Preparation and Characterization of Cinnamomum Essential Oil–Chitosan Nanocomposites: Physical, Structural, and Antioxidant Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. CS-CEO
2.2.2. CS-NPs
2.3. Analytical Methods
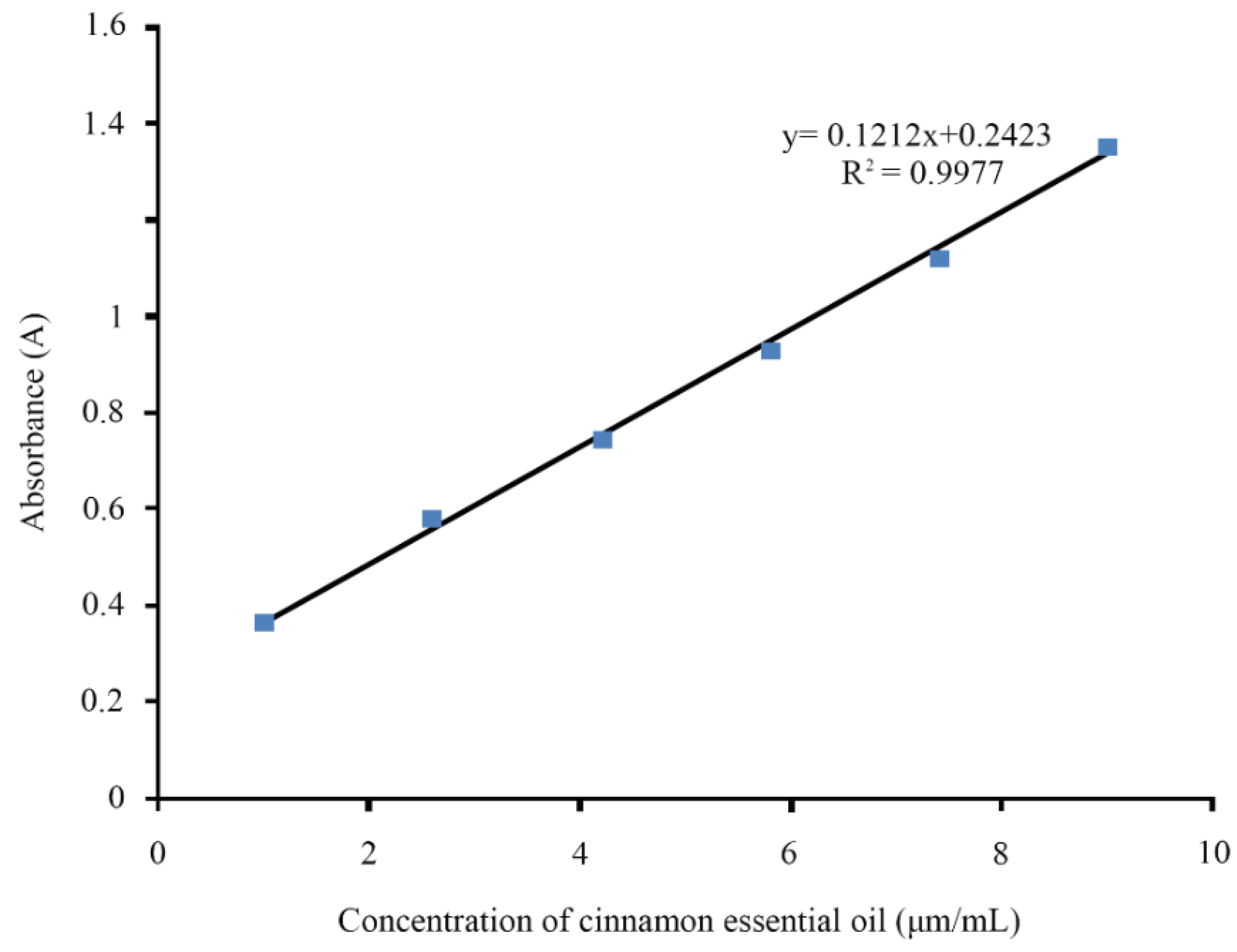
2.3.1. EE and LC
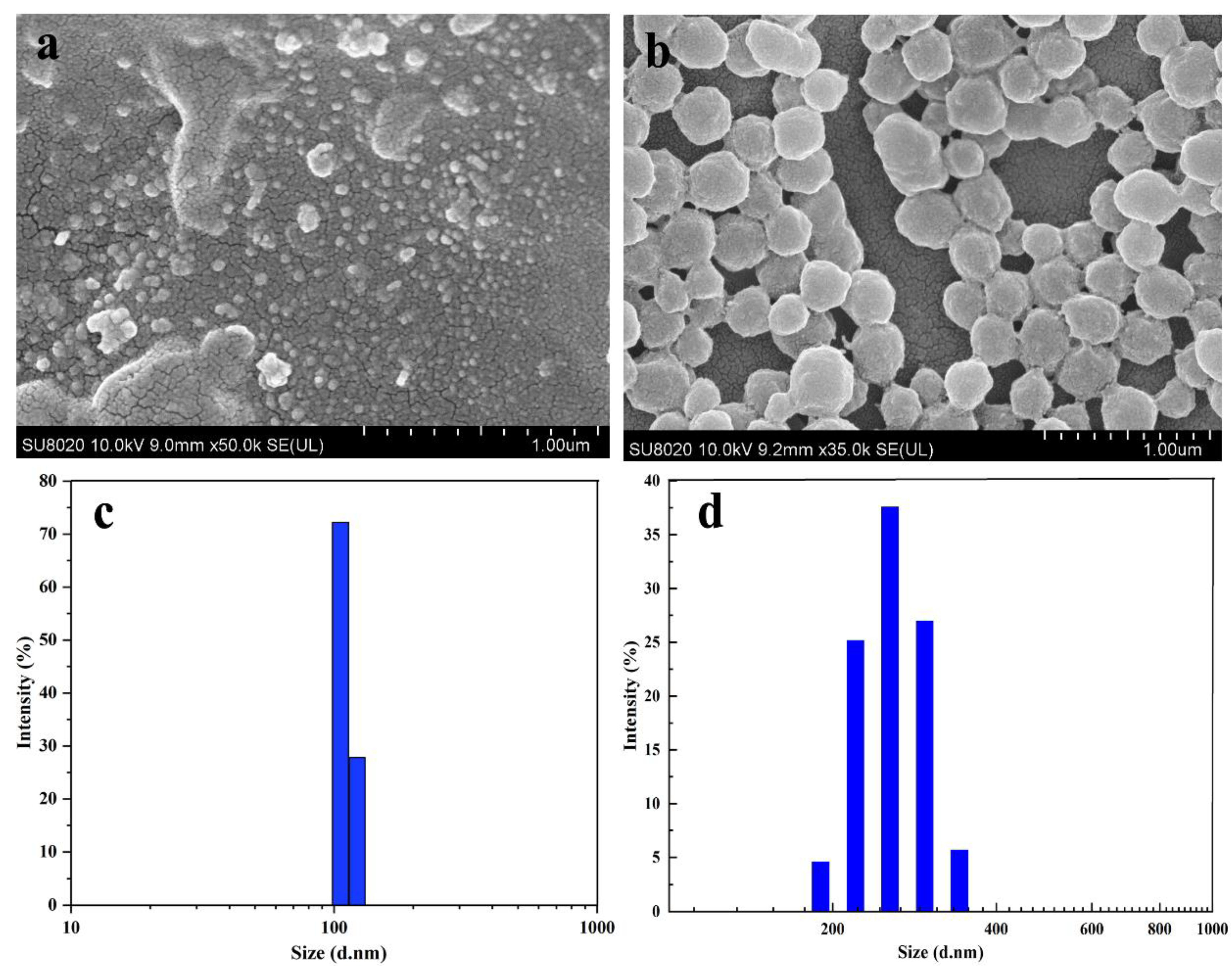
2.3.2. SEM
2.3.3. Particle Size
2.3.4. Zeta Potential Analysis
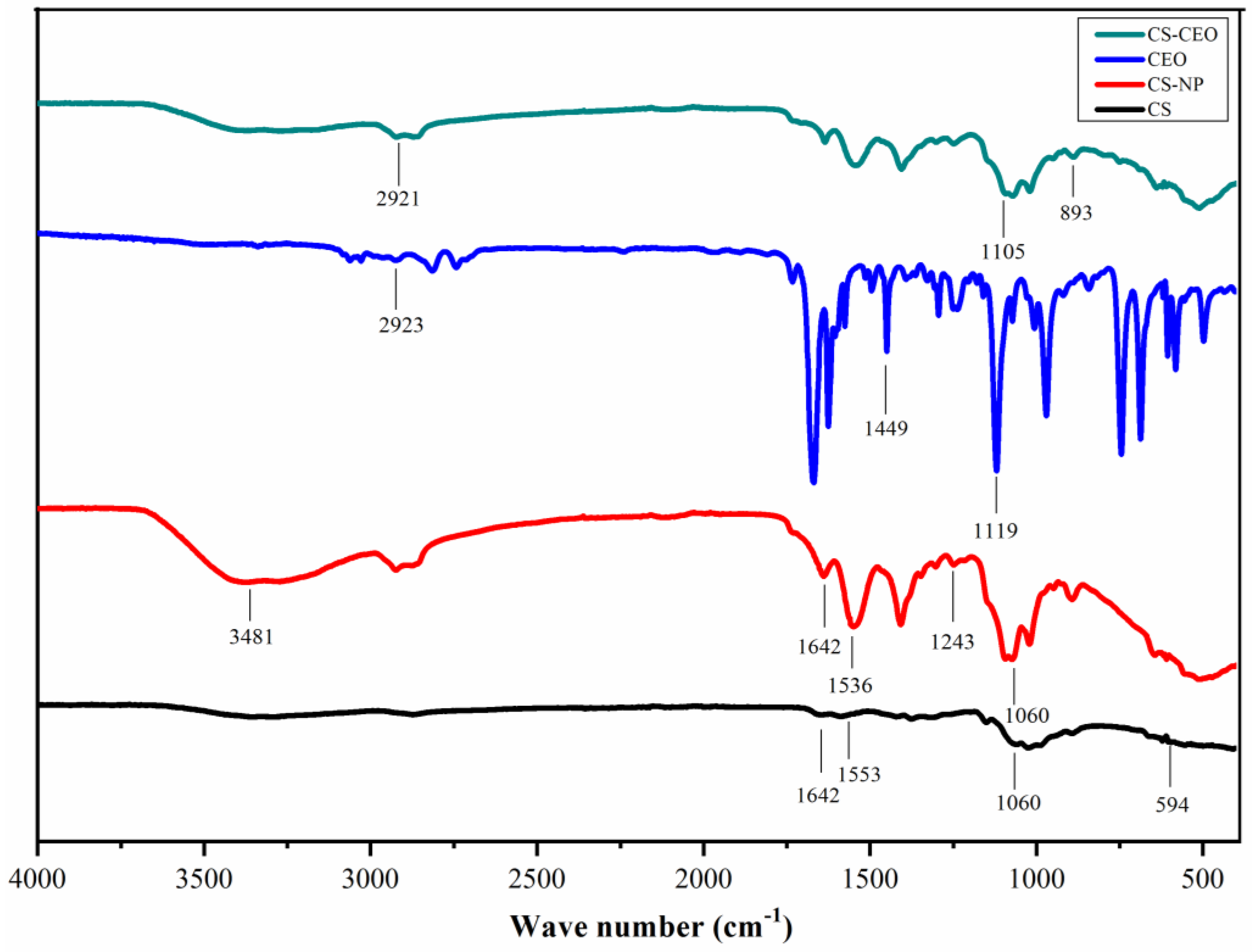
2.3.5. FT–IR
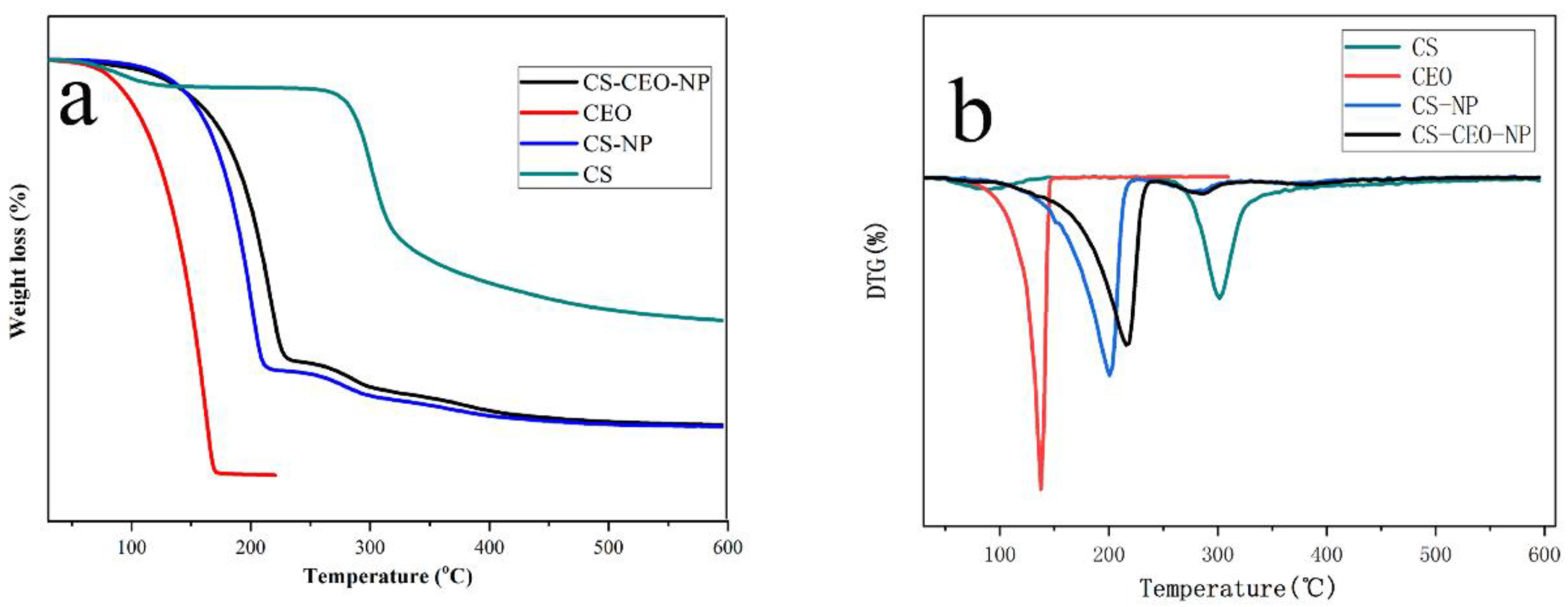
2.3.6. Thermogravimetric Analysis (TGA)
2.3.7. XRD
2.3.8. Antioxidant Activity (AOA)
- 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay and
- Hydroxyl free radical scavenging assay.
2.3.9. Statistical Analysis
3. Results and Discussion
3.1. EE and LC
3.2. Characterization of CS-NPs by SEM, Particle Size Analyzer, Zeta Potentials, FT-IR, TGA, and XRD
3.3. AOA
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Du, W.X.; Olsen, C.W.; Avena-Bustillos, R.J.; McHugh, T.H.; Levin, C.E.; Friedman, M. Effects of allspice, cinnamon, and clove bud essential oils in edible apple films on physical properties and antimicrobial activities. J. Food Sci. 2009, 74, M372–M378. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Popovic-Djordjevic, J.; Solak, M.H. Wild edible mushrooms from Mediterranean region: Metal concentrations and health risk assessment. Ecotoxicol. Environ. Saf. 2020, 190. [Google Scholar] [CrossRef] [PubMed]
- Canan, I.; Gundogdu, M.; Seday, U.; Oluk, C.A.; Karasahin, Z.; Eroglu, E.C.; Yazici, E.; Unlu, M. Determination of antioxidant, total phenolic, total carotenoid, lycopene, ascorbic acid, and sugar contents of Citrus species and mandarin hybrids. Turk. J. Agric. 2016, 40, 894–899. [Google Scholar] [CrossRef]
