Evaluation of the Interactive Effect Pretreatment Parameters via Three Types of Microwave-Assisted Pretreatment and Enzymatic Hydrolysis on Sugar Yield
Abstract
:1. Introduction
2. Material and Methods
2.1. Substrate
2.2. Enzymes and Chemicals
2.3. Microwave-Assisted Pretreatment
2.4. Enzymatic Hydrolysis
2.5. Sugar Analysis
3. Results and Discussion
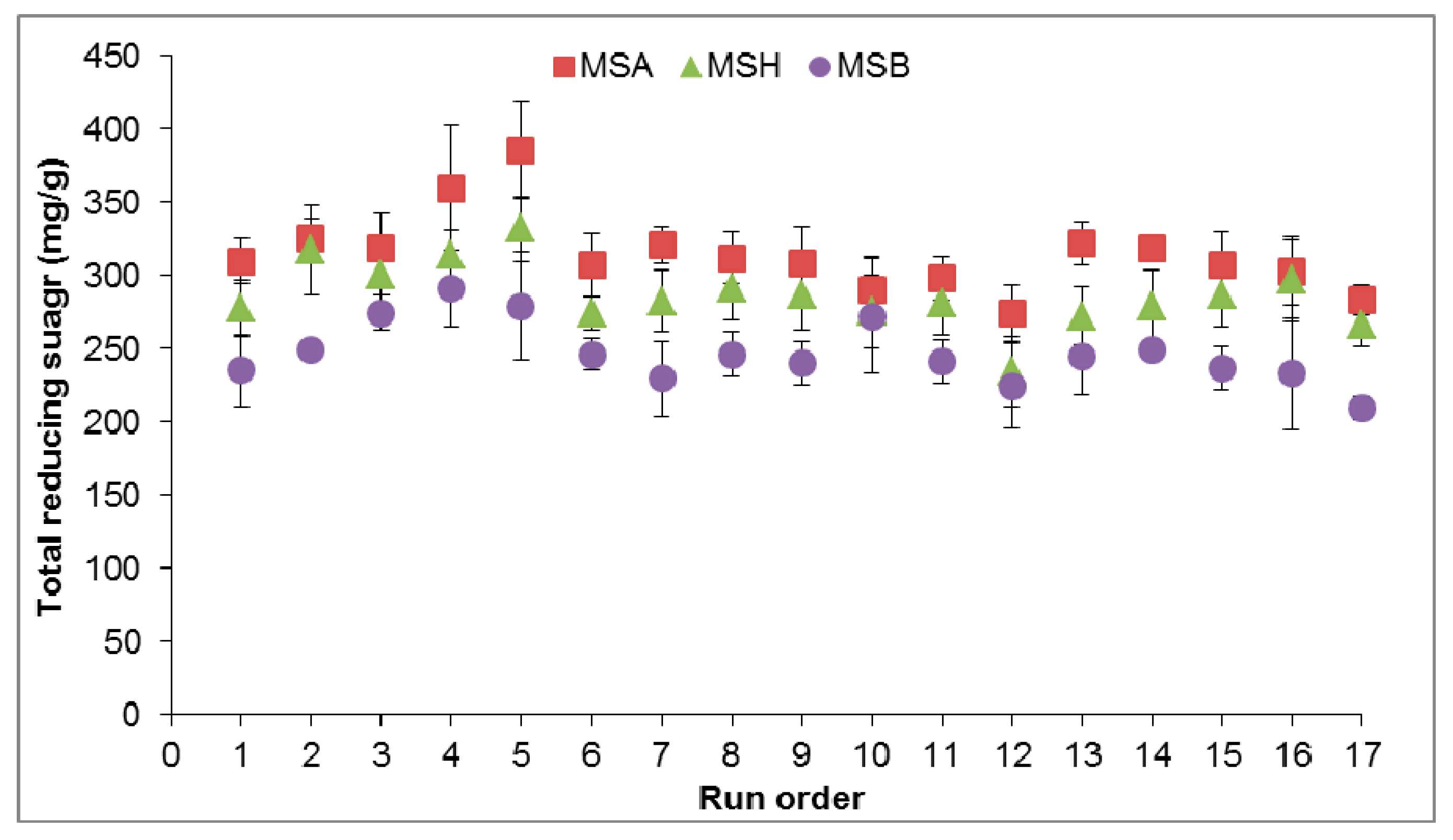
3.1. Effect of Microwave-Assisted Pretreatment Type on Sugar Yield
3.2. Analysis Effect of Microwave-Assisted Pretreatment Parameters Using ANOVA
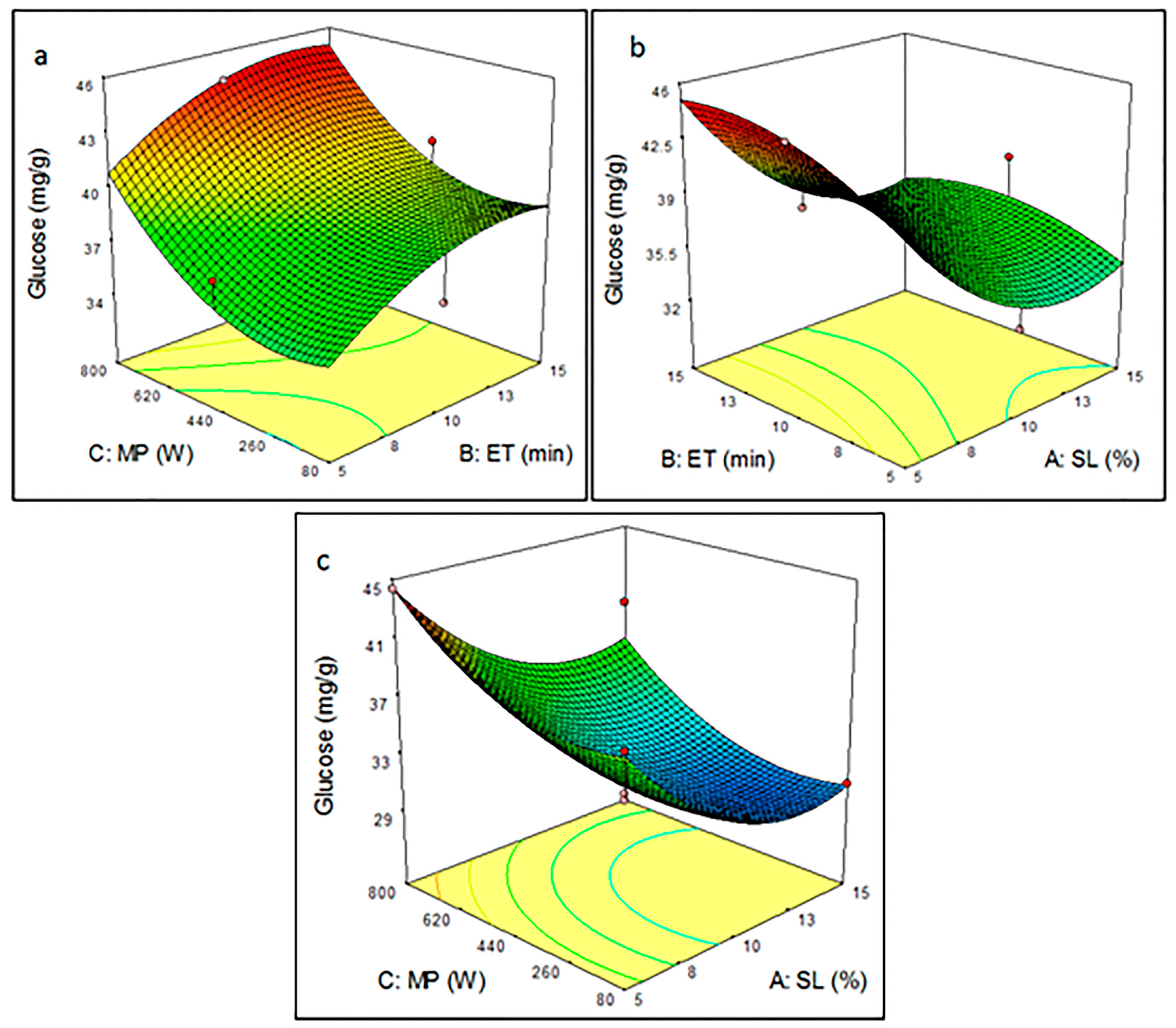
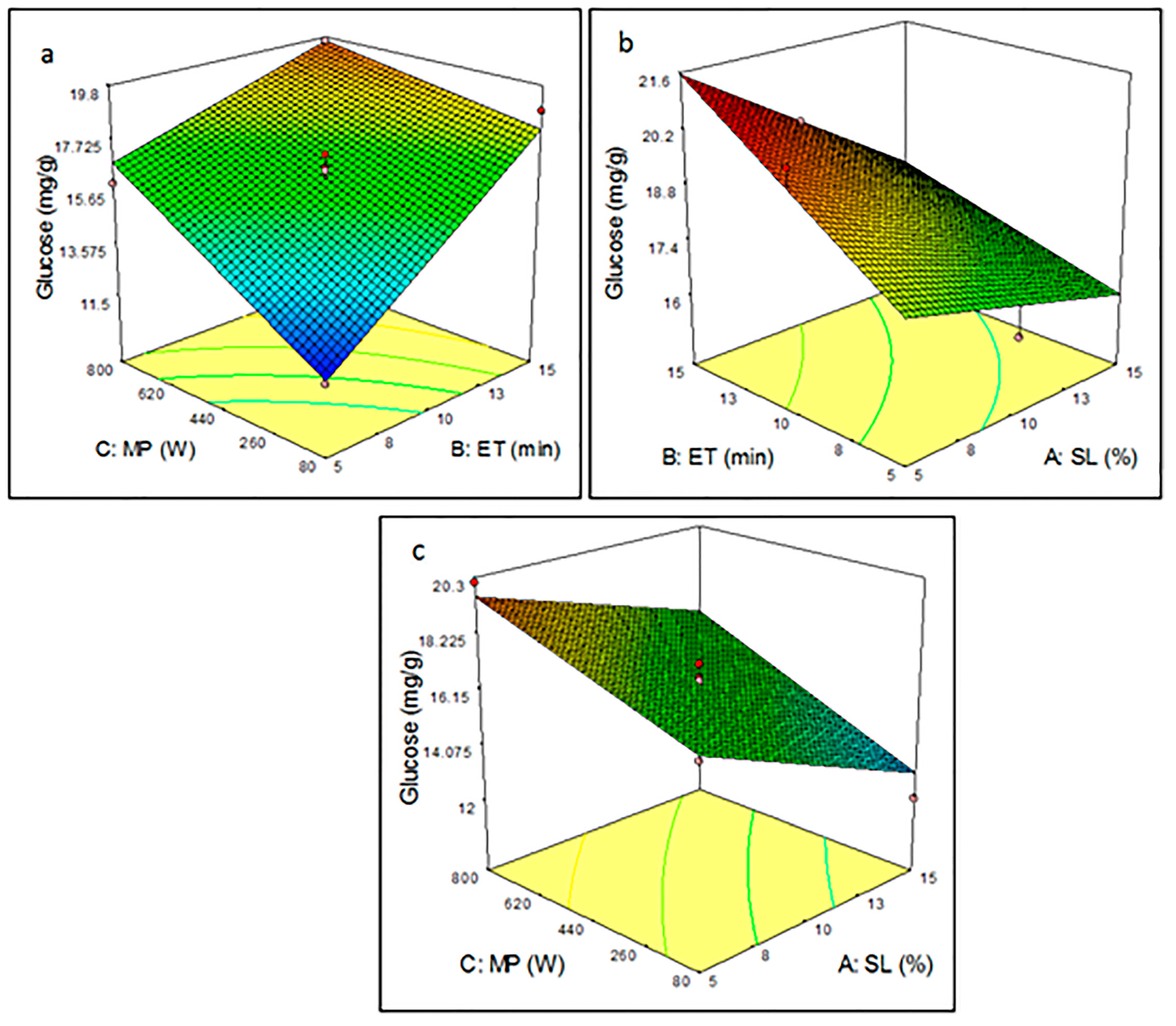
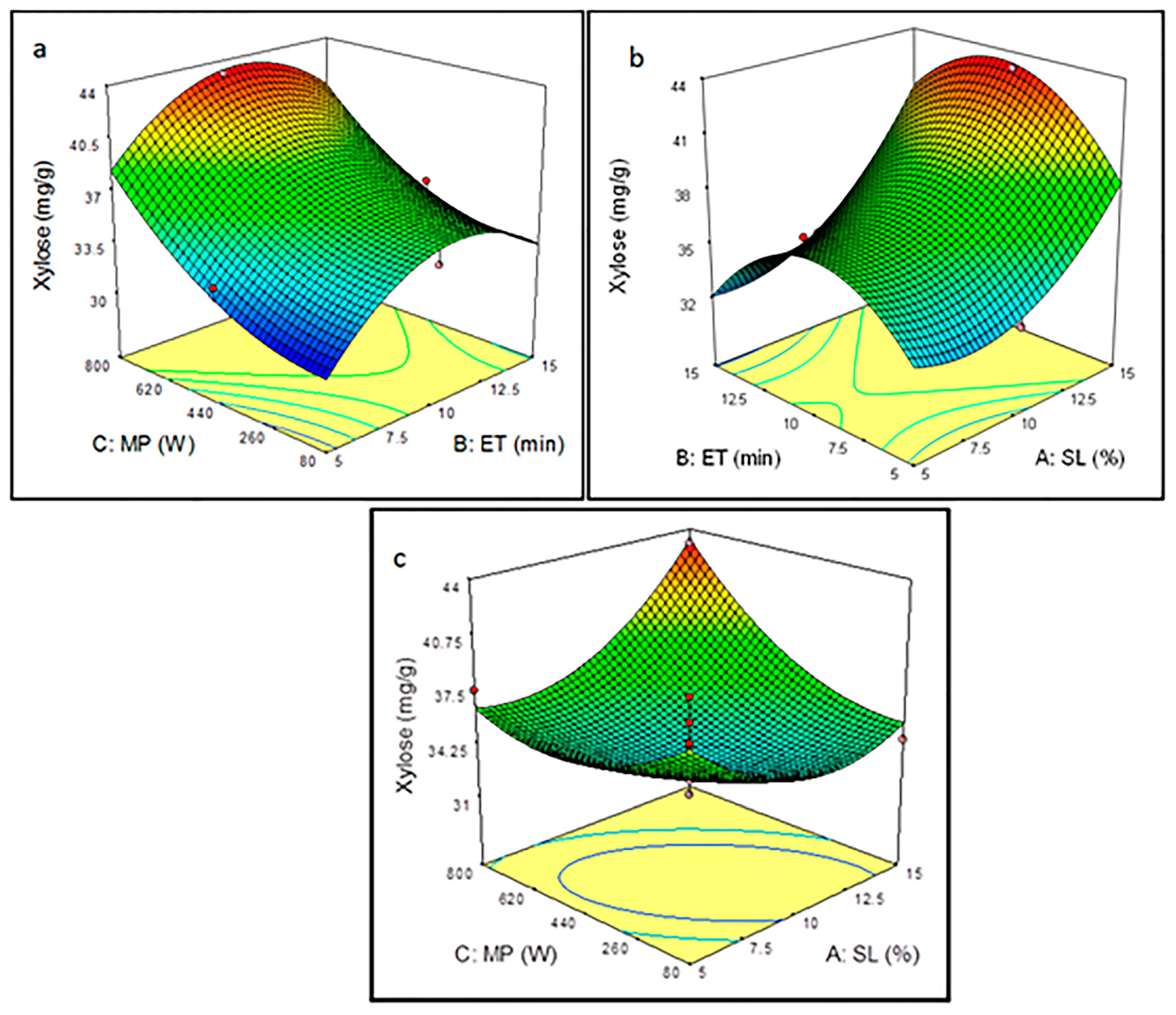
3.3. Analysis Effect of Microwave-Assisted Pretreatment Parameters Using RSM Plots
3.3.1. Pretreatment Parameters on Glucose Yield
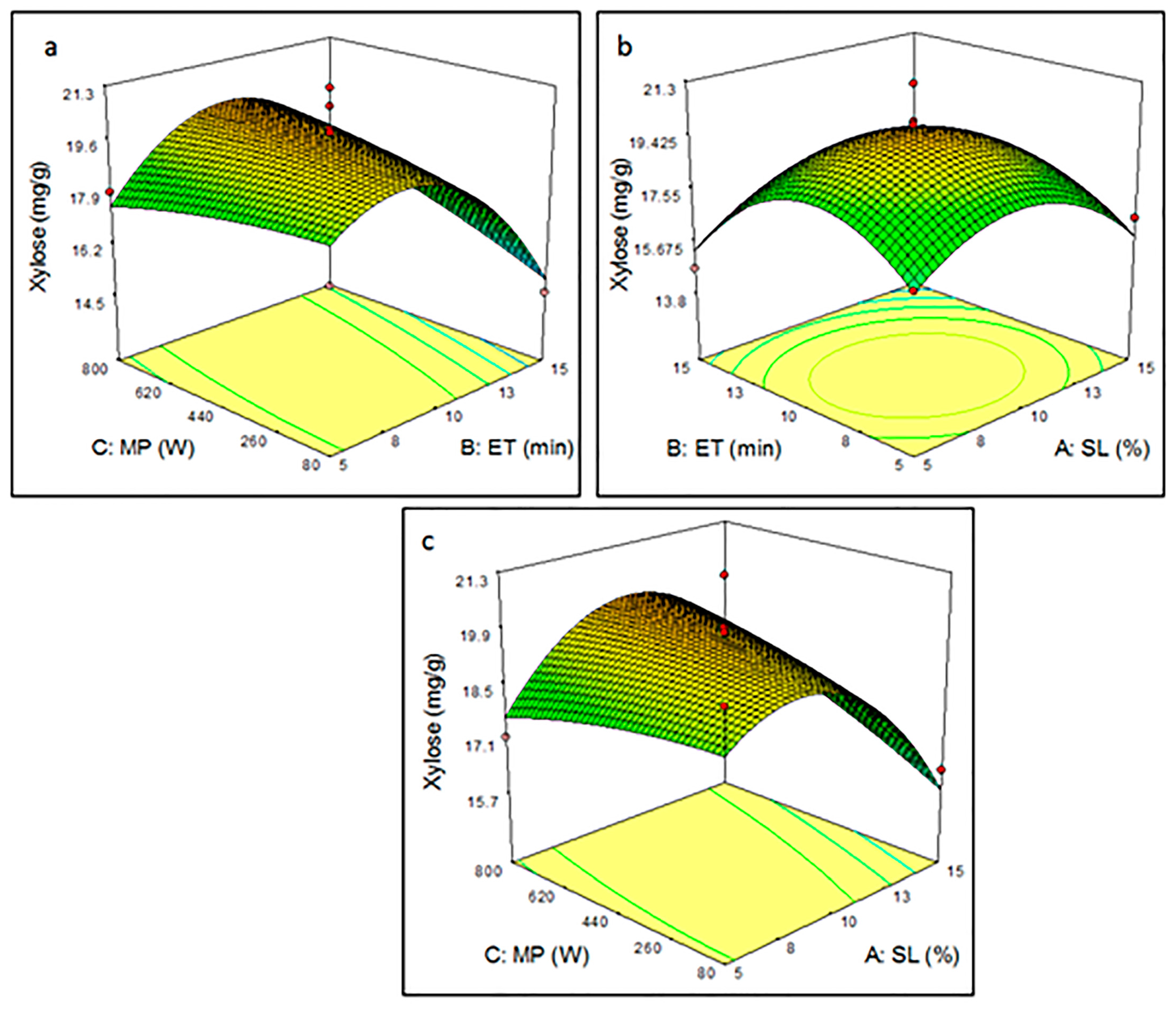
3.3.2. Effect of Pretreatment Parameters on Xylose Yield
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ethaib, S. Solid Waste Situation in Thi-Qar Governorate. In IOP Conference Series: Material Science Engineering; IOP Publishing: Bristol, UK, 2019; p. 584012023. [Google Scholar]
- Ethaib, S.; Omar, R.; Mazlina, M.; Radiah, A.; Syafiie, S.; Harun, M.Y. Effect of microwave-assisted acid or alkali pretreatment on sugar release from Dragon fruit foliage. Int. Food Res. J. 2016, 23, S149–S154. [Google Scholar]
- Ethaib, S.; Erabee, I.K.; Abdulsahib, A.A. Removal of Methylene Blue Dye from Synthetic Wastewater using Kenaf Core and Activated Carbon. Int. J. Eng. Technol. 2018, 7, 909–913. [Google Scholar] [CrossRef] [Green Version]
- Ethaib, S.; Omar, R.; Mustapa Kamal, S.M.; Awang Biak, D.R.; Syam, S.; Harun, M.Y. Microwave-assisted pretreatment of sago palm bark. J. Wood Chem. Technol. 2017, 37, 26–42. [Google Scholar] [CrossRef]
- Jørgensen, H.; Kristensen, J.B.; Felby, C. Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities. Biofuels Bioprod. Biorefin. 2007, 1, 119–134. [Google Scholar] [CrossRef]
