Lipid Production by Yeasts Growing on Commercial Xylose in Submerged Cultures with Process Water Being Partially Replaced by Olive Mill Wastewaters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism, Media and Cultures
2.2. Determinations and Analyses
2.3. Data Analysis
3. Results and Discussion
3.1. Initial Screening on Commercial-Type Xylose
3.2. Lipid Production in High-Xylose Concentration Media Partially Diluted with Olive Mill Wastewaters
3.3. Cellular Lipid Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, H.; Wang, B.; Lv, J.; Jiang, M.; Lin, S.; Deng, Z. Xylitol production from xylose mother liquor: A novel strategy that combines the use of recombinant Bacillus subtilis and Candida maltose. Microb. Cell Fact. 2011, 10, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarris, D.; Papanikolaou, S. Biotechnological production of ethanol: Biochemistry, processes and technologies. Eng. Life Sci. 2016, 16, 307–329. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Arora, N.; Sartaj, K.; Pruthi, V.; Pruthi, P.A. Sustainable biodiesel production from oleaginous yeasts utilizing hydrolysates of various non-edible lignocellulosic biomasses. Renew. Sustain. Energy Rev. 2016, 62, 836–855. [Google Scholar] [CrossRef]
- Patel, A.; Mikes, F.; Bühler, S.; Matsakas, L. Valorization of brewers’ spent grain for the production of lipids by oleaginous yeast. Molecules 2018, 23, 3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An Overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Li, L.; Zhang, L.; An, J.; Chang, H.; Deng, Z. Xylitol production from waste xylose mother liquor containing miscellaneous sugars and inhibitors: One-pot biotransformation by Candida tropicalis and recombinant Bacillus subtilis. Microb. Cell Fact. 2016, 15, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, L.; Liu, L.; Zeng, A.-P.; Wei, D. From low-cost substrates to single cell oils synthesized by oleaginous yeasts. Bioresour. Technol. 2017, 245, 1507–1519. [Google Scholar] [CrossRef]
- Diwan, B.; Parkhey, P.; Gupta, P. From agro-industrial wastes to single cell oils: A step towards prospective biorefinery. Folia Microbiol. 2018, 63, 547–568. [Google Scholar] [CrossRef]
- Mantzavinos, D.; Kalogerakis, N. Treatment of olive mill effluents. Environ. Int. 2005, 31, 289–295. [Google Scholar] [CrossRef]
- Crognale, S.; D’Annibale, A.; Federici, F.; Fenice, M.; Quaratino, D.; Petruccioli, M. Olive oil mill wastewater valorisation by fungi. J. Chem. Technol. Biotechnol. 2006, 81, 1547–1555. [Google Scholar] [CrossRef]
- Sarris, D.; Stoforos, N.G.; Mallouchos, A.; Kookos, I.K.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Production of added-value metabolites by Yarrowia lipolytica growing in olive mill wastewater-based media under aseptic and non-aseptic conditions. Eng. Life Sci. 2017, 17, 695–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarris, D.; Rapti, A.; Papafotis, N.; Koutinas, A.A.; Papanikolaou, S. Production of added-value chemical compounds through bioconversions of olive-mill wastewaters blended with crude glycerol by a Yarrowia lipolytica strain. Molecules 2019, 24, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, P.A.; Fernández, P.M.; de Figueroa, L.I.C.; Pajot, H.F. Exploitation alternatives of olive mill wastewater: Production of value-added compounds useful for industry and agriculture. Biofuel Res. J. 2019, 6, 980–994. [Google Scholar] [CrossRef]
- Alfano, A.; Corsuto, L.; Finamore, R.; Savarese, M.; Ferrara, F.; Falco, S.; Santabarbara, G.; De Rosa, M.; Schiraldi, C. Valorization of olive mill wastewater by membrane processes to recover natural antioxidant compounds for cosmeceutical and nutraceutical applications or functional foods. Antioxidants 2018, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- D’Annibale, A.; Sermanni, G.G.; Federici, F.; Petruccioli, M. Olive-mill wastewaters: A promising substrate for microbial lipase production. Bioresour. Technol. 2006, 97, 1828–1833. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Galiotou-Panayotou, M.; Fakas, S.; Komaitis, M.; Aggelis, G. Citric acid production by Yarrowia lipolytica cultivated on olive-mill wastewater-based media. Bioresour. Technol. 2008, 99, 2419–2428. [Google Scholar] [CrossRef]
