Electrochemical Hydrogen Production Using Separated-Gas Cells for Soybean Oil Hydrogenation
Abstract
:1. Introduction
Zirfon
2. Experimental Development
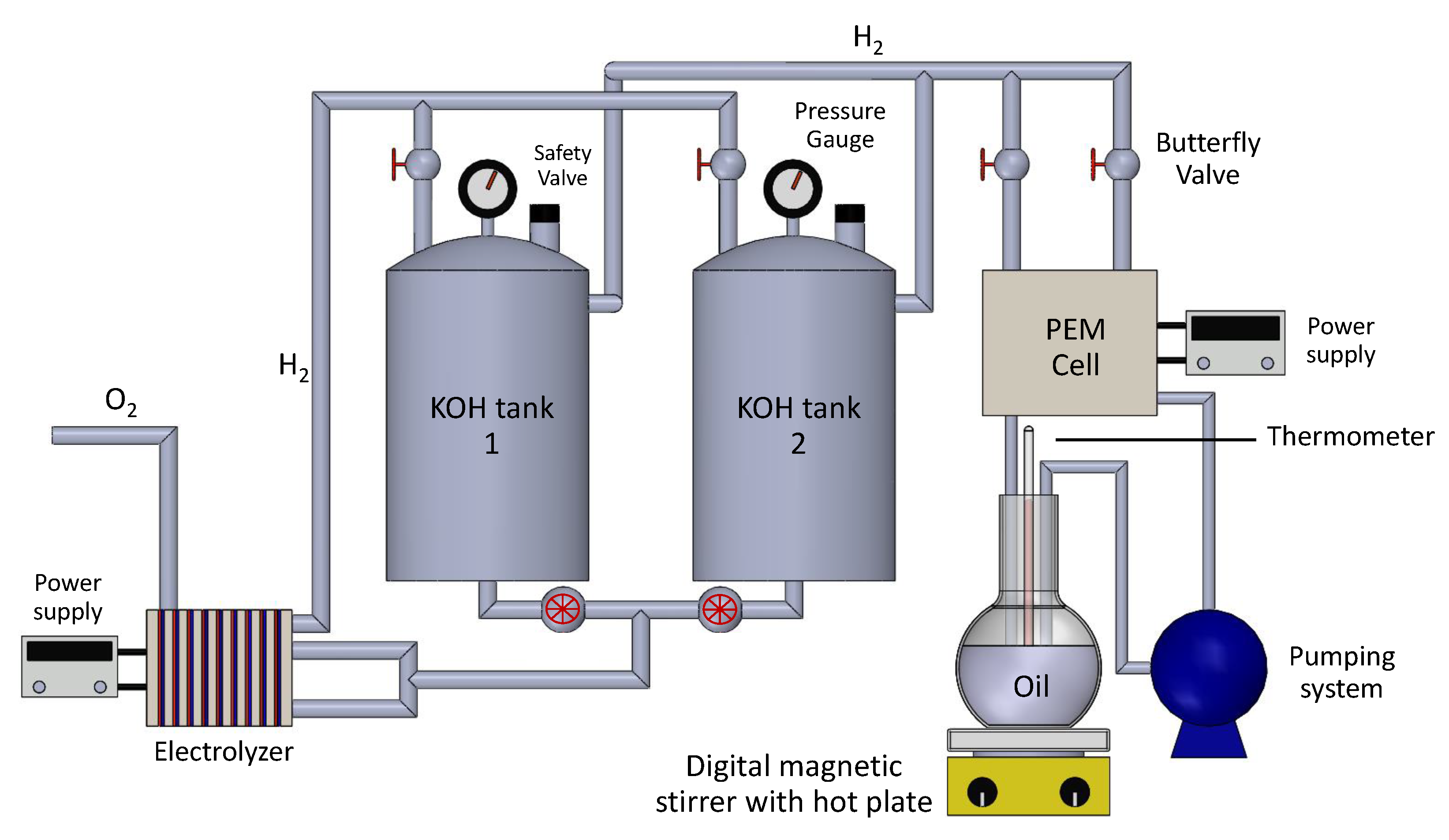
2.1. Experimental Procedure
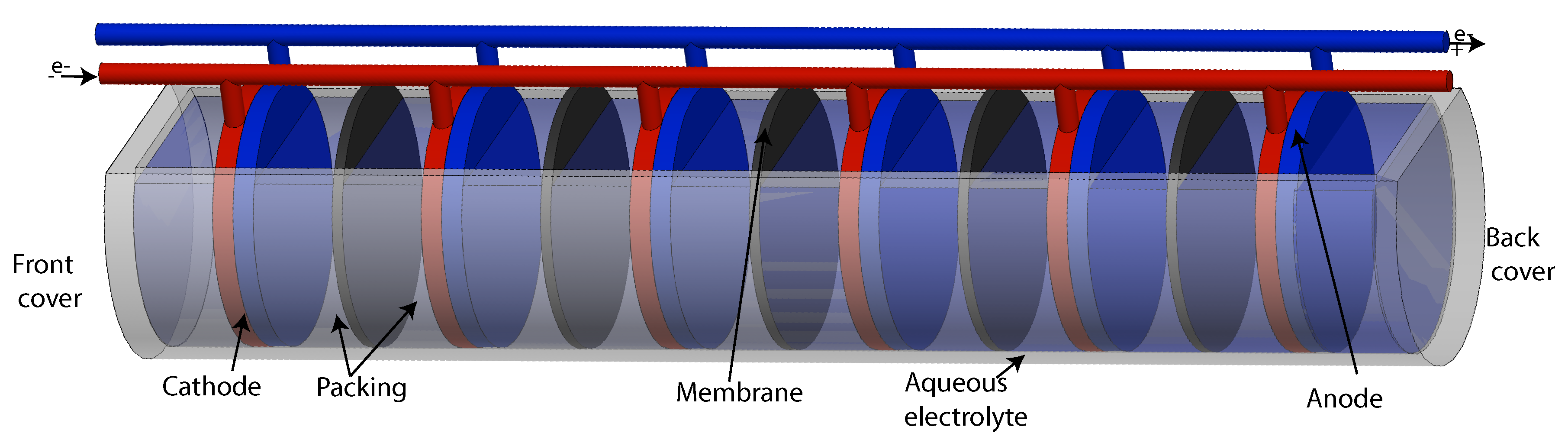
2.2. Design of Separate Gas Electrolyzer and Oil Hydrogenation PEM Reactor
2.3. Chemical Analysis
- I is the iodine number;
- m is the sample mass (g);
- is the volume of sodium thiosulfate expended in blank titration (mL);
- is the volume of sodium thiosulfate expended in sample titration (mL);
- N is the normality of the sodium thiosulfate sample (eq/L), and iodine constant with the value of 0.1269.
3. Results and Discussion
3.1. Hydrogen Production in a Separate Gas Electrolyzer
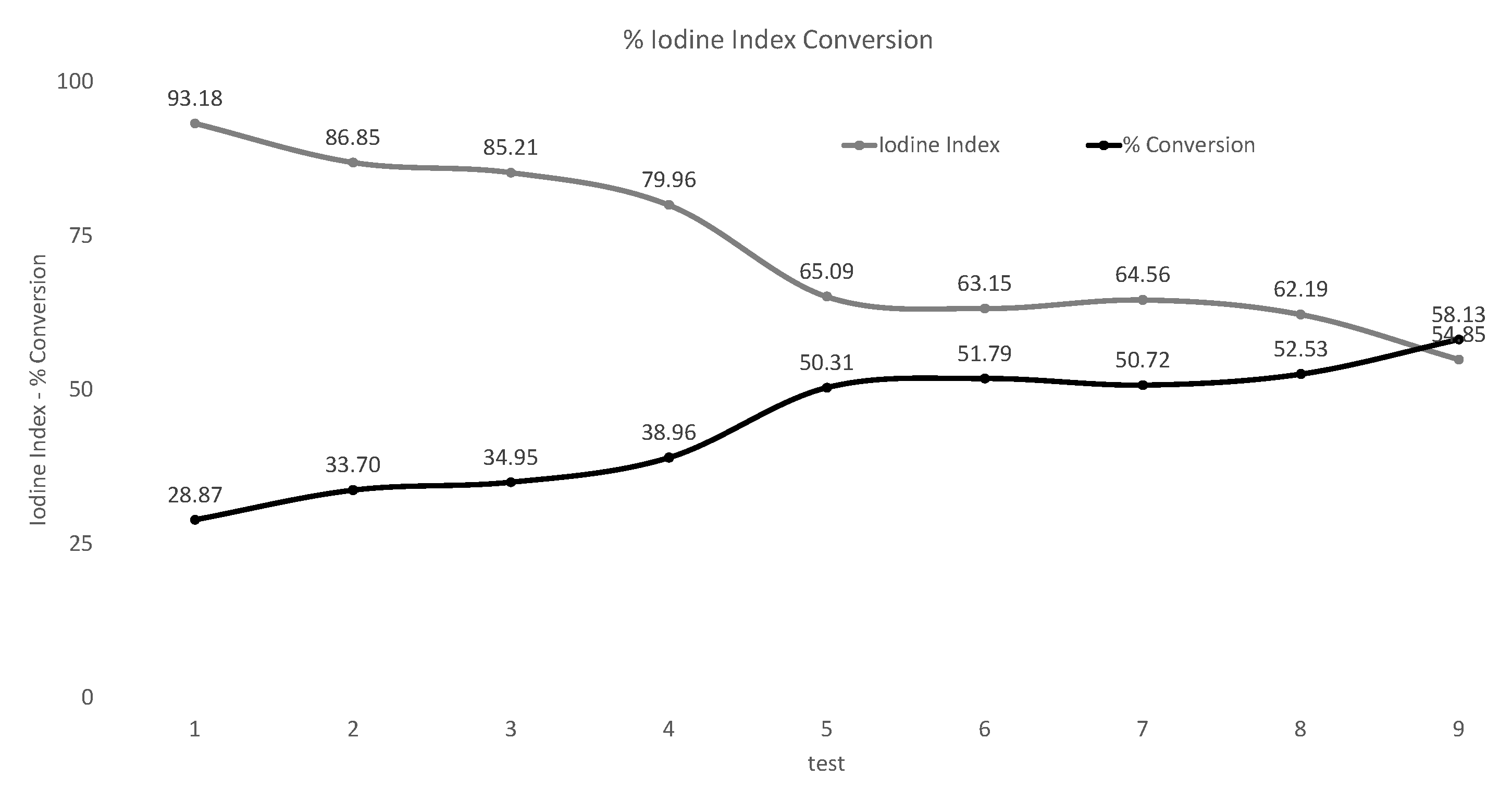
3.2. Iodine Index Results
4. Conclusions
5. Future Works
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Potential under standard conditions (potentials are tabulated for different reduction reactions) | |
| Potential cathode energy | |
| Potential Anode Energy | |
| E | Corrected cell potential |
| R | Gas constant in J/mole K |
| T | Absolute temperature (Kelvin scale) |
| n | Amount of mole of electrons involved in the reaction |
| F | Faraday’s constant in C/mole |
| Q | Reaction ratio in the Nernst equation. |
| Electrical charge calculation in C/min |
References
- Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8, 248. [Google Scholar] [CrossRef] [Green Version]
- DemirbaŞ, A. Fuel conversional aspects of palm oil and sunflower oil. Energy Sources 2003, 25, 457–466. [Google Scholar] [CrossRef]
- Vermeiren, P.; Adriansens, W.; Leysen, R. Zirfon: A new separator for Ni-H {sub 2} batteries and alkaline fuel cells. Int. J. Hydrogen Energy 1996, 21, 679–684. [Google Scholar] [CrossRef]