- Öhgren, K.; Bura, R.; Saddler, J.; Zacchi, G. Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover. Bioresour. Technol. 2007, 98, 2503–2510. [Google Scholar] [CrossRef]
- Blue, D.; Fortela, D.L.; Holmes, W.; LaCour, D.; LeBoeuf, S.; Stelly, C.; Revellame, E.D. Valorization of Industrial Vegetable Waste Using Dilute HCl Pretreatment. Processes 2019, 7, 853. [Google Scholar] [CrossRef]
- Han, L.; Feng, J.; Zhang, S.; Ma, Z.; Wang, Y.; Zhang, X. Alkali pretreated of wheat straw and its enzymatic hydrolysis. Braz. J. Microbiol. 2012, 43, 53–61. [Google Scholar] [CrossRef]
- Min, D.Y.; Xu, R.S.; Hou, Z.; Lv, J.Q.; Huang, C.X.; Jin, Y.C.; Yong, Q. Minimizing inhibitors during pretreatment while maximizing sugar production in enzymatic hydrolysis through a two-stage hydrothermal pretreatment. Cellulose 2015, 22, 1253–1261. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Mustapa Kamal, S.M.; Awang Biak, D.R. Comparison of sodium hydroxide and sodium bicarbonate pretreatment methods for characteristic and enzymatic hydrolysis of sago palm bark. Energy Source Part A Recovery Util. Environ. Eff. 2020, 1–11. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Kamal, S.M.; Biak, D.A. Microwave-assisted pretreatment of lignocellulosic biomass: A review. J. Eng. Sci. Technol. 2015, 2, 97–109. [Google Scholar]
- Mokhtar, N.M.; Ethaib, S.; Omar, R. Effects of microwave absorbers on the products of microwave pyrolysis of oily sludge. J. Eng. Sci. Technol. 2018, 13, 3313–3330. [Google Scholar]
- Rahimi, M.A.; Omar, R.; Ethaib, S.; Mazlina, M.S.; Biak, D.A.; Aisyah, R.N. Microwave-Assisted Extraction of Lipid from Fish Waste. In IOP Conference Series: Material Science Engineering; IOP Publishing: Bristol, UK, 2017; Volume 206, p. 012096. [Google Scholar]