- Sarris, D.; Galiotou-Panayotou, M.; Koutinas, A.A.; Komaitis, M.; Papanikolaou, S. Citric acid, biomass and cellular lipid production by Yarrowia lipolytica strains cultivated on olive mill wastewater-based media. J. Chem. Technol. Biotechnol. 2011, 86, 1439–1448. [Google Scholar] [CrossRef]
- Sarris, D.; Giannakis, M.; Philippoussis, A.; Komaitis, M.; Koutinas, A.A.; Papanikolaou, S. Conversions of olive mill wastewater-based media by Saccharomyces cerevisiae through sterile and non-sterile bioprocesses: Bioconversions of olive-mill wastewaters by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2013, 88, 958–969. [Google Scholar] [CrossRef]
- Papadaki, E.; Tsimidou, M.Z.; Mantzouridou, F.T. Changes in phenolic compounds and phytotoxicity of the Spanish-style green olive processing wastewaters by Aspergillus niger B60. J. Agric. Food Chem. 2018, 66, 4891–4901. [Google Scholar] [CrossRef]
- Papadaki, E.; Mantzouridou, F.T. Current status and future challenges of table olive processing wastewater valorization. Biochem. Eng. J. 2016, 112, 103–113. [Google Scholar] [CrossRef]
- Papadaki, E.; Mantzouridou, F.T. Citric acid production from the integration of spanish-style green olive processing wastewaters with white grape pomace by Aspergillus niger. Bioresour. Technol. 2019, 280, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Sources of microbial oils with emphasis to Mortierella (Umbelopsis) isabellina fungus. World J. Microbiol. Biotechnol. 2019, 35, 63. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, S.; Zullaikah, S.; Tran-Nguyen, P.L.; Ismadji, S.; Ju, Y.-H. Lipomyces starkeyi: Its current status as a potential oil producer. Fuel Proc. Technol. 2018, 177, 39–55. [Google Scholar] [CrossRef]
- Athenaki, M.; Gardeli, C.; Diamantopoulou, P.; Tchakouteu, S.S.; Sarris, D.; Philippoussis, A.; Papanikolaou, S. Lipids from yeasts and fungi: Physiology, production and analytical considerations. J Appl. Microbiol. 2018, 124, 336–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellou, S.; Makri, A.; Sarris, D.; Michos, K.; Rentoumi, P.; Celik, A.; Papanikolaou, S.; Aggelis, G. The olive mill wastewater as substrate for single cell oil production by Zygomycetes. J. Biotechnol. 2014, 170, 50–59. [Google Scholar] [CrossRef]
- Bellou, S.; Triantaphyllidou, I.-E.; Aggeli, D.; Elazzazy, A.M.; Baeshen, M.N.; Aggelis, G. Microbial oils as food additives: Recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr. Opin. Biotechnol. 2016, 37, 24–35. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Chevalot, I.; Komaitis, M.; Aggelis, G.; Marc, I. Kinetic profile of the cellular lipid composition in an oleaginous Yarrowia lipolytica capable of producing a cocoa-butter substitute from industrial fats. Antonie Van Leeuwenhoek 2001, 80, 215–224. [Google Scholar] [CrossRef]
- Valta, K.; Kosanovic, T.; Malamis, D.; Moustakas, K.; Loizidou, M. Overview of water usage and wastewater management in the food and beverage industry. Desalin. Water Treat. 2015, 53, 3335–3347. [Google Scholar] [CrossRef]
- Gardeli, C.; Athenaki, M.; Xenopoulos, E.; Mallouchos, A.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Lipid production and characterization by Mortierella (Umbelopsis) isabellina cultivated on lignocellulosic sugars. J. Appl. Microbiol. 2017, 123, 1461–1477. [Google Scholar] [CrossRef]
