Abstract
CoO/Co3O4 nanoparticles (NPs) were synthesized by using a fresh egg white-assisted combustion method which acts as a new approach for green synthesis of this composite. This method was carried out by the direct heat of cobalt precursor with egg white at low temperature for very short period. In fact, this route is a novel, cheap and appropriate technique yielding nanoparticle-based materials. CoO/Co3O4 nanoparticles were characterized by examining the structure and identifying the elements and determining the morphology via XRD, FTIR, SEM, EDS and TEM techniques. The sample magnetic observations were measured through the use of a vibrating sample magnetometer (VSM). The results of XRD, EDS, SEM and TEM confirmed the positive synthesis of the cubic CoO/Co3O4 NPs with sponge crystals which proceed. For the as synthesized composite, 57.75 m2/g, 0.0148 cc/g and 10.31 nm were identified to be the SBET, Vp and ȓ, respectively. The cobalt oxide particles in their nature were polycrystalline, and the crystallite sizes varied from 10 to 20 nm. The magnetic measurement showed that the prepared nanocomposite displays room temperature ferromagnetism with an optimum value, 3.45 emu/g, of saturation magnetization.
1. Introduction
Solar energy is an important and vital source of energy because it is a renewable, inexpensive and infinite energy source. It constitutes an attractive source of energy for electrical, thermal and thermochemical applications [1]. In many countries of the world, solar energy may be absent for long periods, so thermal storage of solar energy has become a necessity. Therefore, thermal energy storage (TES) technologies were important and had a special place in the research process. More such technologies are regarded into three categories, based on sensitive, latent and thermochemical heat absorption and release [2]. Solar energy thermochemical storage systems (TCS) consist of reversible chemical reactions which absorb the solar heat during an endothermal step and release it through an exothermic reaction. TCS has the benefit of higher energy density stored (up to fifteen times greater than sensitive and six times greater than latent TES) [3]. Nevertheless, the energy storage density is one of the most significant factors of a TES system. CoO/Co3O4 redox pairs have great potential as materials for high temperature solar energy storage [4]. For Mn2O3/Mn3O4, CuO/Cu2O and CoO/Co3O4 the energy density was 202 kJ/kg, 811 kJ/kg and 844 kJ/kg, respectively [5].
Spinel Co3O4 as a p-type semiconductor with various nano structural forms such as nano rods, nano sheets and ordered nano flowers has been synthesized [6,7,8,9]. Co3O4-based promising material has been investigated with different applications such as catalysis processes, gas-sensing, solar energy absorption, magneto-electronics and electro chromic devices [10,11,12,13,14]. It may appear at first glance that the normal spinel structure of Co3O4 is closely related to that of the inverse spinel Fe3O4. Nonetheless, compared to Fe3O4, Co3O4 exhibits strikingly different magnetic order. In particular, Co3O4 does not exhibit ferrimagnetic ordering of the type demonstrated in Fe3O4 since Co3+ ions are in low spin S = 0 on octahedral B sites [15]. Alternatively, it demonstrates antiferromagnetic ordering on site A with each Co2+ ion requiring four adjacent Co2+ ions with reverses spins [16].
Co3O4 and CoO particles are generated largely by a broad range of wet chemical techniques such as spray-pyrolysis, freeze-drying, solubility-controlled synthesis, coprecipitation, combustion synthesis and sol–gel, [17,18,19,20,21,22,23]. To date, an information concerning synthesis of cobalt oxides varies with absence of studies on egg whites as fuel in preparation process of these oxides. In addition, knowledge of the egg-white-assisted green synthesis of cobalt oxides powders is scant. For this reason, the current work was confined to the synthesis of CoO/Co3O4 NPs by using egg white which had not recently been reported in this reverence. Characterization and magnetic properties of the as synthesized nanocomposite were also decided.
2. Experimental
2.1. Materials
Cobalt (II) nitrate hexahydrate with linear formula Co(NO3)2·6H2O was the only chemical material and was supplied by Sigma-Aldrich Company. As this reagent was analytical grade, it was used without further cleansing. The egg white was extracted from local hens’ fresh eggs.
2.2. Preparation Route
One sample of cobalt oxides was synthesized by mixing a detected weight of cobalt nitrate (2.91 g) with 5 mL of egg white, followed by continuous stirring at 80 °C in a Pyrex beaker that puted on hotplate to evaporate the water crystals, subsequently maximizing the viscosity of the resulting precursor sol as shown in Figure 1a. This sol was then heated at 120 °C to establish a gel (Figure 1b). The corresponding precursor gel was calcined for 15 min in a furnace at 350 °C, and then sparks appeared at one corner as shown in Figure 1c. These sparks spread across the entire prepared block, generating a good deal of foam which finishes by a black superfine massive powder (Figure 1d). Thus, the egg-white-assisted combustion method led to formation of a voluminous and flabby material containing porous cobalt oxides composite with a carbonaceous byproduct.

Figure 1.