- Lu, Z.; Cherepakhin, V.; Kapenstein, T.; Williams, T.J. Upgrading biodiesel from vegetable oils by hydrogen transfer to its fatty esters. ACS Sustain. Chem. Eng. 2018, 6, 5749–5753. [Google Scholar] [CrossRef]
- Xu, L.; Yu, Y.; Li, W.; You, Y.; Xu, W.; Zhang, S. The influence of manufacturing parameters and adding support layer on the properties of Zirfon® separators. Front. Chem. Sci. Eng. 2014, 8, 295–305. [Google Scholar] [CrossRef]
- Vermeiren, P.; Adriansens, W.; Moreels, J.; Leysen, R. Evaluation of the Zirfon® separator for use in alkaline water electrolysis and Ni-H2 batteries. Int. J. Hydrogen Energy 1998, 23, 321–324. [Google Scholar] [CrossRef]
- Nikolic, V.M.; Tasic, G.S.; Maksic, A.D.; Saponjic, D.P.; Miulovic, S.M.; Kaninski, M.P.M. Raising efficiency of hydrogen generation from alkaline water electrolysis–Energy saving. Int. J. Hydrogen Energy 2010, 35, 12369–12373. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Stojić, D.L.; Marčeta, M.P.; Sovilj, S.P.; Miljanić, Š.S. Hydrogen generation from water electrolysis—possibilities of energy saving. J. Power Sources 2003, 118, 315–319. [Google Scholar] [CrossRef]
- Vermeiren, P.H.; Leysen, R.; Beckers, H.; Moreels, J.P.; Claes, A. The influence of manufacturing parameters on the properties of macroporous Zirfon® separators. J. Porous Mater. 2008, 15, 259–264. [Google Scholar] [CrossRef]
- Verma, D.; Rana, B.S.; Kumar, R.; Sibi, M.; Sinha, A.K. Diesel and aviation kerosene with desired aromatics from hydroprocessing of jatropha oil over hydrogenation catalysts supported on hierarchical mesoporous SAPO-11. Appl. Catal. A Gen. 2015, 490, 108–116. [Google Scholar] [CrossRef]
- Aerts, P.; Kuypers, S.; Genne, I.; Leysen, R.; Mewis, J.; Vankelecom, I.; Jacobs, P. Polysulfone- ZrO2 Surface Interactions. The Influence on Formation, Morphology and Properties of Zirfon-Membranes. J. Phys. Chem. B 2006, 110, 7425–7430. [Google Scholar] [CrossRef] [PubMed]