- Fakas, S.; Papanikolaou, S.; Galiotou-Panayotou, M.; Komaitis, M.; Aggelis, G. Lipids of Cunninghamella echinulata with emphasis to γ-linolenic acid distribution among lipid classes. Appl. Microbiol. Biotechnol. 2006, 73, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Rontou, M.; Belka, A.; Athenaki, M.; Gardeli, C.; Mallouchos, A.; Kalantzi, O.; Koutinas, A.A.; Kookos, I.K.; Zeng, A.-P.; et al. Conversion of biodiesel-derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng. Life Sci. 2017, 17, 262–281. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulou, P.; Filippousi, R.; Antoniou, D.; Varfi, E.; Xenopoulos, E.; Sarris, D.; Papanikolaou, S. Production of added-value microbial metabolites during growth of yeast strains on media composed of biodiesel-derived crude glycerol and glycerol/xylose blends. FEMS Microbiol. Lett. 2020, 367, fnaa063. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulou, P.; Papanikolaou, S.; Katsarou, E.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Mushroom polysaccharides and lipids synthesized in liquid agitated and static cultures. Part II: Study of Volvariella volvacea. Appl. Biochem. Biotechnol. 2012, 167, 1890–1906. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Yanniotis, S.; Kookos, I.; Koutinas, A.A. Utilisation of by-products from sunflower-based biodiesel production processes for the production of fermentation feedstock. Waste Biomass Valor. 2013, 4, 529–537. [Google Scholar] [CrossRef]
- Aggelis, G.; Iconomou, D.; Christou, M.; Bokas, D.; Kotzailias, S.; Christou, G.; Tsagou, V.; Papanikolaou, S. Phenolic Removal in a model olive oil mill wastewater using Pleurotus ostreatus in bioreactor cultures and biological evaluation of the process. Water Res. 2003, 37, 3897–3904. [Google Scholar] [CrossRef]
- Sayadi, S.; Ellouz, R. Decolourization of olive mill waste-waters by the white-rot fungus Phanerochaete chrysosporium: Involvement of the lignin-degrading system. Appl. Microbiol. Biotechnol. 1992, 37, 813–817. [Google Scholar] [CrossRef]
- Tchakouteu, S.S.; Kalantzi, O.; Gardeli, C.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Lipid production by yeasts growing on biodiesel-derived crude glycerol: Strain selection and impact of substrate concentration on the fermentation efficiency. J. Appl. Microbiol. 2015, 118, 911–927. [Google Scholar] [CrossRef]
- Filippousi, R.; Antoniou, D.; Tryfinopoulou, P.; Nisiotou, A.A.; Nychas, G.-J.; Koutinas, A.A.; Papanikolaou, S. Isolation, identification and screening of yeasts towards their ability to assimilate biodiesel-derived crude glycerol: Microbial production of polyols, endopolysaccharides and lipid. J. Appl. Microbiol. 2019, 127, 1080–1100. [Google Scholar] [CrossRef]
- Makri, A.; Fakas, S.; Aggelis, G. Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour. Technol. 2010, 101, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. The citric acid production from raw glycerol by Yarrowia lipolytica yeast and its regulation. Appl. Microbiol. Biotechnol. 2013, 97, 7387–7397. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Ashoor, S.; Taccari, M.; Comitini, F.; Antonucci, M.; Truzzi, C.; Scarponi, G.; Ciani, M. Conversion of raw glycerol to microbial lipids by new Metschnikowia and Yarrowia lipolytica strains. Ann. Microbiol. 2016, 66, 1409–1418. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Fakas, S.; Galiotou-Panayotou, M.; Komaitis, M.; Aggelis, G.; Papanikolaou, S. Commercial sugars as substrates for lipid accumulation in Cunninghamella echinulata and Mortierella isabellina fungi. Eur. J. Lipid Sci. Technol. 2010, 112, 1048–1057. [Google Scholar] [CrossRef]
- Kothri, M.; Mavrommati, M.; Elazzazy, A.M.; Baeshen, M.N.; Moussa, T.A.A.; Aggelis, G. Microbial sources of polyunsaturated fatty acids (PUFAs) and the prospect of organic residues and wastes as growth media for PUFA-producing microorganisms. FEMS Microbiol. Lett. 2020, 367, fnaa028. [Google Scholar] [CrossRef]
- Tsakona, S.; Skiadaresis, A.G.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Valorisation of side streams from wheat milling and confectionery industries for consolidated production and extraction of microbial lipids. Food Chem. 2016, 198, 85–92. [Google Scholar] [CrossRef]