- Celik, A.; Ercisli, S.; Turgut, N. Some physical, pomological and nutritional properties of kiwifruit cv. Hayward. Int. J. Food Sci. Nutr. 2007, 58, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Sardeshpande, M.; Shackleton, C. Wild Edible Fruits: A Systematic Review of an Under-Researched Multifunctional NTFP (Non-Timber Forest Product). Forests 2019, 10, 467. [Google Scholar] [CrossRef] [Green Version]
- Ercisli, S.; Tosun, M.; Duralija, B.; Voca, S.; Sengu, M.; Turan, M. Phytochemical Content of Some Black (Morus nigra L.) and Purple (Morus rubra L.) Mulberry Genotypes. Food Technol. Biotech. 2010, 48, 102–106. [Google Scholar]
- Rop, O.; Ercisli, S.; Mlcek, J.; Jurikova, T.; Hoza, I. Antioxidant and radical scavenging activities in fruits of 6 sea buckthorn (Hippophae rhamnoides L.) cultivars. Turk. J. Agric. 2014, 38, 224–232. [Google Scholar] [CrossRef]
- Zorenc, Z.; Veberic, R.; Stampar, F.; Koron, D.; Mikulic-Petkovsek, M. Changes in berry quality of northern highbush blueberry (Vaccinium corymbosum L.) during the harvest season. Turk. J. Agric. 2016, 40, 855–864. [Google Scholar] [CrossRef]
- Mari, M.; Bautista-Banos, S.; Sivakumar, D. Decay control in the postharvest system: Role of microbial and plant volatile organic compounds. Postharvest Biol. Technol. 2016, 122, 70–81. [Google Scholar] [CrossRef]
- Lee, J.; Park, G.; Chang, Y.H. Nutraceuticals and antioxidant properties of Lonicera japonica Thunb. as affected by heating time. Int. J. Food Prop. 2019, 22, 630–645. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.H.; Wang, L.P.; Hu, Y.Q.; Chen, S.G.; Liu, D.H.; Ye, X.Q. Edible coating from citrus essential oil-loaded nanoemulsions: Physicochemical characterization and preservation performance. RSC Adv. 2016, 6, 20892–20900. [Google Scholar] [CrossRef]