- Komolwanich, T.; Tatijarern, P.; Prasertwasu, S.; Khumsupan, D.; Chaisuwan, T.; Luengnaruemitchai, A. Comparative potentiality of Kans grass (Saccharum spontaneum) and Giant reed (Arundo donax) as lignocellulosic feedstocks for the release of monomeric sugars by microwave/chemical pretreatment. Cellulose 2014, 21, 1327–1340. [Google Scholar] [CrossRef]
- Alexandre, A.M.; Matias, A.A.; Bronze, M.R.; Cocero, M.J.; Mato, R. Phenolic Compounds Extraction of Arbutus unedo L.: Process Intensification by Microwave Pretreatment. Processes 2020, 8, 298. [Google Scholar] [CrossRef] [Green Version]
- Ethaib, S.; Omar, R.; Mazlina, M.K.S.; Radiah, A.B.D.; Syafiie, S. Microwave-assisted Dilute Acid Pretreatment and Enzymatic Hydrolysis of Sago Palm Bark. BioResources 2016, 11, 5687–5702. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Liu, W.; Chen, X.; Wu, Y.; Yu, Z. Enhanced enzymatic saccharification of rice straw by microwave pretreatment. Bioresour. Technol. 2009, 100, 1279–1284. [Google Scholar] [CrossRef]
- Vani, S.; Binod, P.; Kuttiraja, M.; Sindhu, R.; Sandhya, S.V.; Preeti, V.E.; Pandey, A. Energy requirement for alkali assisted microwave and high pressure reactor pretreatments of cotton plant residue and its hydrolysis for fermentable sugar production for biofuel application. Bioresour. Technol. 2012, 112, 300–307. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W.L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Mazlina, M.K.S.; Radiah, A.B.D.; Syafiie, S. Development of a hybrid PSO–ANN model for estimating glucose and xylose yields for microwave-assisted pretreatment and the enzymatic hydrolysis of lignocellulosic biomass. Neural Comput. Appl. 2018, 30, 1111–1121. [Google Scholar] [CrossRef]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crop. Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- Chadni, M.; Bals, O.; Ziegler-Devin, I.; Brosse, N.; Grimi, N. Microwave-assisted extraction of high-molecular-weight hemicelluloses from spruce wood. Comptes Rendus Chim. 2019, 22, 574–584. [Google Scholar] [CrossRef]
