholar] [CrossRef]
- Rebodos, R.L.; Vikesland, P.J. Effects of Oxidation on the Magnetization of Nanoparticulate Magnetite. Langmuir 2010, 26, 16745–16753. [Google Scholar] [CrossRef]
- Kim, W.; Suh, C.-Y.; Cho, S.-W.; Roh, K.-M.; Kwon, H.; Song, K.; Shon, I.-J. A new method for the identification and quantification of magnetite–maghemite mixture using conventional X-ray diffraction technique. Talanta 2012, 94, 348–352. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Sun, X.; Wang, L.; Sun, X. Evaluation of zeolites synthesized from fly ash as potential adsorbents for wastewater containing heavy metals. J. Environ. Sci. 2009, 21, 127–136. [Google Scholar] [CrossRef]
- Belova, T. Adsorption of heavy metal ions (Cu2+, Ni2+, Co2+ and Fe2+) from aqueous solutions by natural zeolite. Heliyon 2019, 5, e02320. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.B.; Ma, Y.; Chen, L.; Xian, K. Adsorption of aqueous Cd2+, Pb2+, Cu2+ ions by nano-hydroxyapatite: Single- and multi-metal competitive adsorption study. Geochem. J. 2010, 44, 233–239. [Google Scholar] [CrossRef]
- El-Dib, F.I.; Mohamed, D.E.; El-Shamy, O.A.A.; Mishrif, M.R. Study the adsorption properties of magnetite nanoparticles in the presence of different synthesized surfactants for heavy metal ions removal. Egypt. J. Pet. 2020, 29, 1–7. [Google Scholar] [CrossRef]
- Ferroudj, N.; Nzimoto, J.; Davidson, A.; Talbot, D.; Briot, E.; Dupuis, V.; Bée, A.; Medjram, M.S.; Abramson, S. Maghemite nanoparticles and maghemite/silica nanocomposite microspheres as magnetic Fenton catalysts for the removal of water pollutants. Appl. Catal. B 2013, 136, 9–18. [Google Scholar] [CrossRef]
- Fopase, R.; Pandey, L.M. Iron oxide magnetic nanomaterials for biomedical applications. In Magnetochemisty Materials and Applications; Boddula, I.R., Asiri, A.M., Eds.; Materials Research Forum LLC.: Millesville, PA, USA, 2020; Volume 66, pp. 276–322. [Google Scholar]
- Guivar, J.A.R.; Sadrollahi, E.; Menzel, D.; Fernandes, E.G.R.; López, E.O.; Torres, M.M.; Arsuaga, J.M.; Arencibia, A.; Litterst, F.J. Magnetic, structural and surface properties of functionalized maghemite nanoparticles for copper and lead adsorption. RSC Adv. 2017, 7, 28763–28779. [Google Scholar] [CrossRef]
- Pignatello, J.J. Dark and photoassisted Fe3+-catalysed degradation of chlorophenoxy herbicides by hydrogen peroxide. Environ. Sci. Technol. 1992, 26, 944. [Google Scholar] [CrossRef]
- Rusevova, K.; Kopinke, F.-D.; Georgi, A. Nano-sized magnetic iron oxides as catalysts for heterogeneous Fenton-like reactions—Influence of Fe(II)/Fe(III) ratio on catalytic performance. J. Hazard. Mater. 2012, 241, 433–440. [Google Scholar] [CrossRef]



| Sample | SBET, m2/g | Smicro, m2/g | Sextern, m2/g | Vtotal, cm3/g | Vmicro, cm3/g | Vmeso, cm3/g | dmicro, Å | dmeso, Å |
|---|---|---|---|---|---|---|---|---|
| CFAZ | 486 | 334 | 166 | 0.307 | 0.133 | 0.174 | 14 | 42 |
| MNP-CFAZ | 264 | 137 | 127 | 0.246 | 0.057 | 0.189 | 14 | 49 |
| Cu-CFAZ | 67 | 20 | 47 | 0.080 | 0.009 | 0.071 | 13 | 51 |
| Co-CFAZ | 213 | 96 | 117 | 0.200 | 0.040 | 0.160 | 13 | 48 |