- Kallel, I.; Hadrich, B.; Gargouri, B.; Chaabane, A.; Lassoued, S.; Gdoura, R.; Bayoudh, A.; Ben Messaoud, E. Optimization of Cinnamon (Cinnamomum zeylanicum Blume) Essential Oil Extraction: Evaluation of Antioxidant and Antiproliferative Effects. Evid. Based Complement. Altern. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atares, L.; Bonilla, J.; Chiralt, A. Characterization of sodium caseinate-based edible films incorporated with cinnamon or ginger essential oils. J. Food Eng. 2010, 100, 678–687. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. Antibacterial properties and major bioactive components of cinnamon stick (Cinnamomum burmannii): Activity against foodborne pathogenic bacteria. J. Agric. Food Chem. 2007, 55, 5484–5490. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Leyva, J.M.; Ortega-Ramirez, L.A.; Carrazco-Lugo, D.K.; Perez-Carlon, J.J.; Melgarejo-Flores, B.G.; Gonzalez-Aguilar, G.A.; Miranda, M.R.A. Pectin-cinnamon leaf oil coatings add antioxidant and antibacterial properties to fresh-cut peach. Flavour Frag. J. 2013, 28, 39–45. [Google Scholar] [CrossRef]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H.; Imran, M. Encapsulation of Cardamom Essential Oil in Chitosan Nano-composites: In-vitro Efficacy on Antibiotic-Resistant Bacterial Pathogens and Cytotoxicity Studies. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Azizi, M.H. Nanoencapsulation Approach to Improve Antimicrobial and Antioxidant Activity of Thyme Essential Oil in Beef Burgers during Refrigerated Storage. Food Bioprocess Technol. 2016, 9, 1187–1201. [Google Scholar] [CrossRef]
- Esmaeili, A.; Asgari, A. In vitro release and biological activities of Carum copticum essential oil (CEO) loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2015, 81, 283–290. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Huang, J.W.; Qin, J.Z.; Zhang, P.; Chen, X.M.; You, X.R.; Zhang, F.; Zuo, B.Q.; Yao, M. Facile preparation of a strong chitosan-silk biocomposite film. Carbohydr. Polym. 2020, 229. [Google Scholar] [CrossRef]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohydr. Polym. 2020, 231. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Yilmaz, A.; Akman, P.K.; Bozkurt, F.; Dertli, E.; Basahel, A.; Al-Sasi, B.; Taylan, O.; Sagdic, O. Electrospraying method for fabrication of essential oil loaded-chitosan nanoparticle delivery systems characterized by molecular, thermal, morphological and antifungal properties. Innov. Food Sci. Emerg. 2019, 52, 166–178. [Google Scholar] [CrossRef]
- Khalili, S.T.; Mohsenifar, A.; Beyki, M.; Zhaveh, S.; Rahmani-Cherati, T.; Abdollahi, A.; Bayat, M.; Tabatabaei, M. Encapsulation of Thyme essential oils in chitosan-benzoic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. LWT-Food Sci. Technol. 2015, 60, 502–508. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Chitosan nanoparticles loaded with Cinnamomum zeylanicum essential oil enhance the shelf life of cucumber during cold storage. Postharvest Biol. Tecnol. 2015, 110, 203–213. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Liu, X.Y.; Wang, Y.F.; Jiang, P.P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Vicente, S.; Neto, C.; Castro, P.M.; Veiga, M.; Madureira, R.; Tavaria, F.; Pintado, M.M. Chitosan nanoparticles as alternative anti-staphylococci agents: Bactericidal, antibiofilm and antiadhesive effects. Mater. Sci. Eng. C-Mater. 2017, 79, 221–226. [Google Scholar] [CrossRef]
- Feyzioglu, G.C.; Tornuk, F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT-Food Sci. Technol. 2016, 70, 104–110. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Bala, A.; Singh, B. Concomitant production of cellulase and xylanase by thermophilic mould Sporotrichum thermophile in solid state fermentation and their applicability in bread making. World J. Microbiol. Biotechnol. 2017, 33. [Google Scholar] [CrossRef]
- Pan, J.F.; Shen, H.X.; Luo, Y.K. Cryoprotective Effects of Trehalose on Grass Carp (Ctenopharyngodon Idellus) Surimi during Frozen Storage. J. Food Process. Preserv. 2010, 34, 715–727. [Google Scholar] [CrossRef]