Process flowchart for fabricating the as-prepared sample: (a) precursor sol; (b) precursor gel; (c) flaming gel and (d) combusted product.
2.3. Characterization Techniques
A BRUKER D8 advance diffractometer (Bruker, Karlsruhe, Karlsruhe, Germany) used to measure the various mixed solids using an X-ray. The patterns were measured at 40 kV with Cu Kα radiation and 40 mA, with a scanning speed of 2° min−1 in 2θ. The crystallite sizes of Co3O4 and CoO were defined by a broadening of the X-ray diffraction line using Scherer equation [24].
where d is the examined process’s average crystallite thickness, B is the Scherrer constant (0.89), λ is the X-ray wavelength, β is the diffraction’s full-width half limit (FWHM) and θ is the Bragg angle.
d = Bλ/βcosθ
PerkinElmer Spectrophotometer (type 1430, Liantrisant, CF72 8YW, United Kingdom) was used to determine the Fourier-transform infrared spectrum (FTIR) of different solids. At wavelength from 4000 to 400 cm−1 the spectra for IR were collected. Two mg of each solid sample were mixed with 200 mg of Potassium Bromide (KBr) grade vacuum-dried IR. Dispersed by grinding in a vibratory ball mill for 3 min, the mixture was inserted in a steel die 13 mm in diameter and exposed to a 12-ton strain. The sample discs were inserted in the double grating IR spectrometer holder.
A JEOL JAX-840A scanning electron microanalyzer (SEM) and JEOL Model 1230 (JEOL, Akishima, Tokyo, Japan) transmittance electron microanalyzer (TEM) captured the scanning and transmittance electron micrographs of the as-prepared solid, respectively. At first, the solid was washed in ethanol, and then ultrasonically adjusted to disperse individual particles over a small piece double-stick carbon tape putted over mount holders.
Energy dispersive X-ray analysis (EDS) was recorded on JEOL (JED-2200 Series) electron microscope with a Delta Kevex system attached (JEOL, Akishima, Tokyo, Japan).. The variables were as follows: 15 kV voltage acceleration, 100 s time accumulation, 8 μm width of window. The structure of surface molars was calculated by the Asa method, ZAF-correction, Gaussian extrapolation.
The surface properties of numerous solid catalysts—namely that surface basic area (SBET), total volume of pore (Vp) and mean radius of pore (ȓ)—were calculated from isotherms measured at 77 K using a Nova 2000, Quanta Chrome (commercial BET unit, Boynton Beach, FL 33426. USA). Before undertaking these measurements, each sample was degassed for 2 h at 200 °C under a reduced pressure of 10−5 Torr,.
The magnetic features of the examined solids were measured in an optimum applied field of ±2000 Oe at ambient temperature using a vibrating sample magnetometer (VSM; Lake Shore Model 7410, Weistron, West Hollywood, Hollywood, Los Angeles, CA, USA). A hysteresis loop acquired resulted in saturation magnetization (Ms), remanence magnetization (Mr), coercivity (Hc) and squareness (Mr/Ms) being calculated. The magnetic field was measured in the hysteresis loop with a relative error smaller than 0.0001%.
3. Results
3.1. XRD Study
The XRD pattern of the as-synthesized CoO/Co3O4 NPs is shown in Figure 2. The XRD trajectory demonstrates nanocomposite formation, which contained CoO and Co3O4. A series of diffraction peaks at degree 2θ = 31.19, 36.75, 38.25, 44.73, 55.47, 59.36, 65.37, 73.48, 76.67 and 77.7 characteristic to indexing planes (220), (311), (222), (400), (422), (511), (440), (620), (533) and (622), respectively. These planes agree with the values reported for Co3O4 NPs (PDF file No. 78-1970) [25]. In addition, these diffraction peaks can be indexed as typical cubic spinel structure for Co3O4 (Major phase). Another series of diffraction peaks at degree 2θ = 36.37, 42.24, 61.28, 73.39 and 77.24 characteristic to indexing planes (111), (200), (220), (311) and (222), respectively. These planes agree with the values reported for CoO NPs (PDF file No. 71-1187) [26]. However, these diffraction peaks can be indexed as typical cubic structure for CoO NPs. No other phases were observed in the XRD patterns. Moreover, in this study, one cannot ignore presence of a carbonaceous byproduct as impurities depended upon using egg white in the preparation method. This expectation will be confirmed in SEM and EDS sections.
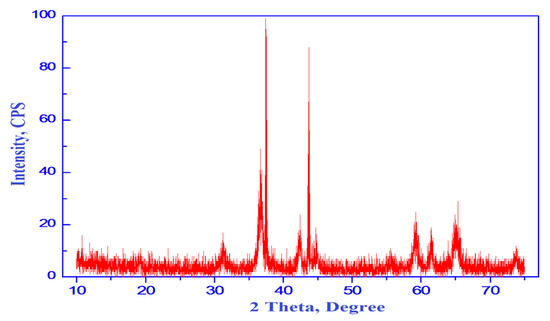
Figure 2.