- Sevda, S.; Dominguez-Benetton, X.; Vanbroekhoven, K.; Sreekrishnan, T.; Pant, D. Characterization and comparison of the performance of two different separator types in air—Cathode microbial fuel cell treating synthetic wastewater. Chem. Eng. J. 2013, 228, 1–11. [Google Scholar] [CrossRef]
- Trasarti, A.; Segobia, D.; Apesteguia, C.; Santoro, F.; Zaccheria, F.; Ravasio, N. Selective hydrogenation of soybean oil on copper catalysts as a tool towards improved bioproducts. J. Am. Oil Chem. Soc. 2012, 89, 2245–2252. [Google Scholar] [CrossRef]
- Shin, H.Y.; Ryu, J.H.; Bae, S.Y.; Kim, Y.C. Biodiesel production from highly unsaturated feedstock via simultaneous transesterification and partial hydrogenation in supercritical methanol. J. Supercrit. Fluids 2013, 82, 251–255. [Google Scholar] [CrossRef]
- Pintauro, P.; Gil, M.P.; Warner, K.; List, G.; Neff, W. Electrochemical hydrogenation of soybean oil with hydrogen gas. Ind. Eng. Chem. Res. 2005, 44, 6188–6195. [Google Scholar] [CrossRef]
- Mondal, K.; Lalvani, S. Low temperature soybean oil hydrogenation by an electrochemical process. J. Food Eng. 2008, 84, 526–533. [Google Scholar] [CrossRef]
- Pletcher, D.; Li, X. Prospects for alkaline zero gap water electrolysers for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 15089–15104. [Google Scholar] [CrossRef] [Green Version]
- Darjat, D.; Sulistyo, S.; Triwiyatno, A.; Sudjadi; Kurniahadi, A. Designing Hydrogen and Oxygen Flow Rate Control on a Solid Oxide Fuel Cell Simulator Using the Fuzzy Logic Control Method. Processes 2020, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Watson, S.; Infield, D. Comparison of electrical energy efficiency of atmospheric and high-pressure electrolysers. Int. J. Hydrogen Energy 2006, 31, 1964–1979. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Gong, X.; Guo, Z. The intensification technologies to water electrolysis for hydrogen production—A review. Renew. Sustain. Energy Rev. 2014, 29, 573–588. [Google Scholar] [CrossRef]