- Carvalho, R.L.; Cabral, M.F.; Germano, T.A.; de Carvalho, W.M.; Brasil, I.M.; Gallao, M.I.; Moura, C.F.H.; Lopes, M.M.A.; de Miranda, M.R.A. Chitosan coating with trans-cinnamaldehyde improves structural integrity and antioxidant metabolism of fresh-cut melon. Postharvest Biol. Technol. 2016, 113, 29–39. [Google Scholar] [CrossRef]
- Dong, H.M.; Zhang, Q.; Li, L.; Liu, J.; Shen, L.W.; Li, H.Y.; Qin, W. Antioxidant activity and chemical compositions of essential oil and ethanol extract of Chuanminshen violaceum. Ind. Crop. Prod. 2015, 76, 290–297. [Google Scholar] [CrossRef]
- Vilas, V.; Philip, D.; Mathew, J. Essential oil mediated synthesis of silver nanocrystals for environmental, anti-microbial and antioxidant applications. Mater. Sci. Eng. C-Mater. 2016, 61, 429–436. [Google Scholar] [CrossRef]
- Kavaz, D.; Idris, M.; Onyebuchi, C. Physiochemical characterization, antioxidative, anticancer cells proliferation and food pathogens antibacterial activity of chitosan nanoparticles loaded with Cyperus articulatus rhizome essential oils. Int. J. Biol. Macromol. 2019, 123, 837–845. [Google Scholar] [CrossRef]
- Abreu, F.O.M.S.; Oliveira, E.F.; Paula, H.C.B.; de Paula, R.C.M. Chitosan/cashew gum nanogels for essential oil encapsulation. Carbohydr. Polym. 2012, 89, 1277–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donsi, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT-Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Bai, Z.F.; Cristancho, D.E.; Rachford, A.A.; Reder, A.L.; Williamson, A.; Grzesiak, A.L. Controlled Release of Antimicrobial ClO2 Gas from a Two-Layer Polymeric Film System. J. Agric. Food Chem. 2016, 64, 8647–8652. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Jin, T.; Fan, X.T.; Liu, L.S.; Yam, K.L. Development of Chlorine Dioxide Releasing Film and Its Application in Decontaminating Fresh Produce. J. Food Sci. 2013, 78, M276–M284. [Google Scholar] [CrossRef]