XRD pattern of CoO/Co3O4 nanoparticles.
Thus, the XRD result displays the formation of CoO/Co3O4 nanocomposite. Indeed, most of the peaks present in XRD pattern belong to Co3O4 phase with fraction of cubic CoO. The height of the diffraction peaks of Co3O4 (311) and CoO (200) planes can be used for calculation of the relative content or the calculated fraction (F) of CoO crystallites in the porous CoO/Co3O4 nanocomposite depending upon the following relation:
where Icubic is the count of I(200) of cubic CoO phase and Itotal is the sum of the counts of I(311) and I(200) for Co3O4 and CoO phases, respectively. The fraction of cubic CoO phase present in porous CoO/Co3O4 nanocomposite was found to be 32%. Depending on the calculations with fullprof program for Rietveld analysis (structure profile refinement) of X-ray powder diffraction data, the values of the crystallite size (d), X-ray density (D), unit cell volume (V), lattice constants (a) and dislocation density (ρd) are tabulated in Table 1 [27].
F = Icubic/Itotal

Table 1.
Structural properties of the as-synthesized cobalt oxides.
The Miller indices, d spacing and the values of 2θ for the crystalline phases which present in the as-synthesized cobalt oxides are listed in Table 2.

Table 2.
Miller indices, d spacing and the values of 2θ for the crystalline phases (cobalt oxides) in the as-synthesized solid.
3.2. FTIR Analysis
A spectrum FTIR of the as-prepared sample was shown in Figure 3. Examination of this figure indicated that: (i) this sample’s spectra shows different bands at 3415, 1650, 1163, 643, 572 and 513 cm−1. These bands enabled us to identify of functional group and structure elucidation for the investigated samples; (ii) the observed wide bands belonging to the mixed oxides at 3415 cm−1 and 1650 cm−1 were due to the stretching and bending vibration of H2O group OH [28]. However, the 1163 cm−1 band can be correlated during the cooling process to the chemisorption’s of O2- on the sample surface particularly carbon surface; (iii) The spectra show a sharp 513-cm−1 band with two 643-cm−1 and 572 cm−1 shoulder bands. These bands correspond to the Co3O4 spinel structure [29]. It is understood that the characteristic bands of single and/or mixed oxides in the 1000 and 400 cm−1 ranges are usually attributed to the vibration of metallic ions in the crystal lattice [29]. The spinel materials, however, are known to have two fundamental IR active modes in the vibration spectrum, which are high frequency bands around 600 cm−1 at the tetrahedral (A) site and low frequency bands around 400 cm−1 at octahedral (B) sites [30]. The bands in the obtained spectrum at 513 and 643 cm−1 are correlated with vibrations of both the Co3+ in an octahedral hole and the Co2+ in a tetrahedral hole in the spinel lattice, respectively [31,32]. (iv) The divalent octahedral metal ion and oxygen ion complexes indicate the presence of the shoulder band at 572 cm−1 [33]. In this analysis, the presence of some Co2+ ions at B-site could be due to this shoulder band.
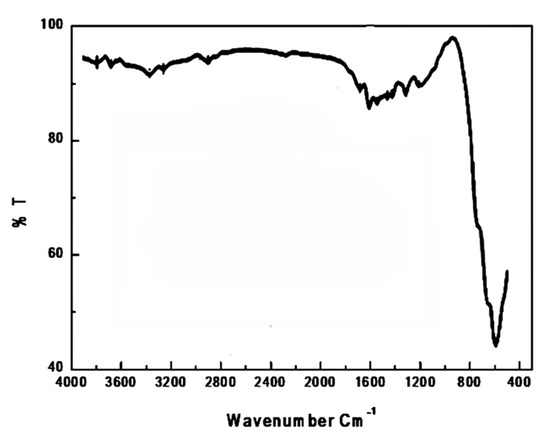
Figure 3.
FTIR spectrum of CoO/Co3O4 nanoparticles.
3.3. Energy Dispersive X-ray Spectrometry (EDS) Analysis
In order to confirm the existence of cobalt oxides and also to analyze the chemical structure and composition of the synthesized sample, EDS analysis was achieved. Illustration Figure 4 shows the EDS sequence of nanoparticles as synthesized. The characteristic signals of the elements of cobalt (Co), oxygen (O) and carbon (C) were calculated in Figure 4. Co: 76.7 wt percent, O: 20.2 wt percent and C: 3.1, respectively, were the weights percent of cobalt, oxygen and carbon measured from EDS. However, the value about 0.512 and 0.25 keV corresponds to the binding energies of oxygen (O-Kα) and (C-Kα), while the peaks at binding energies of 0.78, 6.92 and 7.68 keV correspond to CoL, CoKα and CoKβ, respectively. This finding confirms that the as-prepared sample consisted entirely of mixed cobalt oxides and with a carbonaceous byproduct.
