Magnetic and Characterization Studies of CoO/Co3O4 Nanocomposite
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation Route
2.3. Characterization Techniques
3. Results
3.1. XRD Study
3.2. FTIR Analysis
3.3. Energy Dispersive X-ray Spectrometry (EDS) Analysis
3.4. Morphologic Analysis
3.5. Surface Properties
3.6. Magnetic Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Romero, M.; Steinfeld, A. Concentrating solar thermal power and thermochemical fuels. Energy Environ. Sci. 2012, 5, 9234–9245. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sust. Energ. Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Sattler, C. Hybrid sensible/thermochemical solar energy storge concepts based on porous ceramic structures and redox pair oxides chemistry. Energy Procedia 2015, 69, 706–715. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Sattler, C. Exploitation of thermochemical cycles based on solid oxide redox systems for thermochemical storage of solar heat. Sol. En. 2014, 102, 189–211. [Google Scholar] [CrossRef]
- Wong, B. Thermochemical Heat Storage for Concentrated Solar Power; U.S. Department of Energy. General Atomics Project 30314; 2011. Available online: https://www.osti.gov/biblio/1039304 (accessed on 11 May 2020).
- Gangopadhyay, S.; Hadjipanayis, G.C.; Sorensen, C.M.; Klabunde, K.J. Exchange anisotropy in oxide passivated Co fine particles. J. Appl. Phys. 1993, 73, 6964–6966. [Google Scholar] [CrossRef]
- Resnick, D.A.; Gilmore, K.; Idzerda, Y.U.; Klem, M.T.; Allen, M.; Douglas, T.; Arenholz, E.; Young, M. Magnetic properties of Co3O4 nanoparticles mineralized in Listeria innocua Dps. J. Appl. Phys. 2006, 99, 08Q501–08Q503. [Google Scholar] [CrossRef]
- He, L.; Chen, C.; Wang, N.; Zhou, W.; Guo, L. Finite size effect on N’eel temperature with Co3O4 nanoparticles. J. Appl. Phys. 2007, 102, 103911–103914. [Google Scholar] [CrossRef]
- Salabas, E.L.; Rumplecker, A.; Kleitz, F.; Radu, F.; Schüth, F. Exchange anisotropy in nanocasted Co3O4 nanowires. Nano Lett. 2006, 6, 2977–2981. [Google Scholar] [CrossRef]
- Duan, X.; Huang, Y.; Agarwal, R.; Lieber, C.M. Single-nanowire electrically driven lasers. Nature 2003, 421, 241–245. [Google Scholar] [CrossRef]
- Kim, H.; Park, D.W.; Woo, H.C.; Chung, J.S. Reduction of SO2 by CO to elemental sulfurover Co3O4-TiO2 catalysts. Appl. Catal. B 1998, 19, 233–243. [Google Scholar] [CrossRef]
- Li, W.Y.; Xu, L.N.; Chen, J. Co3O4 nanomaterials in lithium-ion batteries and gas sensors. Adv. Funct. Mater. 2005, 15, 851–857. [Google Scholar] [CrossRef]
- Elliott, D.W.; Zhang, W.X. Field Assessment of Nanoscale Bimetallic Particles for Groundwater Treatment. Environ. Sci. Technol. 2001, 35, 4922–4926. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Brus, L.; Murray, C.B. Synthesis of Monodisperse Nanoparticles of Barium Titanate: Toward a Generalized Strategy of Oxide Nanoparticle Synthesis. J. Am. Chem. Soc. 2001, 123, 12085–12086. [Google Scholar] [CrossRef]
- Roth, W.L. The magnetic structure of Co3O4. J. Phys. Chem. Solids. 1964, 25, 1–10. [Google Scholar] [CrossRef]
- Dutta, P.; Seehra, M.S.; Thota, S.; Kumar, J. A comparative study of the magnetic properties of bulk and nanocrystalline Co3O4. J. Phys. Condens Matter. 2008, 20, 1–8. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Konstantinov, K.; Yuan, L.; Liu, H.K.; Dou, S.X. In-situ fabrication of nanostructured cobalt oxide powders by spray pyrolysis technique. J. Nanosci. Nanotechnol. 2004, 8, 861–866. [Google Scholar] [CrossRef]