- Tchakouteu, S.S.; Chatzifragkou, A.; Kalantzi, O.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Oleaginous yeast Cryptococcus curvatus exhibits interplay between biosynthesis of intracellular sugars and lipids: Biochemical features of Cryptococcus curvatus. Eur. J. Lipid Sci. Technol. 2015, 117, 657–672. [Google Scholar] [CrossRef]
- Harde, S.M.; Wang, Z.; Horne, M.; Zhu, J.Y.; Pan, X. Microbial lipid production from SPORL-pretreated douglas fir by Mortierella isabellina. Fuel 2016, 175, 64–74. [Google Scholar] [CrossRef]
- Carota, E.; Petruccioli, M.; D’Annibale, A.; Gallo, A.M.; Crognale, S. Orange peel waste-based liquid medium for biodiesel production by oleaginous yeasts. Appl. Microbiol. Biotechnol. 2020, 104, 4617–4628. [Google Scholar] [CrossRef]
- Tsakona, S.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Formulation of fermentation media from flour-rich waste streams for microbial lipid production by Lipomyces starkeyi. J. Biotechnol. 2014, 189, 36–45. [Google Scholar] [CrossRef]
- Evans, C.T.; Ratledge, C. Phosphofructokinase and the regulation of the flux of carbon from glucose to lipid in the oleaginous yeast Rhodosporidium toruloides. J. Gen. Microbiol. 1984, 130, 3251–3264. [Google Scholar] [CrossRef] [Green Version]
- Park, W.-S.; Murphy, P.A.; Glatz, B.A. Lipid metabolism and cell composition of the oleaginous yeast Apiotrichum curvatum grown at different carbon to nitrogen ratios. Can. J. Microbiol. 1990, 36, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Zikou, E.; Chatzifragkou, A.; Koutinas, A.A.; Papanikolaou, S. Evaluating glucose and xylose as cosubstrates for lipid accumulation and γ-linolenic acid biosynthesis of Thamnidium elegans. J. Appl. Microbiol. 2013, 114, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Fernández, D.; Aguiar, T.Q.; Martín, V.I.; Romaní, A.; Silva, R.; Domingues, L.; Revuelta, J.L.; Jiménez, A. Microbial lipids from industrial wastes using xylose-utilizing Ashbya gossypii strains. Bioresour. Technol. 2019, 293, 122054. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Sarantou, S.; Komaitis, M.; Aggelis, G. Repression of reserve lipid turnover in Cunninghamella echinulata and Mortierella isabellina cultivated in multiple-limited media. J. Appl. Microbiol. 2004, 97, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Diamantopoulou, P.; Chatzifragkou, A.; Philippoussis, A.; Aggelis, G. Suitability of low-cost sugars as substrates for lipid production by the fungus Thamnidium elegans. Energy Fuel. 2010, 24, 4078–4086. [Google Scholar] [CrossRef]
- Fakas, S.; Galiotou-Panayotou, M.; Papanikolaou, S.; Komaitis, M.; Aggelis, G. Compositional shifts in lipid fractions during lipid turnover in Cunninghamella echinulata. Enzyme Microb. Technol. 2007, 40, 1321–1327. [Google Scholar] [CrossRef]
- Chatzifragkou, A.; Makri, A.; Belka, A.; Bellou, S.; Mavrou, M.; Mastoridou, M.; Mystrioti, P.; Onjaro, G.; Aggelis, G.; Papanikolaou, S. Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 2011, 36, 1097–1108. [Google Scholar] [CrossRef]
- Bellou, S.; Moustogianni, A.; Makri, A.; Aggelis, G. Lipids containing polyunsaturated fatty acids synthesized by Zygomycetes grown on glycerol. Appl. Biochem. Biotechnol. 2012, 166, 146–158. [Google Scholar] [CrossRef]
- Aggelis, G.; Sourdis, J. Prediction of lipid accumulation-degradation in oleaginous micro-organisms growing on vegetable oils. Antonie Van Leeuwenhoek 1997, 72, 159–165. [Google Scholar] [CrossRef]
- Serrano-Carreon, L.; Hathout, Y.; Bensoussan, M.; Belin, J.-M. Lipid accumulation in Trichoderma species. FEMS Microbiol. Lett. 1992, 93, 181–187. [Google Scholar] [CrossRef]