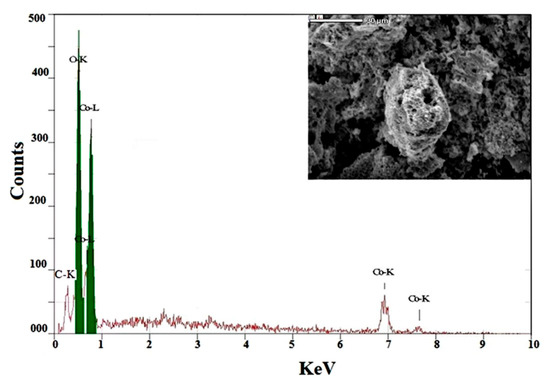
Figure 4.
EDS pattern of CoO/Co3O4 nanoparticles.
3.4. Morphologic Analysis
Scanning electron microscopic (SEM) analysis resulted in the surface morphology of the synthesized CoO/Co3O4 nanoparticles. SEM images of the as-prepared nanoparticles with various magnifications are illustrated from Figure 5a–e. A higher tendency of agglomeration of many particles was observed. These agglomerations are gathered as sponge carrots. Such islands are like porous bodies with various types of pores and voids. Growth of the island was clearly seen from the tightly packed structures. Careful examination of SEM images indicates different contrast light of the as synthesized sample. Indeed, high contrast light nanoparticles, cobalt oxides, are clearly seen supported on a lower contrast darker porous material which is a carbonaceous byproduct. These features are also clearly visible in the lower magnification images. In addition, Figure 5f, there is histogram for particle size distribution of the as-prepared sample based on SEM micrographs [34]. This figure shows that the size of the as-prepared material was ranged between 10 and 140 nm with average sized located at 90 nm. This confirms formation of agglomerated particles in the as-prepared system.
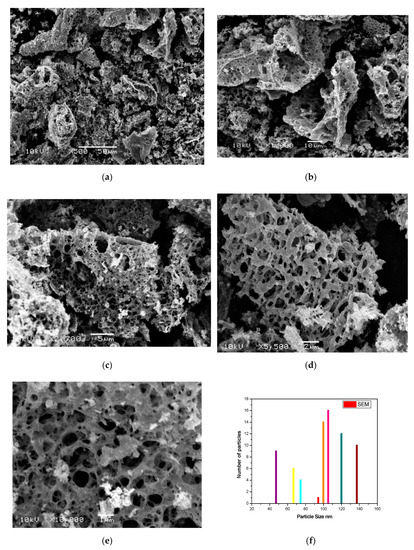
Figure 5.
(a–e) SEM images of the as-prepared sample with different magnifications and (f) its histogram for particle size distribution.
Transmission electron microscopic (TEM) analysis has made a clear picture for morphologic characteristics of the as synthesized sample as shown in Figure 6a. Indeed, TEM enables data to be captured regarding the sample’s inner structure, where SEM provides data about the surface of the analyzed object. However, this figure shows that the porous material (a carbonaceous byproduct with low contrast light) is mixed with high contrast light CoO/Co3O4 nanoparticles. However, the synthesized material had homogenous structures and sizes ranged in the nanometer scale. In addition, Figure 6b, there is histogram for particle size distribution of the as-prepared sample based on TEM micrograph [34]. This figure confirms that the size of particle was in the range from 10 to 20 nm. Finally, these observations report the polycrystalline CoO/Co3O4 NPs and also are consistent with XRD and EDS measurements.
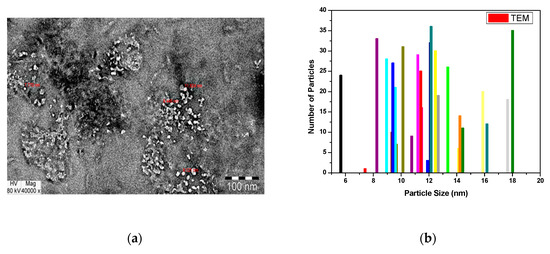
Figure 6.
(a) TEM image of the as-prepared material and (b) its histogram for particle size distribution.
3.5. Surface Properties
Different surface properties were investigated from N2 adsorption isotherms conducted at 77 K, namely SBET, Vp and ȓ, for a synthesized composite. The SBET, Vp and ȓ, values were 57.75 m2/g, 0.0148 cc/g and 10.31 nm, respectively, for the as synthesized sample. These results confirm that the composite as-prepared is porous, with a powerful surface area depending upon presence of a carbonaceous byproduct and nature of the preparation method.
3.6. Magnetic Properties
A vibrating sample magnetometer (VSM) measured the magnetic properties of the CoO/Co3O4 nanocrystals at room temperature. The magnetization plots (Ms) as a function of the applied magnetic field (H) are shown in Figure 7. This figure indicates a period of hysteresis estimated at 300 K. Table 3 includes Ms, Mr, Hc and Mr/Ms values of the as synthesized sample. The Hc, Ms and Mr values were calculated as shown in literatures [35,36,37].