- Wu, J.G.; Tu, J.P.; Wang, X.L.; Zhang, W.K. Synthesis of nanoscale CoO particles and their effect on the positive electrodes of nickel–metal hydride batteries. Int. J. Hydrog. Energy 2007, 32, 606–610. [Google Scholar] [CrossRef]
- He, T.; Chen, D.; Jiao, X.; Wang, Y.; Duan, Y. Solubility-controlled synthesis of high-quality C03O4 nanocrystals. Chem. Mater. 2005, 17, 4023–4030. [Google Scholar] [CrossRef]
- Deraz, N.M. Catalytic decomposition of H2O2 on promoted cobaltic oxide catalysts. Mater. Lett. 2002, 57, 914–920. [Google Scholar] [CrossRef]
- Deraz, N.M. The Effect of Calcium Doping on the Surface and Catalytic Properties of Cobaltic Oxide Catalysts. Adsorp. Sci. Technol. 2003, 21, 229–243. [Google Scholar] [CrossRef]
- Deraz, N.M.; Fouda, M.M.G. Fabrication and Magnetic Properties of Cobalt-Copper Nano-Composite. Int. J. Electrochem. Sci. 2013, 8, 2682–2690. [Google Scholar]
- Toniolo, J.C.; Takimi, A.S.; Bergmann, C.P. Nanostructured cobalt oxides (Co3O4 and CoO) and metallic Co powders synthesized by the solution combustion method. Mater. Res. Bull. 2010, 45, 672–676. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesly Publishing Co. Inc., Techpark: Singapore, 1976; Ch. 14. [Google Scholar]
- Will, G.; Masciocchi, N.; Parrish, W.; Hart, M. Refinement of simple crystal structures from synchrotron radiation powder diffraction data. J. Appl. Cryst. 1987, 20, 394–401. [Google Scholar] [CrossRef]
- Sasaki, S.; Fujino, K.; Takeuchi, Y. X-Ray Determination of Electron-Density Distributions in Oxides, MgO, MnO, CoO, and NiO, and Atomic Scattering Factors of their Constituent Atoms. Proc Jpn. Acad 1979, 55, 43–48. [Google Scholar] [CrossRef]
- Boultif, A.; Louer, D. Powder pattern indexing with the dichotomy method. J. Appl. Cryst. 2004, 37, 724–731. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Yu, J.; Wu, T.; Wang, G. Surface structure and catalytic behavior of silica-supported copper catalysts prepared by impregnation and sol–gel methods. Appl. Catal. A-Gen. 2003, 239, 87–94. [Google Scholar] [CrossRef]
- Waldron, R.D. Infrared Spectra of Ferrites. Phys. Rev. 1955, 99, 1725–1727. [Google Scholar] [CrossRef]
- Ouaguenouni, H.S.M.; Benadda, A.; Kiennemann, A.; Barama, A. Preparation and Catalytic Activity of Nickel –manganese oxide catalysts in the reaction of partial oxidation of methane. Comptes Rendus Chim. 2009, 12, 740–747. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Ge, B.; Zhou, H.; Yuan, A.; Shen, X. Concave Co3O4 octahedral mesocrystal: Polymer-mediated synthesis and sensing properties. Cryst. Eng. Comm. 2012, 14, 6264–6270. [Google Scholar] [CrossRef]
- He, T.; Chen, D.; Jiao, X.; Xu, Y.; Gu, Y. Controlled Synthesis of Co3O4 Nanoparticles through Oriented Aggregation. Chem. Mater. 2004, 16, 737–743. [Google Scholar] [CrossRef]
- Berchmans, L.J.; Selvan, R.K.; Kumar, P.N.S.; Augustin, C.O. Structural and electrical properties of Ni1−x MgxFe2O4 synthesized by citrate gel process. J. Magn. Magn. Mater. 2004, 279, 103–110. [Google Scholar] [CrossRef]
- Rasband, W.S. Image J; U.S. National Institutes of Health: Bethesda, MD, USA, 1997–2012. Available online: https://Imagej.nih.gov/i/ (accessed on 11 May 2020).
- Sandomirski, S. Calculation and Analysis the Magnetic Parameters of the Minors Hysteresis Loop for Steels from the Basic Magnetic Parameters. 19th World Conference on Non-Destructive Testing. 2016. Available online: https://scholar.google.com.hk/scholar?hl=zh-TW&as_sdt=0%2C5&q=Calculation+and+Analysis+the+Magnetic+Parameters+of+the+Minors+Hysteresis+Loop+for+Steels+from+the+Basic+Magnetic+Parameters&btnG= (accessed on 11 May 2020).