- Holdsworth, J.E.; Veenhuis, M.; Ratledge, C. Enzyme activities in oleaginous yeasts accumulating and utilizing exogenous or endogenous lipids. J. Gen. Microbiol. 1988, 134, 2907–2915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holdsworth, J.E.; Ratledge, C. Lipid turnover in oleaginous yeasts. J. Gen. Microbiol. 1988, 134, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Lopez Garcia, I.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S.K. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, M.; Papapostolou, H.; Vlysidis, A.; Gardeli, C.; Komaitis, M.; Papanikolaou, S.; Koutinas, A.A. Extraction of phenolic compounds and succinic acid production from spent sulphite liquor. J. Chem. Technol. Biotechnol. 2016, 91, 2751–2760. [Google Scholar] [CrossRef]
- Alexandri, M.; Papapostolou, H.; Komaitis, M.; Stragier, L.; Verstraete, W.; Danezis, G.P.; Georgiou, C.A.; Papanikolaou, S.; Koutinas, A.A. Evaluation of an integrated biorefinery based on fractionation of spent sulphite liquor for the production of an antioxidant-rich extract, lignosulphonates and succinic acid. Bioresour. Technol. 2016, 214, 504–513. [Google Scholar] [CrossRef]
- Yousuf, A.; Sannino, F.; Addorisio, V.; Pirozzi, D. Microbial conversion of olive oil mill wastewaters into lipids suitable for biodiesel production. J. Agric. Food Chem. 2010, 58, 8630–8635. [Google Scholar] [CrossRef]
- Dourou, M.; Kancelista, A.; Juszczyk, P.; Sarris, D.; Bellou, S.; Triantaphyllidou, I.-E.; Rywinska, A.; Papanikolaou, S.; Aggelis, G. Bioconversion of olive mill wastewater into high-added value products. J. Clean. Prod. 2016, 139, 957–969. [Google Scholar] [CrossRef]
- Tsioulpas, A.; Dimou, D.; Iconomou, D.; Aggelis, G. Phenolic removal in olive oil mill wastewater by strains of Pleurotus spp. in respect to their phenol oxidase (laccase) activity. Bioresour. Technol. 2002, 84, 251–257. [Google Scholar] [CrossRef]
- Tzirita, M.; Kremmyda, M.; Sarris, D.; Koutinas, A.A.; Papanikolaou, S. Effect of salt addition upon the production of metabolic compounds by Yarrowia lipolytica cultivated on biodiesel-derived glycerol diluted with olive-mill wastewaters. Energies 2019, 12, 3649. [Google Scholar] [CrossRef] [Green Version]
- Lanciotti, R.; Gianotti, A.; Baldi, D.; Angrisani, R.; Suzzi, G.; Mastrocola, D.; Guerzoni, M.E. Use of Yarrowia lipolytica strains for the treatment of olive mill wastewater. Bioresour. Technol. 2005, 96, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Federici, F.; Montedoro, G.; Servili, M.; Petruccioli, M. Pectic enzyme production by Cryptococcus albidus var. albidus on olive vegetation waters enriched with sunflower calathide meal. Biol. Wastes 1988, 25, 291–301. [Google Scholar] [CrossRef]
- Ettayebi, K.; Errachidi, F.; Jamai, L.; Tahri-Jouti, M.A.; Sendide, K.; Ettayebi, M. Biodegradation of polyphenols with immobilized Candida tropicalis under metabolic induction. FEMS Microbiol. Lett. 2003, 223, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Chtourou, M.; Ammar, E.; Nasri, M.; Medhioub, K. Isolation of a yeast, Trichosporon cutaneum, able to use low molecular weight phenolic compounds: Application to olive mill waste water treatment. J. Chem. Technol. Biotechnol. 2004, 79, 869–878. [Google Scholar] [CrossRef]
- Lakhtar, H.; Ismaili-Alaoui, M.; Philippoussis, A.; Perraud-Gaime, I.; Roussos, S. Screening of strains of Lentinula edodes grown on model olive mill wastewater in solid and liquid state culture for polyphenol biodegradation. Int. Biodeterior. Biodegrad. 2010, 64, 167–172. [Google Scholar] [CrossRef]
- Anschau, A.; Xavier, M.C.A.; Hernalsteens, S.; Franco, T.T. Effect of feeding strategies on lipid production by Lipomyces starkeyi. Bioresour. Technol. 2014, 157, 214–222. [Google Scholar] [CrossRef]
- Evans, C.T.; Ratledge, C. A comparison of the oleaginous yeast, Candida curvata, grown on different carbon sources in continuous and batch culture. Lipids 1983, 18, 623–629. [Google Scholar] [CrossRef]
- Ykema, A.; Verbree, E.C.; Kater, M.M.; Smit, H. Optimization of lipid production in the oleaginous yeast Apiotrichum curvatum in whey permeate. Appl. Microbiol. Biotechnol. 1988, 29, 211–218. [Google Scholar] [CrossRef]