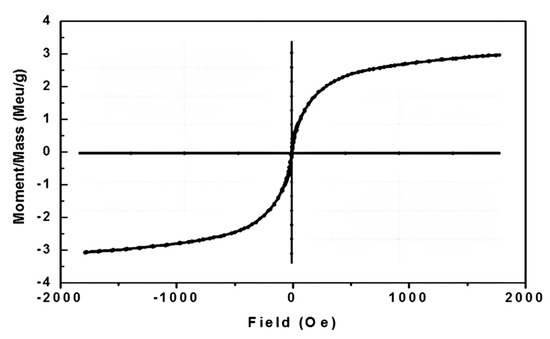
Figure 7.
M-H curve for CoO/Co3O4 nanoparticles.

Table 3.
Magnetic properties of the as-synthesized nanocomposite.
4. Discussion
There are three well-known valence states in the cobalt oxide system: cobalt oxide (CoO), cobalt oxide (Co2O3) and cobalt cobaltite (Co3O4). The most stable phase in this system is a mixed-valence compound with ordinary spinel structure [Co2+Co3+ 2O4] [38]. In the current study, the egg-white-assisted green synthesis resulted in formation of nanocomposite containing CoO and Co3O4. The crystallite sizes of CoO/Co3O4 mixed solids were ranged between 10 and 20 nm. The well-defined diffraction peaks involved in the spectrum of XRD indicate to formation of thin crystalline cubic phases. The preparation method led to formation fine size cobalt oxide powders depending upon on the peak broadening at each reflection. However, it can be seen from Table 1 that there was dislocation in the crystal lattice of CoO/Co3O4 system. A crystallographic defect (irregularity) caused by the dislocation within a crystal structure that leads to changes in material properties. This fault distorts the ordinary atomic array of a perfect crystal [39,40,41].
A range of the synthesized CoO/Co3O4 nanoparticles with overgrowth of clusters was observed in the SEM results. Spongy and fragile agglomerations with different pores and voids were observed. It was evident from the EDS spectrum that the CoO/Co3O4 nanoparticles were synthesized successfully depending upon the major constituents of the as-prepared sample were Co and O elements with traces of carbon. In addition, the CoO/Co3O4 composite consisted entirely of nanocrystalline particles based on the strong, intense, narrow width and sharp peaks involved in the EDS spectrum.
Table 3 reveals that the synthesized sample exhibits coercive magnetic hysteresis loops (85.032 Oe) and magnetization of saturation (3.45 emu/g). This indicates the ferromagnetism of the CoO/Co3O4 NPs at room temperature. In other expressions, the behavior of the room temperature ferromagnetic (RTFM), as synthesized CoO/Co3O4 NPs, showed very strong. Indeed, the efficient working of the spintronic devices relies on the semiconductor nanoparticles RTFM. Nonetheless, the origin of RTFM for the as synthesized CoO/Co3O4 NPs, depending upon the presence of the unpaired electrons spin, could be attributed to the following reasons: (i) Surface uncompensated Co2+ ions [42]; (ii) Range of the particles within nano scale [43]; (iii) Surface oxygen vacancies/or particle interface [44,45,46,47,48]; the oxygen vacancies in CoO/Co3O4 nanoparticles were still a critical factor in the presence of RTFM. The oxygen vacancies play an important role in producing ferromagnetism especially in nanometric scale system. Opposite behavior was observed in a bulk system. This indicates the reduction of oxygen vacancies in the bulk system due to reduced oxygen vacancies density. To put it another way, ferromagnetism is inversely proportional to particle size. In nanosized materials, the loss of translational symmetry and weak surface coordination contribute to the occurrence of vacancies of oxygen with subsequent density increases. Furthermore, the oxygen vacancies are found primarily on the surface of the particles. Resultantly, it is assumed that these vacancies play a key role for the ferromagnetism identified in nanosized cobalt oxides. This could be due to trapping unpaired electrons in the oxygen vacancies and their spins will polarize together through interactions of exchange that create ferromagnetic order [49,50].
Additionally, one of the important unique properties which depend on its anisotropy is the squareness of a material. The squareness is also a function of the hysteresis loop forming the square. In the results of this study, CoO/Co3O4 NPs, squareness was below 0.5. This result demonstrates that the particles interact by magnetostatic interaction, and anisotropy in the crystal lattice decreases [51,52,53]. However, it could explore the possibility of dealing with superparamagnetic particles in a blocked state due to the small size of their nanocrystallites in the range of 10–20 nm [54]. In addition, cobalt nanoparticles provide a significantly greater anisotropy barrier elsewhere compared with average atomic thermal energy, and then the particle magnetization is stuck or “blocked” in a given direction until an external magnetic field reverses it. In other words, the possible mechanism for reduction of the squareness could be a direct consequence of the effect of the thermal fluctuations on change the direction of the magnetization for superparamagnetic particles. Similar results were observed in the case of spinel nickel manganites [55].