- Alaiz-Moretón, H.; Jove, E.; Casteleiro-Roca, J.L.; Quintián, H.; López García, H.; Benítez-Andrades, J.A.; Novais, P.; Calvo-Rolle, J.L. Bioinspired Hybrid Model to Predict the Hydrogen Inlet Fuel Cell Flow Change of an Energy Storage System. Processes 2019, 7, 825. [Google Scholar] [CrossRef] [Green Version]
- Morán-Durán, A.; Martínez-Sibaja, A.; Rodríguez-Jarquin, J.P.; Posada-Gómez, R.; González, O.S. PEM Fuel Cell Voltage Neural Control Based on Hydrogen Pressure Regulation. Processes 2019, 7, 434. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verastegui, J.E.E.; Zamora Antuñano, M.A.; Resendiz, J.R.; García García, R.; Kañetas, P.J.P.; Ordaz, D.L. Electrochemical Hydrogen Production Using Separated-Gas Cells for Soybean Oil Hydrogenation. Processes 2020, 8, 832. https://doi.org/10.3390/pr8070832
Verastegui JEE, Zamora Antuñano MA, Resendiz JR, García García R, Kañetas PJP, Ordaz DL. Electrochemical Hydrogen Production Using Separated-Gas Cells for Soybean Oil Hydrogenation. Processes. 2020; 8(7):832. https://doi.org/10.3390/pr8070832
Chicago/Turabian StyleVerastegui, Jorge Eduardo Esquerre, Marco Antonio Zamora Antuñano, Juvenal Rodríguez Resendiz, Raul García García, Pedro Jacinto Paramo Kañetas, and Daniel Larrañaga Ordaz. 2020. "Electrochemical Hydrogen Production Using Separated-Gas Cells for Soybean Oil Hydrogenation" Processes 8, no. 7: 832. https://doi.org/10.3390/pr8070832
APA StyleVerastegui, J. E. E., Zamora Antuñano, M. A., Resendiz, J. R., García García, R., Kañetas, P. J. P., & Ordaz, D. L. (2020). Electrochemical Hydrogen Production Using Separated-Gas Cells for Soybean Oil Hydrogenation. Processes, 8(7), 832. https://doi.org/10.3390/pr8070832