- Sandomirski, S.G. The residual magnetization of the ferromagnetic body magnetized in an open magnetic circuit. Defektoskopiia 1997, 8, 50–59. [Google Scholar]
- Sandomirski, S.G. Computing the Magnetization Curve and the Minors of the Hysteresis Loop for Structural Ferromagnetic Materials from the Basic Magnetic Parameters. Elektritchestvo 2010, 1, 61–64. [Google Scholar]
- Shinde, V.R.; Mahadik, S.B.; Gujar, T.P.; Lokhande, C.D. Supercapacitive cobalt oxide (Co3O4) thin films by spray pyrolysis. Appl. Surf. Sci. 2006, 252, 7487–7492. [Google Scholar] [CrossRef]
- Rendale, M.K.; Mathad, S.N.; Puri, V. Thick films of magnesium zinc ferrite with lithium substitution: Structural characteristics. Int. J. Self-Propag. High-Temp. Synth. 2015, 24, 78–82. [Google Scholar] [CrossRef]
- Mathad, S.N.; Jadhav, R.N.; Patil, N.D.; Puri, V. Structural and mechanical properties of Sr+2 doped bismuth manganite thick films. Int. J. Self-Prop. High-Temp. Synth. 2013, 22, 180–184. [Google Scholar] [CrossRef]
- Mathad, S.N.; Jadhav, R.N.; Phadatare, V.; Puri, V. Structural and mechanical properties of Sr-doped barium niobate thick films. Int. J. Self-Propag. High-Temp. Synth. 2014, 23, 145–150. [Google Scholar] [CrossRef]
- Xavier, S.; Thankachan, S.; Jacob, B.P.; Mohammed, E.M. Effect of Samarium Substitution on the Structural and Magnetic Properties of Nanocrystalline Cobalt Ferrite. J. Nanosci. 2013, ID 524380, 1–7. [Google Scholar] [CrossRef]
- Singh, J.; Chae, K. d° Ferromagnetism of Magnesium Oxide. Condens. Matter 2017, 2, 1–13. [Google Scholar]
- Zou, G.F.; Li, H.; Zhang, D.W.; Xiong, K.; Dong, C.; Qian, Y.T. Well-aligned arrays of CuO nanoplatelets. J. Phys. Chem. B 2006, 110, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Gao, D.; Zhang, J.; Yang, Z.; Zhang, Z.; Rao, J.; Xue, D. Observation of room temperature ferromagnetism in pure La2O3 nanoparticles. Appl. Phys. A-Mater. Sci. 2014, 116, 1293–1298. [Google Scholar] [CrossRef]
- Kisan, B.; Alagarsamy, P. Room temperature ferromagnetism in finite sized ZnO nanoparticles. Phys. B Condens. Matter 2014, 448, 115–119. [Google Scholar] [CrossRef]