5. Conclusions
Green synthesis based on egg white brought about formation of CoO/Co3O4 nanoparticles for the first time. This approach has many economic benefits for the synthesis of CoO/Co3O4 nanoparticles without using any additives, such as technical feasibility, unexpectedness to scale up and less time consuming. From XRD results, the as synthesized material consisted entirely of CoO and Co3O4 phases with cubic structure. The elemental and morphologic characteristics of the prepared sample were characterized using EDS and SEM techniques. The as-prepared CoO/Co3O4 NPs have coercivity (85.032 Oe) and saturation magnetization (3.45 emu/g) with RTFM behavior.
Author Contributions
Conceptualization, N.M.D., G.M.A.-S. and O.H.A.-E.; methodology, N.M.D., G.M.A.-S. and O.H.A.-E.; software, O.H.A.-E.; validation, N.M.D., G.M.A.-S.,and O.H.A.-E.; formal analysis, N.M.D. and O.H.A.-E.; investigation, N.M.D., G.M.A.-S. and O.H.A.-E.; resources, N.M.D., G.M.A.-S. and O.H.A.-E.; data curation, N.M.D., G.M.A.-S. and O.H.A.-E.; writing—original draft preparation, N.M.D., G.M.A.-S. and O.H.A.-E.; writing—review and editing, N.M.D., G.M.A.-S. and O.H.A.-E.; visualization, N.M.D., G.M.A.-S. and O.H.A.-E.; supervision, N.M.D., G.M.A.-S. and O.H.A.-E.; project administration N.M.D., G.M.A.-S.; funding acquisition, G.M.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at Princes Nourah bint Abdulrahman University through the fast-track research-funding program.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Romero, M.; Steinfeld, A. Concentrating solar thermal power and thermochemical fuels. Energy Environ. Sci. 2012, 5, 9234–9245. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sust. Energ. Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Sattler, C. Hybrid sensible/thermochemical solar energy storge concepts based on porous ceramic structures and redox pair oxides chemistry. Energy Procedia 2015, 69, 706–715. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Sattler, C. Exploitation of thermochemical cycles based on solid oxide redox systems for thermochemical storage of solar heat. Sol. En. 2014, 102, 189–211. [Google Scholar] [CrossRef]
- Wong, B. Thermochemical Heat Storage for Concentrated Solar Power; U.S. Department of Energy. General Atomics Project 30314; 2011. Available online: https://www.osti.gov/biblio/1039304 (accessed on 11 May 2020).
- Gangopadhyay, S.; Hadjipanayis, G.C.; Sorensen, C.M.; Klabunde, K.J. Exchange anisotropy in oxide passivated Co fine particles. J. Appl. Phys. 1993, 73, 6964–6966. [Google Scholar] [CrossRef]
- Resnick, D.A.; Gilmore, K.; Idzerda, Y.U.; Klem, M.T.; Allen, M.; Douglas, T.; Arenholz, E.; Young, M. Magnetic properties of Co3O4 nanoparticles mineralized in Listeria innocua Dps. J. Appl. Phys. 2006, 99, 08Q501–08Q503. [Google Scholar] [CrossRef]
- He, L.; Chen, C.; Wang, N.; Zhou, W.; Guo, L. Finite size effect on N’eel temperature with Co3O4 nanoparticles. J. Appl. Phys. 2007, 102, 103911–103914. [Google Scholar] [CrossRef]
- Salabas, E.L.; Rumplecker, A.; Kleitz, F.; Radu, F.; Schüth, F. Exchange anisotropy in nanocasted Co3O4 nanowires. Nano Lett. 2006, 6, 2977–2981. [Google Scholar] [CrossRef]
- Duan, X.; Huang, Y.; Agarwal, R.; Lieber, C.M. Single-nanowire electrically driven lasers. Nature 2003, 421, 241–245. [Google Scholar] [CrossRef]
- Kim, H.; Park, D.W.; Woo, H.C.; Chung, J.S. Reduction of SO2 by CO to elemental sulfurover Co3O4-TiO2 catalysts. Appl. Catal. B 1998, 19, 233–243. [Google Scholar] [CrossRef]
- Li, W.Y.; Xu, L.N.; Chen, J. Co3O4 nanomaterials in lithium-ion batteries and gas sensors. Adv. Funct. Mater. 2005, 15, 851–857. [Google Scholar] [CrossRef]
- Elliott, D.W.; Zhang, W.X. Field Assessment of Nanoscale Bimetallic Particles for Groundwater Treatment. Environ. Sci. Technol. 2001, 35, 4922–4926. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Brus, L.; Murray, C.B. Synthesis of Monodisperse Nanoparticles of Barium Titanate: Toward a Generalized Strategy of Oxide Nanoparticle Synthesis. J. Am. Chem. Soc. 2001, 123, 12085–12086. [Google Scholar] [CrossRef]