- Adney, B.; Baker, J. Measurement of Cellulase Activities. In Laboratory Analytical Procedure; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 1996; Volume 6. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Jeffries, T.W.; Yang, V.W.; Davis, M.W. Comparative study of xylanase kinetics using dinitrosalicylic, arsenomolybdate, and ion chromatographic assays. Appl. Biochem. Biotechnol. 1998, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, W.C.; Li, F.X.; Yu, J.Y. Swelling and dissolution of cellulose in NaOH aqueous solvent systems. Cellul. Chem. Technol. 2013, 47, 671–679. [Google Scholar]
- Box, G.E.; Hunter, J.S. Multi-factor experimental designs for exploring response surfaces. Ann. Math. Stat. 1957, 1, 195–241. [Google Scholar] [CrossRef]
- Manaso, J.; Luengnaruemitchai, A.; Wongkasemjit, S. Optimization of two-stage pretreatment combined with microwave radiation using response surface methodology. World Academy of Science, Engineering and Technology World Academy of Science, Engineering and Technology (WASET). Int. J. Chem. Mol. Eng. 2013, 76, 599. [Google Scholar]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave assisted extraction—An innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Lu, X.; Xi, B.; Zhang, Y.; Angelidaki, I. Microwave pretreatment of rape straw for bioethanol production: Focus on energy efficiency. Bioresour. Technol. 2011, 102, 7937–7940. [Google Scholar] [CrossRef]
- Kabel, M.A.; Bos, G.; Zeevalking, J.; Voragen, A.G.; Schols, H.A. Effect of pretreatment severity on xylan solubility and enzymatic breakdown of the remaining cellulose from wheat straw. Bioresour. Technol. 2007, 98, 2034–2042. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Effect of xylan and lignin removal by batch and flowthrough pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnol. Bioeng. 2004, 86, 88–98. [Google Scholar] [CrossRef]