- Shi, S.; Gao, D.; Xu, Q.; Yang, Z.; Xue, D. Singly-charged oxygen vacancy-induced ferromagnetism in mechanically milled SnO2 powders. RSC Adv. 2014, 4, 45467–45472. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J.Z.; Li, Y.L.; Ma, D.C.; Hou, S.N.; Li., L.Z.; Hao, X.L.; Wang, Z.C. Room Temperature Synthesis of 2D CuO Nanoleaves in Aqueous Solution. Nanotechnology 2011, 22, 115604–115613. [Google Scholar] [CrossRef]
- Herzer, G. Nanocrystalline soft magnetic materials. J. Magn. Mater. 1992, 112, 258–262. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, A. Structural and Magnetic Properties of Cobalt Oxide Nanoparticles at Different Annealing Temperatures. Int. J. Mater. Sci. Eng. 2016, 4, 208–214. [Google Scholar]
- Razmara, Z. Synthesis, Characterization and Magnetic Properties of Bi-metallic Copper Complex, as a Precursor for the Preparation of CuO Nanoparticles and Its Application for Removal of Arsenic from Water. J. Inorg. Org. Poly. Mater. 2018, 28, 1255–1262. [Google Scholar] [CrossRef]
- Zhu, D.Z.J.; Zhang, J.; Liu, T.; Chen, L.; Liu, X.; Ma, R.; Zhang, H.; Qiu, G. Controllable fabrication and magnetic properties of double-shell cobalt oxides hollow particles. Sci. Rep. 2015, 5, 8737. [Google Scholar]
- NirmaleshNaveen, A.N. SubramanianSelladurai. Tailoring structural, optical and magnetic properties of spinel type cobalt oxide (Co3O4) by manganese doping. Physica B 2015, 457, 251–262. [Google Scholar]
- Binns, C. Introduction to Nanoscience and Nanotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; p. 229. [Google Scholar]
- Deraz, N.; Abd-Elkader, O.A.; Yassin, M. Impacts of Egg White Assisted Combustion and Ceramic Methods on Structural, Morphological and Magnetic Properties of Nickel Manganite System. Crystals 2020, 10, 489. [Google Scholar] [CrossRef]






| Properties | Values | |
|---|---|---|
| Co3O4 | CoO | |
| Crystal structure | Cubic | Cubic |
| Lattice constant (a), nm | 0.8093 | 0.4267 |
| Unit cell volume (V), nm3 | 0.5300 | 0.7770 |
| Density (D), g/cm3 | 5.95 | 6.1 |
| Dislocation density (ρd), nm−2 | 4.444 × 10−3 | 6.944 × 10−3 |