- Hassan, M.; Blanc, P.J.; Pareilleux, A.; Goma, G. Production of single-cell oil from prickly-pear juice fermentation by Cryptococcus curvatus grown in batch culture. World J. Microbiol. Biotechnol. 1994, 10, 534–537. [Google Scholar] [CrossRef]
- Meesters, P.A.E.P.; Huijberts, G.N.M.; Eggink, G. High-cell-density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl. Microbiol. Biotechnol. 1996, 45, 575–579. [Google Scholar] [CrossRef]
- Liang, Y.; Cui, Y.; Trushenski, J.; Blackburn, J.W. Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour. Technol. 2010, 101, 7581–7586. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Blackburn, J.W.; Liang, Y. Fermentation optimization for the production of lipid by Cryptococcus curvatus: Use of response surface methodology. Biomass Bioenergy 2012, 47, 410–417. [Google Scholar] [CrossRef]
- Gong, Z.; Shen, H.; Zhou, W.; Wang, Y.; Yang, X.; Zhao, Z.K. Efficient conversion of acetate into lipids by the oleaginous yeast Cryptococcus curvatus. Biotechnol. Biofuels 2015, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Béligon, V.; Poughon, L.; Christophe, G.; Lebert, A.; Larroche, C.; Fontanille, P. Validation of a predictive model for fed-batch and continuous lipids production processes from acetic acid using the oleaginous yeast Cryptococcus curvatus. Biochem. Eng. J. 2016, 111, 117–128. [Google Scholar] [CrossRef]
- Zhou, W.; Gong, Z.; Zhang, L.; Liu, Y.; Yan, J.; Zhao, M. Feasibility of lipid production from waste paper by the oleaginous yeast Cryptococcus curvatus. BioResources 2017, 12, 5249–5263. [Google Scholar] [CrossRef] [Green Version]
- Kopsahelis, N.; Dimou, C.; Papadaki, A.; Xenopoulos, E.; Kyraleou, M.; Kallithraka, S.; Kotseridis, Y.; Papanikolaou, S.; Koutinas, A.A. Refining of wine lees and cheese whey for the production of microbial oil, polyphenol-rich extracts and value-added co-products. J. Chem. Technol. Biotechnol. 2018, 93, 257–268. [Google Scholar] [CrossRef]
- Angerbauer, C.; Siebenhofer, M.; Mittelbach, M.; Guebitz, G.M. Conversion of sewage sludge into lipids by Lipomyces starkeyi for biodiesel production. Bioresour. Technol. 2008, 99, 3051–3056. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, X.; Wang, F.; Jiang, X.; Zhang, S.; Ye, M.; Zhao, Z.K.; Zou, H. The proteome analysis of oleaginous yeast Lipomyces starkeyi: Comparative proteomic analysis of Lipomyces starkeyi. FEMS Yeast Res. 2011, 11, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Oguri, E.; Masaki, K.; Naganuma, T.; Iefuji, H. Phylogenetic and biochemical characterization of the oil-producing yeast Lipomyces starkeyi. Antonie Van Leeuwenhoek 2012, 101, 359–368. [Google Scholar] [CrossRef]
- Lin, J.; Li, S.; Sun, M.; Zhang, C.; Yang, W.; Zhang, Z.; Li, X.; Li, S. Microbial lipid production by oleaginous yeast in D-xylose solution using a two-stage culture mode. RSC Adv. 2014, 4, 34944–34949. [Google Scholar] [CrossRef]
- Matsakas, L.; Sterioti, A.-A.; Rova, U.; Christakopoulos, P. Use of dried sweet sorghum for the efficient production of lipids by the yeast Lipomyces starkeyi CBS 1807. Ind. Crop. Prod. 2014, 62, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Economou, C.N.; Philippoussis, A.N.; Diamantopoulou, P.A. Spent mushroom substrate for a second cultivation cycle of Pleurotus mushrooms and dephenolization of agro-industrial wastewaters. FEMS Microbiol. Lett. 2020, 367, fnaa060. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Araújo, C.; Aguedo, M.; Gomes, N.; Gonçalves, C.; Teixeira, J.A.; Belo, I. The use of olive mill wastewater by wild type Yarrowia lipolytica strains: Medium supplementation and surfactant presence effect. J. Chem. Technol. Biotechnol. 2009, 84, 533–537. [Google Scholar] [CrossRef] [Green Version]