| Sample | 300 K | 77 K | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Comp | IS | QS | MHF | RI | IS | QS | MHF | RI | |
| Iron oxide | Sext (1) | 0.35 | - | 45.6 | 24 | 0.44 | - | 50.5 | 54 |
| MNP | Sext (2) | 0.37 | - | 38.5 | 76 | 0.41 | - | 46.9 | 46 |
| Thermodynamic Models | Model | CFAZ | MNP-CFAZ | Cu-CFAZ | Co-CFAZ |
|---|---|---|---|---|---|
| Parameters | |||||
| Langmuir linear | Qsat, mg/g | 170.1 | 163.7 | 99.8 | 93.9 |
| KL, L/mg | 0.3 | 0.4 | 0.3 | 0.4 | |
| R2 | 0.992 | 0.995 | 0.909 | 0.922 | |
| Langmuir nonlinear | Qsat, mg/g | 159.1 | 157.4 | 93.2 | 92.5 |
| KL, L/mg | 0.5 | 0.7 | 1.6 | 1.4 | |
| R2 | 0.978 | 0.928 | 0.825 | 0.886 | |
| Freundlich | Kf, mg/g | 52.9 | 60.2 | 35.7 | 38.9 |
| n | 3.4 | 4.0 | 4.4 | 4.9 | |
| R2 | 0.903 | 0.781 | 0.851 | 0.820 | |
| Langmuir–Freundlich | Qsat, mg/g | 161.8 | 139.7 | - | 93.0 |
| KL, L/mg | 0.5 | 21.5 | 1.3 | ||
| n | 1.0 | 14.4 | 0.94 | ||
| R2 | 0.978 | 0.953 | 0.886 | ||
| Temkin | b | 88.5 | 94.3 | 204.3 | 209.7 |
| RT/b | 27.7 | 26.0 | 12.0 | 11.7 | |
| At, L/g | 7.1 | 0.8 | 35.2 | 39.8 | |
| R2 | 0.975 | 0.874 | 0.846 | 0.864 |
| Sample | Pseudo First Order | Pseudo Second Order | ||||
|---|---|---|---|---|---|---|
| K1, min−1 | τ1/2, min−1 | R2 | K2,M−1min−1 | τ1/2, min−1 | R2 | |
| CFAZ | 0.277 | 2.50 | 0.982 | 3.154 | 0.016 | 0.979 |
| MNP-CFAZ | 0.203 | 3.41 | 0.987 | 2.946 | 0.017 | 0.975 |
| Cu-CFAZ | 0.213 | 3.25 | 0.954 | 2.819 | 0.018 | 0.993 |
| Co-CFAZ | 0.159 | 4.36 | 0.945 | 3.247 | 0.015 | 0.973 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boycheva, S.; Zgureva, D.; Miteva, S.; Marinov, I.; Behunová, D.M.; Trendafilova, I.; Popova, M.; Václaviková, M. Studies on the Potential of Nonmodified and Metal Oxide-Modified Coal Fly Ash Zeolites For Adsorption of Heavy Metals and Catalytic Degradation of Organics for Waste Water Recovery. Processes 2020, 8, 778. https://doi.org/10.3390/pr8070778
Boycheva S, Zgureva D, Miteva S, Marinov I, Behunová DM, Trendafilova I, Popova M, Václaviková M. Studies on the Potential of Nonmodified and Metal Oxide-Modified Coal Fly Ash Zeolites For Adsorption of Heavy Metals and Catalytic Degradation of Organics for Waste Water Recovery. Processes. 2020; 8(7):778. https://doi.org/10.3390/pr8070778
Chicago/Turabian StyleBoycheva, Silviya, Denitza Zgureva, Simona Miteva, Ivan Marinov, Dominika Marcin Behunová, Ivalina Trendafilova, Margarita Popova, and Miroslava Václaviková. 2020. "Studies on the Potential of Nonmodified and Metal Oxide-Modified Coal Fly Ash Zeolites For Adsorption of Heavy Metals and Catalytic Degradation of Organics for Waste Water Recovery" Processes 8, no. 7: 778. https://doi.org/10.3390/pr8070778
APA StyleBoycheva, S., Zgureva, D., Miteva, S., Marinov, I., Behunová, D. M., Trendafilova, I., Popova, M., & Václaviková, M. (2020). Studies on the Potential of Nonmodified and Metal Oxide-Modified Coal Fly Ash Zeolites For Adsorption of Heavy Metals and Catalytic Degradation of Organics for Waste Water Recovery. Processes, 8(7), 778. https://doi.org/10.3390/pr8070778