- Keshwani, D.R. Microwave Pretreatment of Switchgrass for Bioethanol Production. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2009. [Google Scholar]
- Modenbach, A. Sodium Hydroxide Pretreatment of Corn Stover and Subsequent Enzymatic Hydrolysis: An Investigation of Yields, Kinetic Modeling and Glucose Recovery. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 2013. [Google Scholar]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann Publications Elsevier Ltd.: Oxford, UK, 2012. [Google Scholar]


| RUN | X1 | X2 | X3 | SL (%) | ET (min) | MP (W) |
|---|---|---|---|---|---|---|
| 1 | 0 | −1 | 1 | 10 | 5 | 800 |
| 2 | 0 | 1 | 1 | 10 | 15 | 800 |
| 3 | 0 | 1 | −1 | 10 | 15 | 80 |
| 4 | −1 | 0 | 1 | 5 | 10 | 800 |
| 5 | −1 | 1 | 0 | 5 | 15 | 440 |
| 6 | −1 | −1 | 0 | 5 | 5 | 440 |
| 7 | 1 | 0 | 1 | 15 | 10 | 800 |
| 8 | 0 | 0 | 0 | 10 | 10 | 440 |
| 9 | 0 | 0 | 0 | 10 | 10 | 440 |
| 10 | 1 | 0 | −1 | 15 | 10 | 80 |
| 11 | 0 | 0 | 0 | 10 | 10 | 440 |
| 12 | 1 | −1 | 0 | 15 | 5 | 440 |
| 13 | −1 | 0 | −1 | 5 | 10 | 80 |
| 14 | 0 | 0 | 0 | 10 | 10 | 440 |
| 15 | 0 | 0 | 0 | 10 | 10 | 440 |
| 16 | 1 | 1 | 0 | 15 | 15 | 440 |
| 17 | 0 | −1 | −1 | 10 | 5 | 80 |
| Component % w/w | SPB | |||
|---|---|---|---|---|
| Untreated | MSA | MSH | MSB | |
| Cellulose | 40.79 | 47.23 | 47.1 | 44.92 |
| Hemicellulose | 22.32 | 19.55 | 24.21 | 27.18 |
| Lignin (Removal) | 25.85 | 17.68 (31.6%) | 20.21 (21.8%) | 18.83 (27.1%) |
| others | 11.04 | 15.47 | 8.48 | 9.07 |
| Run | Pretreatment Conditions | MSA Pretreatment | MSH Pretreatment | MSB Pretreatment | |||||
|---|---|---|---|---|---|---|---|---|---|
| SL(%) | ET (min) | MP (W) | Glucose (mg/g) | Xylose (mg/g) | Glucose (mg/g) | Xylose (mg/g) | Glucose (mg/g) | Xylose (mg/g) | |
| 1 | 10 | 5 | 800 | 25.5 ± 5.8 | 32.7 ± 2.8 | 32.9 ± 1.5 | 24.5 ± 0.0 | 16.0 ± 2.0 | 17.9 ± 1.5 |
| 2 | 10 | 15 | 800 | 20.8 ± 1.4 | 33.6 ± 5.8 | 35.8 ± 7.9 | 25.5 ± 0.2 | 19.6 ± 1.8 | 18.9 ± 0.3 |