- Luque-Alcaraz, A.G.; Cortez-Rocha, M.O.; Velazquez-Contreras, C.A.; Acosta-Silva, A.L.; Santacruz-Ortega, H.D.; Burgos-Hernandez, A.; Arguelles-Monal, W.M.; Plascencia-Jatomea, M. Enhanced Antifungal Effect of Chitosan/Pepper Tree (Schinus molle) Essential Oil Bionanocomposites on the Viability of Aspergillus parasiticus Spores. J. Nanomater. 2016. [Google Scholar] [CrossRef] [Green Version]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr. Polym. 2020, 236. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; Zhao, M.M.; Lin, L.Z.; Wang, J.F.; Sun-Waterhouse, D.X.; Dong, Y.; Zhuang, M.Z.; Su, G.W. Identification of antioxidative peptides from defatted walnut meal hydrolysate with potential for improving learning and memory. Food Res. Int. 2015, 78, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, A.; Tomas, V.; Tudela, J.; Miguel, M.G. Comparative study of GC-MS characterization, antioxidant activity and hyaluronidase inhibition of different species of Lavandula and Thymus essential oils. Flavour Frag. J. 2016, 31, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Takshak, S.; Agrawal, S.B. The role of supplemental ultraviolet-B radiation in altering the metabolite profile, essential oil content and composition, and free radical scavenging activities of Coleus forskohlii, an indigenous medicinal plant. Environ. Sci. Pollut. Res. 2016, 23, 7324–7337. [Google Scholar] [CrossRef]
- Qiu, C.; Chang, R.R.; Yang, J.; Ge, S.J.; Xiong, L.; Zhao, M.; Li, M.; Sun, Q.J. Preparation and characterization of essential oil-loaded starch nanoparticles formed by short glucan chains. Food Chem. 2017, 221, 1426–1433. [Google Scholar] [CrossRef]
- Wang, Y.F.; Xia, Y.W.; Zhang, P.Y.; Ye, L.; Wu, L.Q.; He, S.K. Physical Characterization and Pork Packaging Application of Chitosan Films Incorporated with Combined Essential Oils of Cinnamon and Ginger. Food Bioprocess Technol. 2017, 10, 503–511. [Google Scholar] [CrossRef]