- Roth, W.L. The magnetic structure of Co3O4. J. Phys. Chem. Solids. 1964, 25, 1–10. [Google Scholar] [CrossRef]
- Dutta, P.; Seehra, M.S.; Thota, S.; Kumar, J. A comparative study of the magnetic properties of bulk and nanocrystalline Co3O4. J. Phys. Condens Matter. 2008, 20, 1–8. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Konstantinov, K.; Yuan, L.; Liu, H.K.; Dou, S.X. In-situ fabrication of nanostructured cobalt oxide powders by spray pyrolysis technique. J. Nanosci. Nanotechnol. 2004, 8, 861–866. [Google Scholar] [CrossRef]
- Wu, J.G.; Tu, J.P.; Wang, X.L.; Zhang, W.K. Synthesis of nanoscale CoO particles and their effect on the positive electrodes of nickel–metal hydride batteries. Int. J. Hydrog. Energy 2007, 32, 606–610. [Google Scholar] [CrossRef]
- He, T.; Chen, D.; Jiao, X.; Wang, Y.; Duan, Y. Solubility-controlled synthesis of high-quality C03O4 nanocrystals. Chem. Mater. 2005, 17, 4023–4030. [Google Scholar] [CrossRef]
- Deraz, N.M. Catalytic decomposition of H2O2 on promoted cobaltic oxide catalysts. Mater. Lett. 2002, 57, 914–920. [Google Scholar] [CrossRef]
- Deraz, N.M. The Effect of Calcium Doping on the Surface and Catalytic Properties of Cobaltic Oxide Catalysts. Adsorp. Sci. Technol. 2003, 21, 229–243. [Google Scholar] [CrossRef]
- Deraz, N.M.; Fouda, M.M.G. Fabrication and Magnetic Properties of Cobalt-Copper Nano-Composite. Int. J. Electrochem. Sci. 2013, 8, 2682–2690. [Google Scholar]
- Toniolo, J.C.; Takimi, A.S.; Bergmann, C.P. Nanostructured cobalt oxides (Co3O4 and CoO) and metallic Co powders synthesized by the solution combustion method. Mater. Res. Bull. 2010, 45, 672–676. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesly Publishing Co. Inc., Techpark: Singapore, 1976; Ch. 14. [Google Scholar]
- Will, G.; Masciocchi, N.; Parrish, W.; Hart, M. Refinement of simple crystal structures from synchrotron radiation powder diffraction data. J. Appl. Cryst. 1987, 20, 394–401. [Google Scholar] [CrossRef]
- Sasaki, S.; Fujino, K.; Takeuchi, Y. X-Ray Determination of Electron-Density Distributions in Oxides, MgO, MnO, CoO, and NiO, and Atomic Scattering Factors of their Constituent Atoms. Proc Jpn. Acad 1979, 55, 43–48. [Google Scholar] [CrossRef]
- Boultif, A.; Louer, D. Powder pattern indexing with the dichotomy method. J. Appl. Cryst. 2004, 37, 724–731. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Yu, J.; Wu, T.; Wang, G. Surface structure and catalytic behavior of silica-supported copper catalysts prepared by impregnation and sol–gel methods. Appl. Catal. A-Gen. 2003, 239, 87–94. [Google Scholar] [CrossRef]
- Waldron, R.D. Infrared Spectra of Ferrites. Phys. Rev. 1955, 99, 1725–1727. [Google Scholar] [CrossRef]
- Ouaguenouni, H.S.M.; Benadda, A.; Kiennemann, A.; Barama, A. Preparation and Catalytic Activity of Nickel –manganese oxide catalysts in the reaction of partial oxidation of methane. Comptes Rendus Chim. 2009, 12, 740–747. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Ge, B.; Zhou, H.; Yuan, A.; Shen, X. Concave Co3O4 octahedral mesocrystal: Polymer-mediated synthesis and sensing properties. Cryst. Eng. Comm. 2012, 14, 6264–6270. [Google Scholar] [CrossRef]
- He, T.; Chen, D.; Jiao, X.; Xu, Y.; Gu, Y. Controlled Synthesis of Co3O4 Nanoparticles through Oriented Aggregation. Chem. Mater. 2004, 16, 737–743. [Google Scholar] [CrossRef]
- Berchmans, L.J.; Selvan, R.K.; Kumar, P.N.S.; Augustin, C.O. Structural and electrical properties of Ni1−x MgxFe2O4 synthesized by citrate gel process. J. Magn. Magn. Mater. 2004, 279, 103–110. [Google Scholar] [CrossRef]
- Rasband, W.S. Image J; U.S. National Institutes of Health: Bethesda, MD, USA, 1997–2012. Available online: https://Imagej.nih.gov/i/ (accessed on 11 May 2020).
- Sandomirski, S. Calculation and Analysis the Magnetic Parameters of the Minors Hysteresis Loop for Steels from the Basic Magnetic Parameters. 19th World Conference on Non-Destructive Testing. 2016. Available online: https://scholar.google.com.hk/scholar?hl=zh-TW&as_sdt=0%2C5&q=Calculation+and+Analysis+the+Magnetic+Parameters+of+the+Minors+Hysteresis+Loop+for+Steels+from+the+Basic+Magnetic+Parameters&btnG= (accessed on 11 May 2020).