| 3 | 10 | 15 | 80 | 27.1 ± 0.7 | 32.1 ± 7.6 | 32.5 ± 4.7 | 22.4 ± 1.4 | 18.8 ± 2.0 | 14.6 ± 2.3 |
| 4 | 5 | 10 | 800 | 24.4 ± 3.3 | 37.5 ± 5.7 | 44.3 ± 4.8 | 24.6 ± 0.7 | 20.1 ± 1.5 | 17.2 ± 2.4 |
| 5 | 5 | 15 | 440 | 37.5 ± 5.7 | 30.6 ± 3.2 | 40.9 ±3.4 | 24.7 ± 0.0 | 20.6 ± 0.3 | 14.7 ± 0.1 |
| 6 | 5 | 5 | 440 | 23.6 ± 0.8 | 31.4 ± 3.3 | 37.0 ± 1.0 | 15.8 ± 3.8 | 13.6 ± 1.9 | 16.8 ± 2.4 |
| 7 | 15 | 10 | 800 | 20.4 ± 2.1 | 43.1 ± 4.2 | 39.3 ± 1.0 | 22.9 ± 1.4 | 16.6 ± 1.0 | 17.2 ± 1.3 |
| 8 | 10 | 10 | 440 | 28.3 ± 2.4 | 33.8 ± 0.4 | 32.2 ± 1.4 | 19.6 ± 1.0 | 17.1 ± 2.0 | 21.2 ± 3.4 |
| 9 | 10 | 10 | 440 | 27.6 ± 2.2 | 37.1 ± 5.5 | 33.2 ± 1.7 | 18.3 ± 1.7 | 16.2 ± 1.3 | 19.9 ± 0.3 |
| 10 | 15 | 10 | 80 | 22.4 ± 2.3 | 34.5 ± 4.9 | 30.9 ± 5.3 | 17.7 ± 0.3 | 13.0 ± 2.6 | 16.3 ± 0.8 |
| 11 | 10 | 10 | 440 | 27.6 ± 0.8 | 31.1 ± 0.6 | 30.2 ± 1.4 | 18.2 ± 1.4 | 16.2 ± 1.3 | 19.0 ± 0.2 |
| 12 | 15 | 5 | 440 | 29.3 ± 3.9 | 33.0 ± 1.3 | 28.0 ± 1.4 | 14.7 ± 1.5 | 14.4 ± 0.7 | 16.5 ± 0.8 |
| 13 | 5 | 10 | 80 | 29.3 ± 1.5 | 38.9 ± 7.5 | 35.9 ± 3.8 | 19.7 ± 1.6 | 16.5 ± 0.3 | 19.8 ± 0.2 |
| 14 | 10 | 10 | 440 | 27.7 ± 3.7 | 35.5 ± 2.0 | 31.2 ± 2.6 | 19.6 ± 1.0 | 16.5 ± 0.3 | 19.8 ± 0.6 |
| 15 | 10 | 10 | 440 | 28.3 ± 3.0 | 32.0 ± 2.9 | 32.2 ± 1.3 | 18.8 ± 1.4 | 16.6 ± 1.8 | 18.9 ± 0.6 |
| 16 | 15 | 15 | 440 | 27.7 ± 3.9 | 35.5 ± 2.0 | 28.9 ± 0.9 | 17.3 ± 0.9 | 17.1 ± 1.0 | 13.9 ± 0.3 |
| 17 | 10 | 5 | 80 | 23.1 ± 1.2 | 30.1 ± 0.6 | 29.7 ± 1.0 | 14.9 ± 1.0 | 11.5 ± 2.4 | 17.4 ± 1.9 |
| Pretreatment. | Source of Variations | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | R2 |
|---|---|---|---|---|---|---|---|
| MSA | Model | 232.76 | 9 | 25.86 | 6.30 | 0.0120 | 0.8901 |
| X1 (SL) | 28.16 | 1 | 28.16 | 6.86 | 0.0345 | ||
| X2 (ET) | 16.67 | 1 | 16.67 | 4.06 | 0.0837 | ||
| X3 (MP) | 14.95 | 1 | 14.95 | 3.64 | 0.0980 | ||
| X1X2 | 60.31 | 1 | 60.31 | 14.69 | 0.0064 | ||
| X1X3 | 2.02 | 1 | 2.02 | 0.49 | 0.5058 | ||
| X2X3 | 18.91 | 1 | 18.91 | 4.61 | 0.0690 | ||