- Zhaveh, S.; Mohsenifar, A.; Beiki, M.; Khalili, S.T.; Abdollahi, A.; Rahmani-Cherati, T.; Tabatabaei, M. Encapsulation of Cuminum cyminum essential oils in chitosan-caffeic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind. Crop. Prod. 2015, 69, 251–256. [Google Scholar] [CrossRef]
- Wong, L.W.; Loke, X.J.; Chang, C.K.; Ko, W.C.; Hou, C.Y.; Hsieh, C.W. Use of the plasma-treated and chitosan/gallic acid-coated polyethylene film 2 for the preservation of tilapia (Orechromis niloticus) fillets. Food Chem. 2020, 329. [Google Scholar] [CrossRef]



| NO | CEO: CS (w/w) | EE (%) | LC (%) |
|---|---|---|---|
| NP0 | 0:1 | 0 | 0 |
| NP-A | 0.11:1 | 4.6 ± 0.5 | 0.9 ± 0.3 |
| NP-B | 0.25:1 | 22.5 ± 1.5 | 7.8 ± 0.7 |
| NP-C | 0.39:1 | 32.9 ± 2.3 | 10.4 ± 0.7 |
| NP-D | 0.53:1 | 24.6 ± 0.5 | 10.3 ± 1.5 |
| Sample | Zeta Potential (mV) |
|---|---|
| CS-NP | 23.47 ± 1.06 |
| CS-CEO | 17.33 ± 1.16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.; Huang, C.; Liu, Y.; Kong, S.; Wang, J.; Huang, H.; Zhang, B. Preparation and Characterization of Cinnamomum Essential Oil–Chitosan Nanocomposites: Physical, Structural, and Antioxidant Activities. Processes 2020, 8, 834. https://doi.org/10.3390/pr8070834
Su H, Huang C, Liu Y, Kong S, Wang J, Huang H, Zhang B. Preparation and Characterization of Cinnamomum Essential Oil–Chitosan Nanocomposites: Physical, Structural, and Antioxidant Activities. Processes. 2020; 8(7):834. https://doi.org/10.3390/pr8070834
Chicago/Turabian StyleSu, Hongxia, Chongxing Huang, Ying Liu, Song Kong, Jian Wang, Haohe Huang, and Bobo Zhang. 2020. "Preparation and Characterization of Cinnamomum Essential Oil–Chitosan Nanocomposites: Physical, Structural, and Antioxidant Activities" Processes 8, no. 7: 834. https://doi.org/10.3390/pr8070834