| Crystallite size (d), nm | 15 | 12 |
| h | k | l | d spacing Å | 2θ (Obs.) Deg. | 2θ (Cal.) Deg. | Differences | Phases |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 4.676 | 18.977 | 18.9935 | −0.0165 | Co3O4 |
| 0 | 2 | 2 | 2.860 | 31.267 | 31.2608 | 0.0062 | Co3O4 |
| 1 | 1 | 1 | 2.463 | 36.47 | 36.4723 | −0.0023 | CoO |
| 1 | 1 | 3 | 2.463 | 36.841 | 36.8346 | 0.0064 | Co3O4 |
| 2 | 2 | 2 | 2.335 | 38.543 | 38.5356 | 0.0074 | Co3O4 |
| 0 | 0 | 2 | 2.133 | 42.37 | 42.3656 | 0.0044 | CoO |
| 0 | 0 | 4 | 2.022 | 44.804 | 44.7954 | 0.0086 | Co3O4 |
| 3 | 1 | 3 | 1.857 | 49.046 | 49.0665 | −0.0205 | Co3O4 |
| 2 | 2 | 4 | 1.651 | 55.647 | 55.6365 | 0.0105 | Co3O4 |
| 1 | 1 | 5 | 1.557 | 59.348 | 59.3364 | 0.0116 | Co3O4 |
| 0 | 2 | 2 | 1.508 | 61.47 | 61.4634 | 0.0066 | CoO |
| 0 | 4 | 4 | 1.430 | 65.225 | 65.2124 | 0.0126 | Co3O4 |
| 1 | 3 | 5 | 1.367 | 68.618 | 68.604 | 0.014 | Co3O4 |
| 2 | 4 | 4 | 1.348 | 69.731 | 69.7167 | 0.0143 | Co3O4 |
| 1 | 1 | 3 | 1.286 | 73.63 | 73.6285 | 0.0015 | CoO |
| 2 | 0 | 6 | 1.279 | 74.109 | 74.0934 | 0.0156 | Co3O4 |
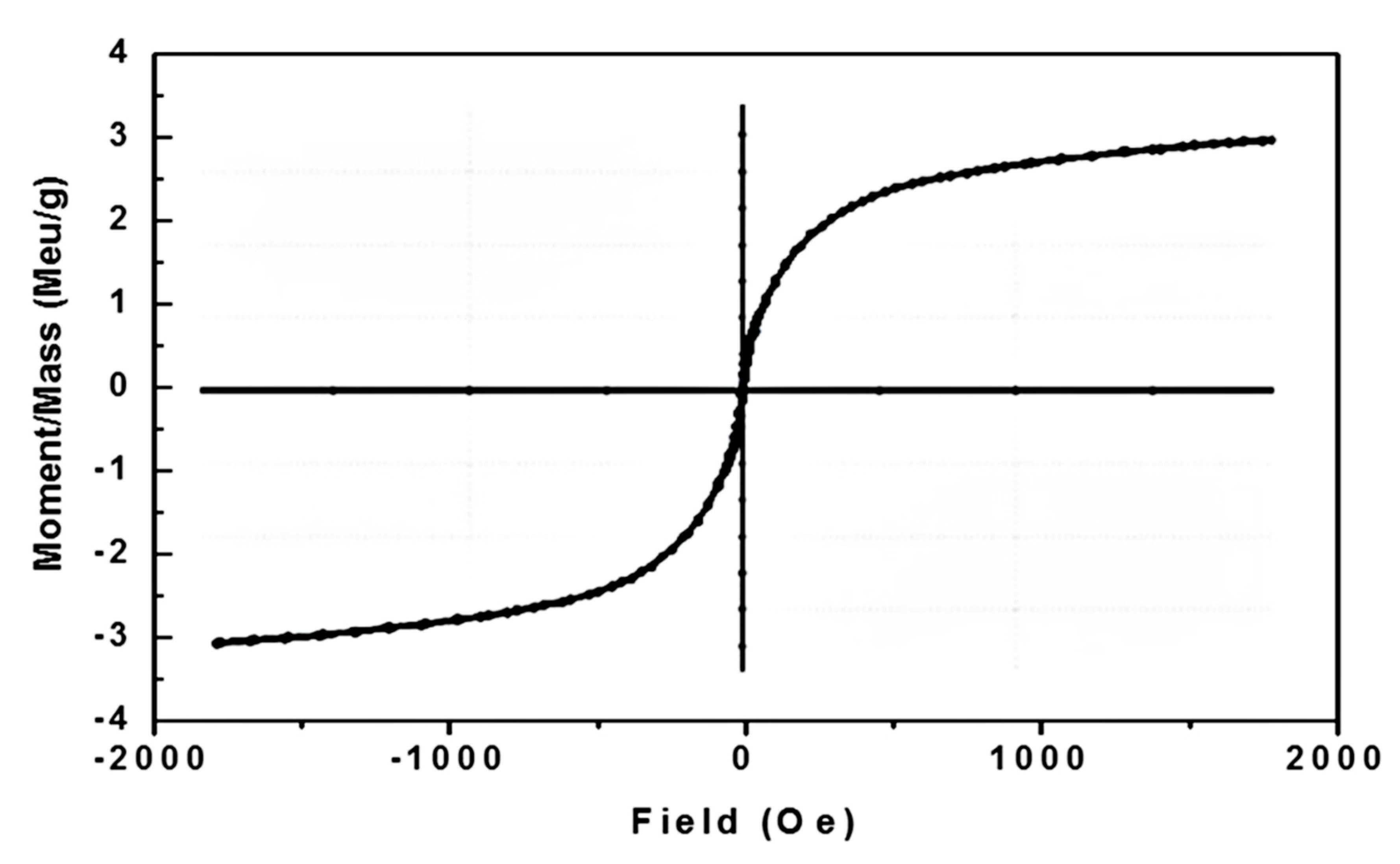
| Properties | Values |
|---|---|
| Saturation magnetization (Ms), emu/g | 3.450 |
| Remanent magnetization (Mr), emu/g | 0.2734 |
| Coercivity (Hc), Oe | 85.032 |
| Squareness (Mr/Ms) | 79.242 × 10−3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Senani, G.M.; Deraz, N.M.; Abd-Elkader, O.H. Magnetic and Characterization Studies of CoO/Co3O4 Nanocomposite. Processes 2020, 8, 844. https://doi.org/10.3390/pr8070844
Al-Senani GM, Deraz NM, Abd-Elkader OH. Magnetic and Characterization Studies of CoO/Co3O4 Nanocomposite. Processes. 2020; 8(7):844. https://doi.org/10.3390/pr8070844
Chicago/Turabian StyleAl-Senani, Ghadah M., Nasrallah M. Deraz, and Omar H. Abd-Elkader. 2020. "Magnetic and Characterization Studies of CoO/Co3O4 Nanocomposite" Processes 8, no. 7: 844. https://doi.org/10.3390/pr8070844
APA StyleAl-Senani, G. M., Deraz, N. M., & Abd-Elkader, O. H. (2020). Magnetic and Characterization Studies of CoO/Co3O4 Nanocomposite. Processes, 8(7), 844. https://doi.org/10.3390/pr8070844