| X1^2 | 2.80 | 1 | 2.80 | 0.68 | 0.4365 | ||
| X2^2 | 2.85 | 1 | 2.85 | 0.70 | 0.4319 | ||
| X3^2 | 88.77 | 1 | 88.77 | 21.63 | 0.0023 | ||
| MSH | Model | 286.65 | 9 | 31.85 | 6.59 | 0.0106 | 0.8944 |
| X1 (SL) | 120.08 | 1 | 120.08 | 24.85 | 0.0016 | ||
| X2 (ET) | 13.82 | 1 | 13.82 | 2.86 | 0.1347 | ||
| X3 (MP) | 68.05 | 1 | 68.05 | 14.08 | 0.0071 | ||
| X1X2 | 2.10 | 1 | 2.10 | 0.43 | 0.5308 | ||
| X1X3 | 0.02073 | 1 | 0.02073 | 0.004289 | 0.9841 | ||
| X2X3 | 0.02621 | 1 | 0.02621 | 0.05422 | 0.9821 | ||
| X1^2 | 48.20 | 1 | 48.20 | 9.97 | 0.0160 | ||
| X2^2 | 9.60 | 1 | 9.60 | 1.99 | 0.2016 | ||
| X3^2 | 24.58 | 1 | 24.58 | 5.09 | 0.0587 | ||
| MSB | Model | 90.99 | 6 | 15.17 | 30.11 | <0.0001 | 0.9476 |
| X1 (SL) | 20.39 | 1 | 20.39 | 40.49 | <0.0001 | ||
| X2 (ET) | 43.28 | 1 | 43.28 | 85.93 | <0.0001 | ||
| X3 (MP) | 22.46 | 1 | 22.46 | 44.61 | <0.0001 | ||
| X1X2 | 1.22 | 1 | 1.22 | 2.41 | 0.1514 | ||
| X1X3 | 0.21 | 1 | 0.21 | 0.42 | 0.5305 | ||
| X2X3 | 3.43 | 1 | 3.43 | 6.82 | 0.0260 |
| Pretreatment | Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | R2 |
|---|---|---|---|---|---|---|---|
| MSA Pretreatment | Model | 155.51 | 9 | 17.28 | 4.18 | 0.0363 | 0.8431 |
| X1 (SL) | 7.68 | 1 | 7.68 | 1.86 | 0.2152 | ||
| X2 (ET) | 2.59 | 1 | 2.59 | 0.63 | 0.4545 | ||
| X3 (MP) | 15.88 | 1 | 15.88 | 3.84 | 0.0909 | ||
| X1X2 | 2.77 | 1 | 2.77 | 0.67 | 0.4402 | ||
| X1X3 | 24.76 | 1 | 24.76 | 5.99 | 0.0443 | ||
| X2X3 | 0.30 | 1 | 0.30 | 0.072 | 0.7956 | ||
| X1^2 | 27.43 | 1 | 27.43 | 6.63 | 0.0367 | ||
| X2^2 | 61.72 | 1 | 61.72 | 14.93 | 0.0062 | ||
| X3^2 | 17.58 | 1 | 17.58 | 4.25 | 0.0782 | ||
| MSH Pretreatment | Model | 201.90 | 9 | 22.43 | 17.89 | 0.0005 | 0.9583 |
| X1 (SL) | 22.13 | 1 | 22.13 | 17.65 | 0.0040 | ||
| X2 (ET) | 56.07 | 1 | 56.07 | 44.72 | 0.0003 | ||
| X3 (MP) | 64.22 | 1 | 64.22 | 51.22 | 0.0002 | ||
| X1X2 | 13.33 | 1 | 13.33 | 10.63 | 0.0138 | ||