- Sandomirski, S.G. The residual magnetization of the ferromagnetic body magnetized in an open magnetic circuit. Defektoskopiia 1997, 8, 50–59. [Google Scholar]
- Sandomirski, S.G. Computing the Magnetization Curve and the Minors of the Hysteresis Loop for Structural Ferromagnetic Materials from the Basic Magnetic Parameters. Elektritchestvo 2010, 1, 61–64. [Google Scholar]
- Shinde, V.R.; Mahadik, S.B.; Gujar, T.P.; Lokhande, C.D. Supercapacitive cobalt oxide (Co3O4) thin films by spray pyrolysis. Appl. Surf. Sci. 2006, 252, 7487–7492. [Google Scholar] [CrossRef]
- Rendale, M.K.; Mathad, S.N.; Puri, V. Thick films of magnesium zinc ferrite with lithium substitution: Structural characteristics. Int. J. Self-Propag. High-Temp. Synth. 2015, 24, 78–82. [Google Scholar] [CrossRef]
- Mathad, S.N.; Jadhav, R.N.; Patil, N.D.; Puri, V. Structural and mechanical properties of Sr+2 doped bismuth manganite thick films. Int. J. Self-Prop. High-Temp. Synth. 2013, 22, 180–184. [Google Scholar] [CrossRef]
- Mathad, S.N.; Jadhav, R.N.; Phadatare, V.; Puri, V. Structural and mechanical properties of Sr-doped barium niobate thick films. Int. J. Self-Propag. High-Temp. Synth. 2014, 23, 145–150. [Google Scholar] [CrossRef]
- Xavier, S.; Thankachan, S.; Jacob, B.P.; Mohammed, E.M. Effect of Samarium Substitution on the Structural and Magnetic Properties of Nanocrystalline Cobalt Ferrite. J. Nanosci. 2013, ID 524380, 1–7. [Google Scholar] [CrossRef]
- Singh, J.; Chae, K. d° Ferromagnetism of Magnesium Oxide. Condens. Matter 2017, 2, 1–13. [Google Scholar]
- Zou, G.F.; Li, H.; Zhang, D.W.; Xiong, K.; Dong, C.; Qian, Y.T. Well-aligned arrays of CuO nanoplatelets. J. Phys. Chem. B 2006, 110, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Gao, D.; Zhang, J.; Yang, Z.; Zhang, Z.; Rao, J.; Xue, D. Observation of room temperature ferromagnetism in pure La2O3 nanoparticles. Appl. Phys. A-Mater. Sci. 2014, 116, 1293–1298. [Google Scholar] [CrossRef]
- Kisan, B.; Alagarsamy, P. Room temperature ferromagnetism in finite sized ZnO nanoparticles. Phys. B Condens. Matter 2014, 448, 115–119. [Google Scholar] [CrossRef]
- Shi, S.; Gao, D.; Xu, Q.; Yang, Z.; Xue, D. Singly-charged oxygen vacancy-induced ferromagnetism in mechanically milled SnO2 powders. RSC Adv. 2014, 4, 45467–45472. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J.Z.; Li, Y.L.; Ma, D.C.; Hou, S.N.; Li., L.Z.; Hao, X.L.; Wang, Z.C. Room Temperature Synthesis of 2D CuO Nanoleaves in Aqueous Solution. Nanotechnology 2011, 22, 115604–115613. [Google Scholar] [CrossRef]
- Herzer, G. Nanocrystalline soft magnetic materials. J. Magn. Mater. 1992, 112, 258–262. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, A. Structural and Magnetic Properties of Cobalt Oxide Nanoparticles at Different Annealing Temperatures. Int. J. Mater. Sci. Eng. 2016, 4, 208–214. [Google Scholar]
- Razmara, Z. Synthesis, Characterization and Magnetic Properties of Bi-metallic Copper Complex, as a Precursor for the Preparation of CuO Nanoparticles and Its Application for Removal of Arsenic from Water. J. Inorg. Org. Poly. Mater. 2018, 28, 1255–1262. [Google Scholar] [CrossRef]
- Zhu, D.Z.J.; Zhang, J.; Liu, T.; Chen, L.; Liu, X.; Ma, R.; Zhang, H.; Qiu, G. Controllable fabrication and magnetic properties of double-shell cobalt oxides hollow particles. Sci. Rep. 2015, 5, 8737. [Google Scholar]
- NirmaleshNaveen, A.N. SubramanianSelladurai. Tailoring structural, optical and magnetic properties of spinel type cobalt oxide (Co3O4) by manganese doping. Physica B 2015, 457, 251–262. [Google Scholar]
- Binns, C. Introduction to Nanoscience and Nanotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; p. 229. [Google Scholar]
- Deraz, N.; Abd-Elkader, O.A.; Yassin, M. Impacts of Egg White Assisted Combustion and Ceramic Methods on Structural, Morphological and Magnetic Properties of Nickel Manganite System. Crystals 2020, 10, 489. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).