- Tiukova, I.A.; Brandenburg, J.; Blomqvist, J.; Sampels, S.; Mikkelsen, N.; Skaugen, M.; Arntzen, M.Ø.; Nielsen, J.; Sandgren, M.; Kerkhoven, E.J. Proteome analysis of xylose metabolism in Rhodotorula toruloides during lipid production. Biotechnol. Biofuels 2019, 12, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]

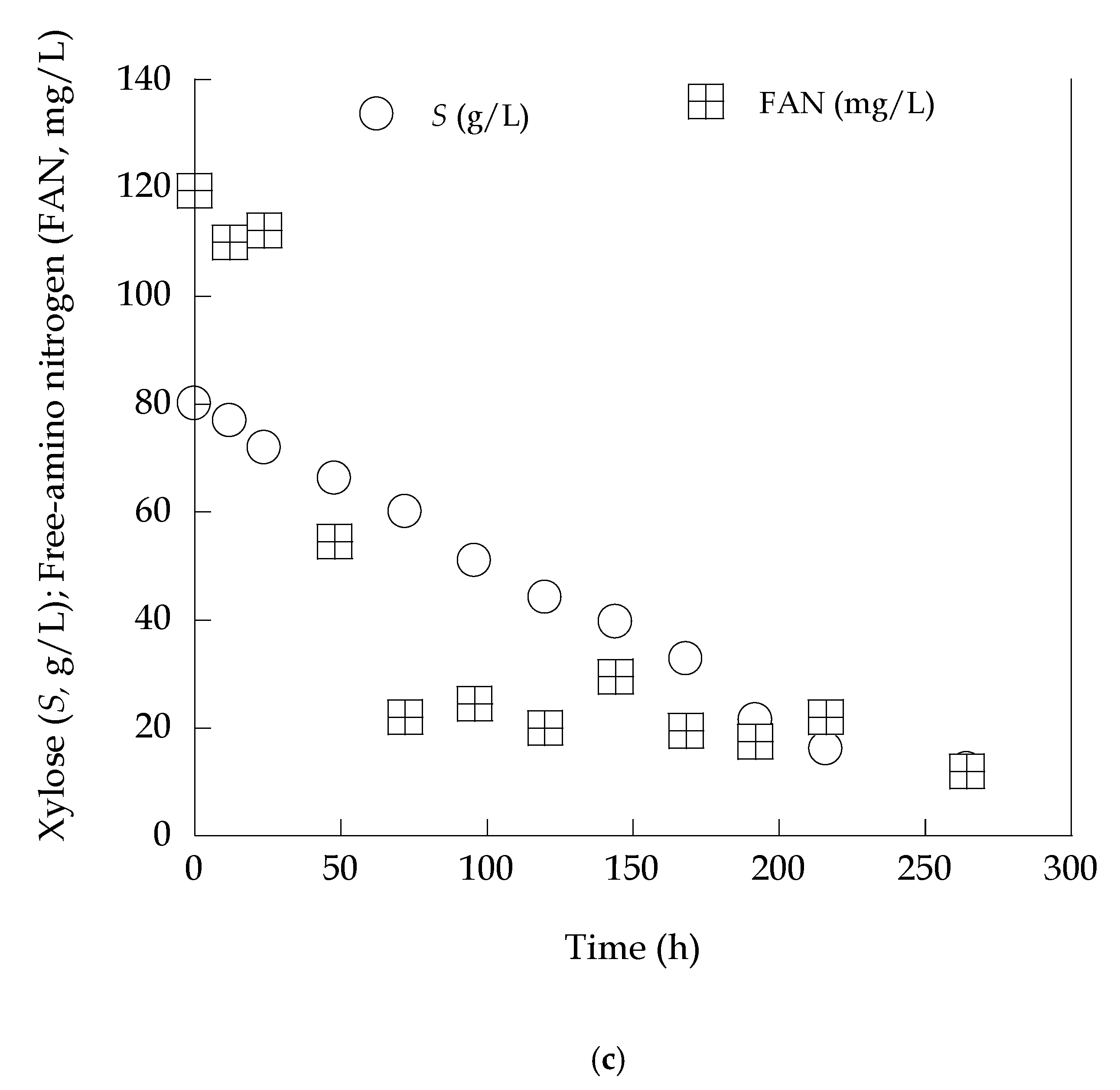
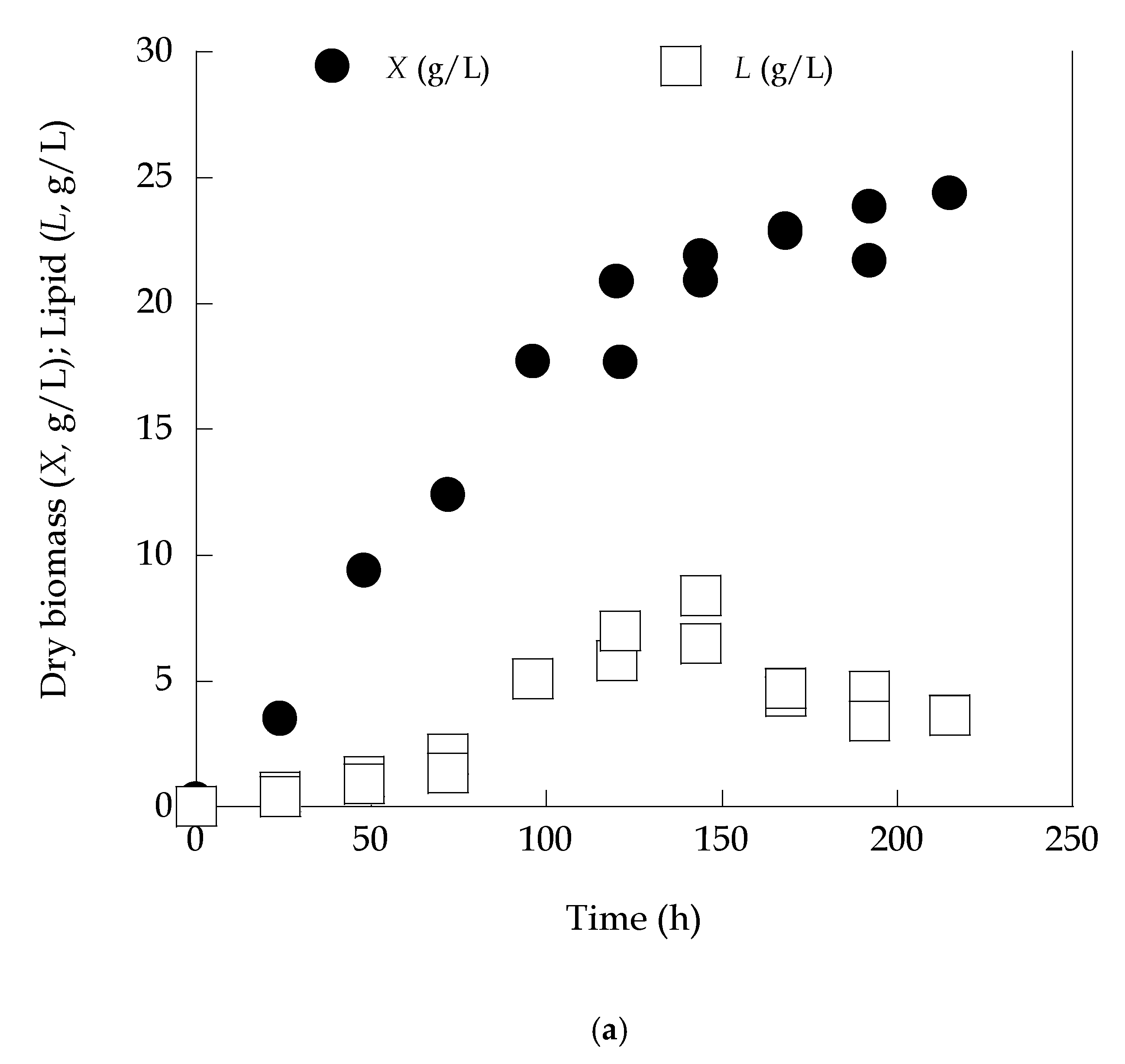
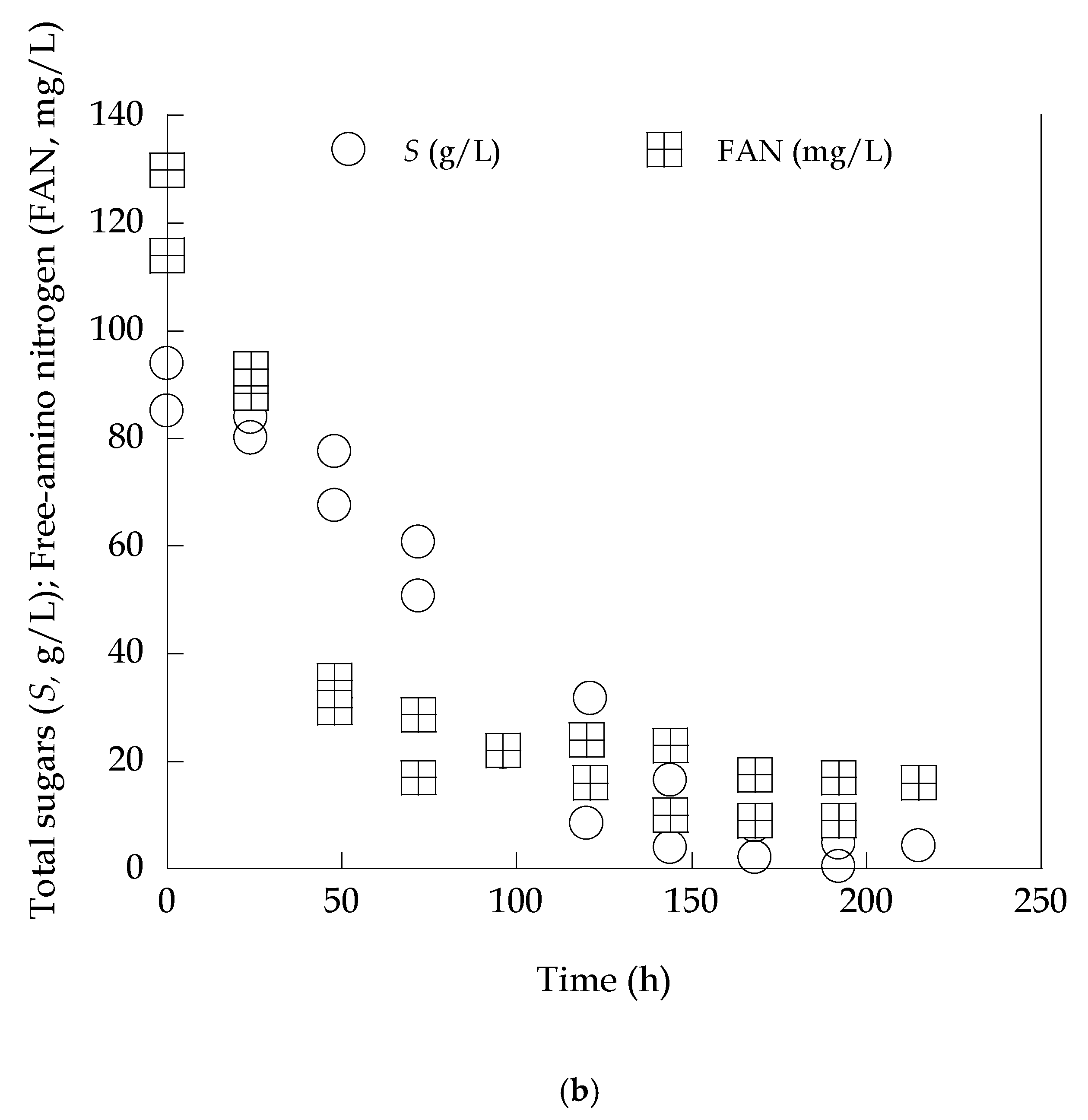

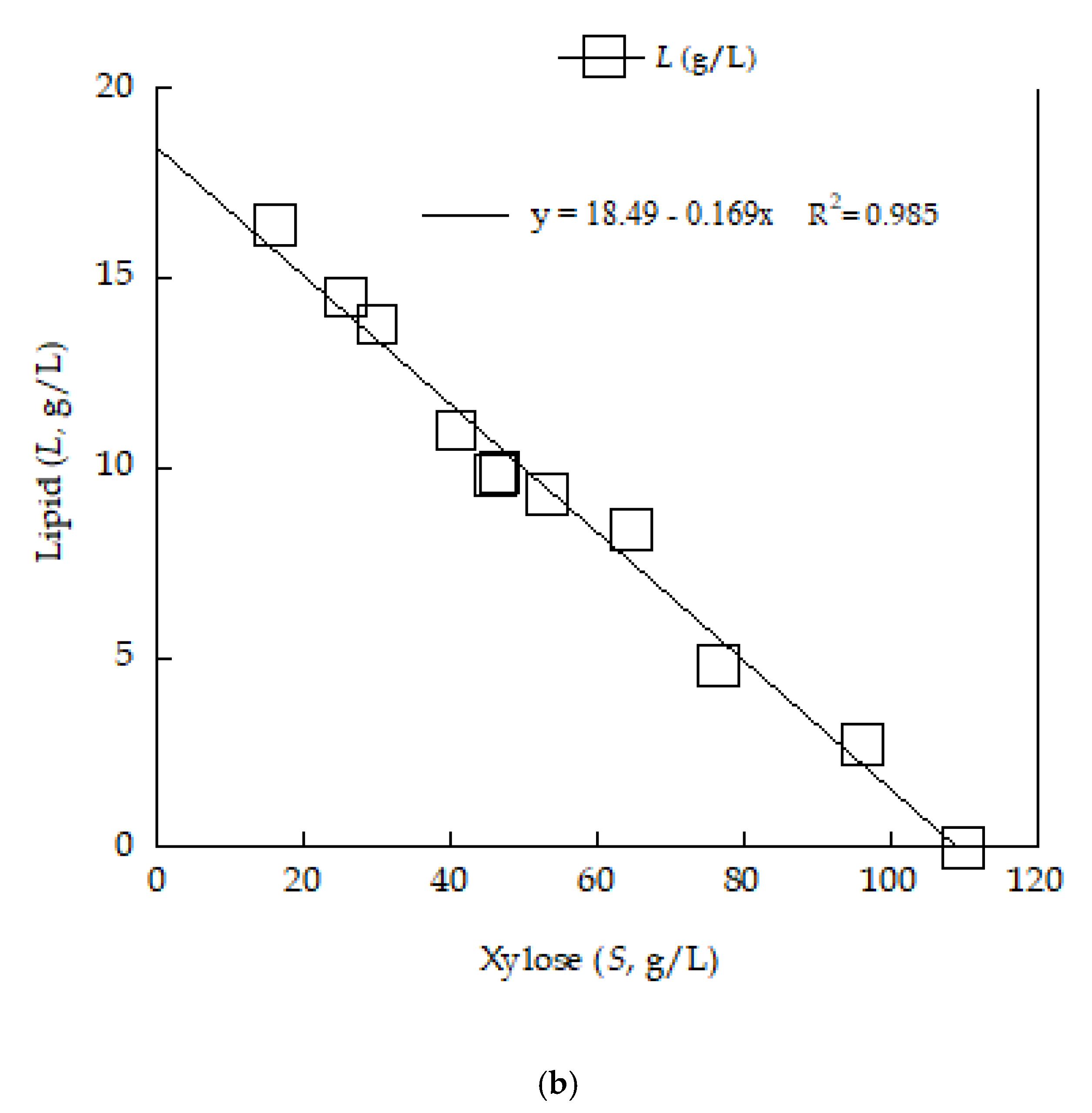


| Yeasts | Time (h) | Scons (g/L) | X (g/L) | L (g/L) | Xyl (g/L) | YL/X (%, w/w) | IPS (g/L) | YIPS/X (%, w/w) | |
|---|---|---|---|---|---|---|---|---|---|
| Rhodosporidium | c | 72 | 26.8 | 5.6 | 0.6 | 4.3 | 10.7 | 1.3 | 23.2 |
| toruloides | d | 120 | 37.2 | 10.7 | 2.8 | 4.0 | 26.2 | 2.3 | 21.4 |
| DSM 4444 | a, b | 264 | 57.8 | 16.5 | 4.8 | 3.1 | 29.1 | 1.7 | 10.3 |
| Rhodosporidium | d | 120 | 40.1 | 11.9 | 1.3 | Tr. * | 10.9 | 3.2 | 26.9 |
| Toruloides NRRL Y-27012 | a, b, c | 264 | 59.0 | 15.6 | 3.0 | Tr. * | 19.5 | 2.7 | 17.3 |
| Rhodotorula glutinis | b | 168 | 41.7 | 10.1 | 1.1 | 0.2 | 9.9 | 1.9 | 12.6 |
| NRRL YB-252 | a, c, d | 264 | 59.0 | 15.1 | 1.0 | 2.0 | 6.6 | 5.0 | 33.1 |
| Cryptococcus curvatus | c, d | 192 | 50.9 | 16.6 | 2.9 | 4.5 | 17.5 | 5.7 | 34.3 |
| NRRL Y-1511 | a, b | 220 | 63.0 | 19.0 | 4.4 | 2.6 | 23.2 | 5.4 | 28.4 |
| Lipomyces starkeyi | c | 144 | 46.4 | 15.6 | 4.0 | 1.0 | 25.6 | 5.8 | 37.1 |
| DSM 70296 | a, b, d | 170 | 56.1 | 17.5 | 5.9 | Tr. * | 33.7 | 6.3 | 36.0 |
| Cryptococcus curvatus | c | 144 | 40.4 | 13.5 | 5.6 | 4.5 | 41.5 | 2.8 | 20.7 |
| ATCC 20509 | a, b, d | 216 | 64.0 | 17.4 | 8.1 | 3.2 | 46.6 | 3.2 | 18.4 |
| PCC0 (g/L) | Time (h) | Scons (g/L) | X (g/L) | L (g/L) | YL/X (%, w/w) | |
|---|---|---|---|---|---|---|
| Cryptococcus curvatus ATCC 20509 | ||||||
| 0.00 * | b | 168 | 87.3 | 21.0 | 10.0 | 47.6 |
| a | 215 | 89.9 | 23.0 | 6.0 | 26.1 | |
| 1.16 | b | 144 | 77.6 | 20.9 | 8.4 | 40.2 |
| a | 215 | 89.7 | 24.4 | 3.6 | 14.8 | |
| 1.65 | b | 240 | 94.8 | 24.7 | 5.0 | 20.2 |
| a | 336 | 97.0 | 25.7 | 1.8 | 7.0 | |
| 1.91 | b | 192 | 92.1 | 23.8 | 2.5 | 10.5 |
| a | 216 | 94.0 | 27.0 | 1.6 | 5.9 | |
| Lipomyces starkeyi DSM 702096 | ||||||
| 0.00 * | b | 216 | 84.1 | 25.3 | 6.0 | 23.7 |
| a | 239 | 92.3 | 26.2 | 4.4 | 16.7 | |
| 1.96 | b | 239 | 92.8 | 21.1 | 5.9 | 27.9 |
| a | 255 | 96.0 | 25.0 | 4.0 | 16.0 | |
| Strain | Culture Mode | Carbon Source | TDCW (g/L) | YL/X (%, w/w) | Reference |
|---|---|---|---|---|---|
| (a) | |||||
| Cryptococcus curvatus | |||||
| D | Continuous bioreactor | Glucose | 13.5 | 29.0 | Evans and Ratledge [77] |
| Xylose | 15.0 | 37.0 | |||
| ATCC 20509 | Batch bioreactor | Whey permeate | 21.6 | 36.0 | Ykema et al. [78] |
| Continuous recycling | 85.0 | 35.0 | |||
| Batch bioreactor | Prickly pear juice | 10.9 | 45.8 | Hassan et al. [79] | |
| Fed-batch bioreactor | Glycerol (pure) | 118.0 | 25.0 | Meesters et al. [80] | |
| Fed-batch bioreactor | Glycerol (crude) | 32.9 | 52.9 | Liang et al. [81] | |
| Fed-batch bioreactor | Glycerol (crude) | 44.9 | 49.0 | Cui et al. [82] | |
| Single-stage continuous | Acetic acid | 5.1 | 66.4 | Gong et al. [83] | |
| NRRL Y-1511 | Shake flasks | Lactose (commercial) | 14.5 | 29.7 | Tchakouteu et al. [47] |
| Shake flasks | Glucose | 13.2 | 19.7 | Harde et al. [48] | |
| Shake flasks | Xylose (pure) | 13.3 | 38.3 | ||
| ATCC 20509 | Continuous bioreactor | Acetic acid | 26.7 | 48–53 | Béligon et al. [84] |
| Shake flasks | WPHL | 17.3 | 52.5 | Zhou et al. [85] | |
| Fed-batch bioreactor | Cheese whey | 66.8 | 49.6 | Kopsahelis et al. [86] | |
| ATCC 20509 | Shake flasks | Xylose (commercial) | 21.0 | 47.6 | Present study |
| Xylose/OMWs | 20.9 | 40.2 | |||
| Batch bioreactor | Xylose (commercial) | 37.0 | 44.3 | ||
| (b) | |||||
| Lipomyces starkeyi | |||||
| DSM 70295 | Shake flasks | Glucose | 9.4 | 68.0 | Angerbauer et al. [87] |
| Glucose/sewage sludge | 9.3 | 72.3 | |||
| AS 2.1560 | Batch bioreactor | Glucose | 30.0 | 46.0 | Liu et al. [88] |