| X1X3 | 0.024 | 1 | 0.024 | 0.019 | 0.8931 | ||
| X2X3 | 10.64 | 1 | 10.64 | 8.49 | 0.0225 | ||
| X1^2 | 1.20 | 1 | 1.20 | 0.96 | 0.3605 | ||
| X2^2 | 0.011 | 1 | 0.011 | 0.08855 | 0.9277 | ||
| X3^2 | 34.73 | 1 | 34.73 | 27.70 | 0.0012 | ||
| MSB Pretreatment | Model | 58.39 | 9 | 6.49 | 4.31 | 0.0335 | 0.8472 |
| X1 (SL) | 2.57 | 1 | 2.57 | 1.71 | 0.2328 | ||
| X2 (ET) | 5.49 | 1 | 5.49 | 3.64 | 0.0979 | ||
| X3 (MP) | 1.19 | 1 | 1.19 | 0.79 | 0.4037 | ||
| X1X2 | 0.094 | 1 | 0.094 | 0.062 | 0.8100 | ||
| X1X3 | 3.23 | 1 | 3.23 | 2.14 | 0.1866 | ||
| X2X3 | 3.58 | 1 | 3.58 | 2.38 | 0.1669 | ||
| X1^2 | 15.71 | 1 | 15.71 | 10.44 | 0.0144 | ||
| X2^2 | 23.53 | 1 | 23.53 | 15.63 | 0.0055 | ||
| X3^2 | 0.19 | 1 | 0.19 | 0.13 | 0.7297 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ethaib, S.; Omar, R.; Siti Mazlina, M.K.; Dayang Radiah, A.B. Evaluation of the Interactive Effect Pretreatment Parameters via Three Types of Microwave-Assisted Pretreatment and Enzymatic Hydrolysis on Sugar Yield. Processes 2020, 8, 787. https://doi.org/10.3390/pr8070787
Ethaib S, Omar R, Siti Mazlina MK, Dayang Radiah AB. Evaluation of the Interactive Effect Pretreatment Parameters via Three Types of Microwave-Assisted Pretreatment and Enzymatic Hydrolysis on Sugar Yield. Processes. 2020; 8(7):787. https://doi.org/10.3390/pr8070787
Chicago/Turabian StyleEthaib, Saleem, Rozita Omar, Mustapa Kamal Siti Mazlina, and Awang Biak Dayang Radiah. 2020. "Evaluation of the Interactive Effect Pretreatment Parameters via Three Types of Microwave-Assisted Pretreatment and Enzymatic Hydrolysis on Sugar Yield" Processes 8, no. 7: 787. https://doi.org/10.3390/pr8070787
APA StyleEthaib, S., Omar, R., Siti Mazlina, M. K., & Dayang Radiah, A. B. (2020). Evaluation of the Interactive Effect Pretreatment Parameters via Three Types of Microwave-Assisted Pretreatment and Enzymatic Hydrolysis on Sugar Yield. Processes, 8(7), 787. https://doi.org/10.3390/pr8070787