| CBS 187 | Shake flask | Xylose (pure) | 12.5 | 80.0 | Oguri et al. [89] |
| DSM 70296 | Fed-batch bioreactor | Glucose/xylose | 82.4 | 46.9 | Anschau et al. [76] |
| Batch bioreactor | SCBHL | 13.9 | 26.7 | ||
| AS 2.1560 | Fed-batch bioreactor | Xylose (pure) | 94.7 | 65.5 | Lin et al. [90] |
| CBS 1807 | Shake flasks | Glucose/fructose | 12.3 | 47.3 | Matsakas et al. [91] |
| SSJc | 21.7 | 29.5 | |||
| DSM 70296 | Fed-batch bioreactor | FRWHL/glucose | 109.8 | 57.8 | Tsakona et al. [50] |
| Shake flasks | FRWHL | 30.5 | 40.4 | ||
| Shake flasks | Glycerol (crude) | 34.4 | 35.9 | Tchakouteu et al. [39] | |
| NRRL Y-11557 | Shake flasks | Glucose/OMWs | 9.5 | 24.5 | Dourou et al. [68] |
| DSM 70296 | Shake flasks | Xylose (commercial) | 25.3 | 23.7 | Present study |
| Xylose/OMWs | 21.1 | 27.9 | |||
| Fatty-Acid Composition of Yeast Lipids (%, w/w) | ||||||
|---|---|---|---|---|---|---|
| Yeast Strain | C16:0 | Δ9C16:1 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | Others |
| Rhodosporidium toruloides DSM 4444 | 29.5 | 0.6 | 7.0 | 51.6 | 7.7 | 3.6 |
| Rhodosporidium toruloides NRRL Y-27012 | 28.4 | 0.5 | 6.9 | 53.7 | 7.5 | 3.0 |
| Rhodotorula glutinis NRRL YB-252 | 20.5 | 1.5 | 2.0 | 55.5 | 9.0 | 11.5 |
| Cryptococcus curvatus NRRL Y-1511 | 22.5 | 0.5 | 14.0 | 49.9 | 13.0 | 0.1 |
| Lipomyces starkeyi DSM 702096 | 28.9 | 5.0 | 5.4 | 50.1 | 5.5 | 5.1 |
| Cryptococcus curvatus ATCC 20509 | 22.5 | 0.9 | 9.0 | 51.1 | 10.8 | 5.7 |
| (a) Cryptococcus curvatus | Fatty-Acid Composition of Cellular Lipids (%, w/w) | |||||
| PCC0 (g/L) | Fermentation Period | C16:0 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | Others |
| 0.0 | LE | 28.0 | 8.6 | 50.0 | 10.5 | 2.9 |
| S | 24.0 | 11.0 | 54.3 | 7.0 | 3.7 | |
| 1.16 | LE | 25.3 | 11.4 | 46.3 | 5.4 | 11.6 |
| S | 21.5 | 13.0 | 54.0 | 7.5 | 4.0 | |
| 1.65 | LE | 25.0 | 12.0 | 49.0 | 6.1 | 7.9 |
| S | 19.7 | 13.7 | 54.4 | 8.2 | 4.0 | |
| 1.91 | LE | 24.8 | 12.2 | 51.2 | 6.8 | 5.0 |
| S | 20.1 | 13.9 | 54.0 | 8.9 | 3.1 | |
| (b) Lipomyces starkeyi | Fatty-Acid Composition of Cellular Lipids (%, w/w) | |||||
| PCC0 (g/L) | Fermentation Period | C16:0 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | Others |
| 0.0 | LE | 31.5 | 3.9 | 49.0 | 5.8 | 9.8 |
| S | 32.0 | 5.0 | 53.0 | 2.7 | 7.3 | |
| 1.96 | LE | 32.3 | 4.7 | 49.7 | 6.6 | 6.7 |
| S | 33.4 | 6.3 | 52.5 | 2.9 | 4.9 | |
| (a) Flask Experiment | |||||
| % w/w | C16:0 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | |
| Total lipid | 26.0 | 9.7 | 53.2 | 7.2 | |
| NLs | 85.2 | 29.0 | 6.5 | 50.8 | 10.8 |
| G+S | 12.6 | 26.0 | 7.2 | 53.4 | 6.1 |
| PLs | 2.2 | 22.1 | 8.0 | 59.0 | 7.0 |
| (b) Bioreactor Experiment | |||||
| % w/w | C16:0 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | |
| Total lipid | 32.1 | 6.0 | 54.4 | 2.9 | |
| NLs | 88.1 | 40.2 | 4.8 | 49.4 | 3.0 |
| G+S | 10.0 | 37.3 | 8.8 | 49.3 | 2.1 |
| PLs | 1.9 | 26.4 | 5.1 | 61.4 | 4.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xenopoulos, E.; Giannikakis, I.; Chatzifragkou, A.; Koutinas, A.; Papanikolaou, S. Lipid Production by Yeasts Growing on Commercial Xylose in Submerged Cultures with Process Water Being Partially Replaced by Olive Mill Wastewaters. Processes 2020, 8, 819. https://doi.org/10.3390/pr8070819
Xenopoulos E, Giannikakis I, Chatzifragkou A, Koutinas A, Papanikolaou S. Lipid Production by Yeasts Growing on Commercial Xylose in Submerged Cultures with Process Water Being Partially Replaced by Olive Mill Wastewaters. Processes. 2020; 8(7):819. https://doi.org/10.3390/pr8070819
Chicago/Turabian StyleXenopoulos, Evangelos, Ioannis Giannikakis, Afroditi Chatzifragkou, Apostolis Koutinas, and Seraphim Papanikolaou. 2020. "Lipid Production by Yeasts Growing on Commercial Xylose in Submerged Cultures with Process Water Being Partially Replaced by Olive Mill Wastewaters" Processes 8, no. 7: 819. https://doi.org/10.3390/pr8070819





