Screening and Application of Chitin Synthase Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
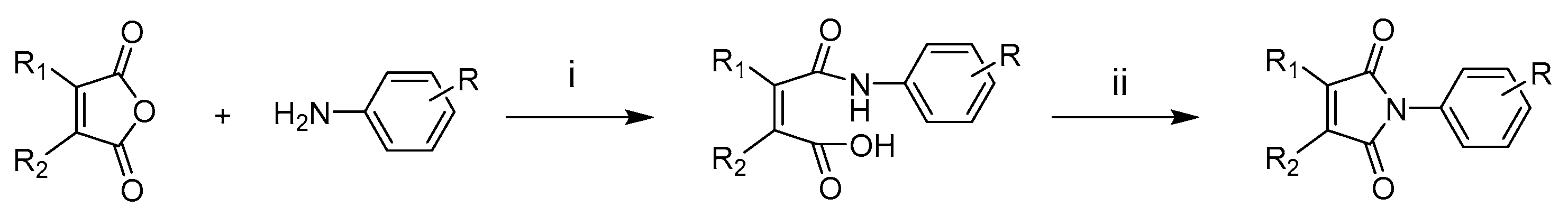
2.2. Chemistry
2.3. Biological Activity Assay
2.3.1. Inhibition on Chitin Synthase Assay
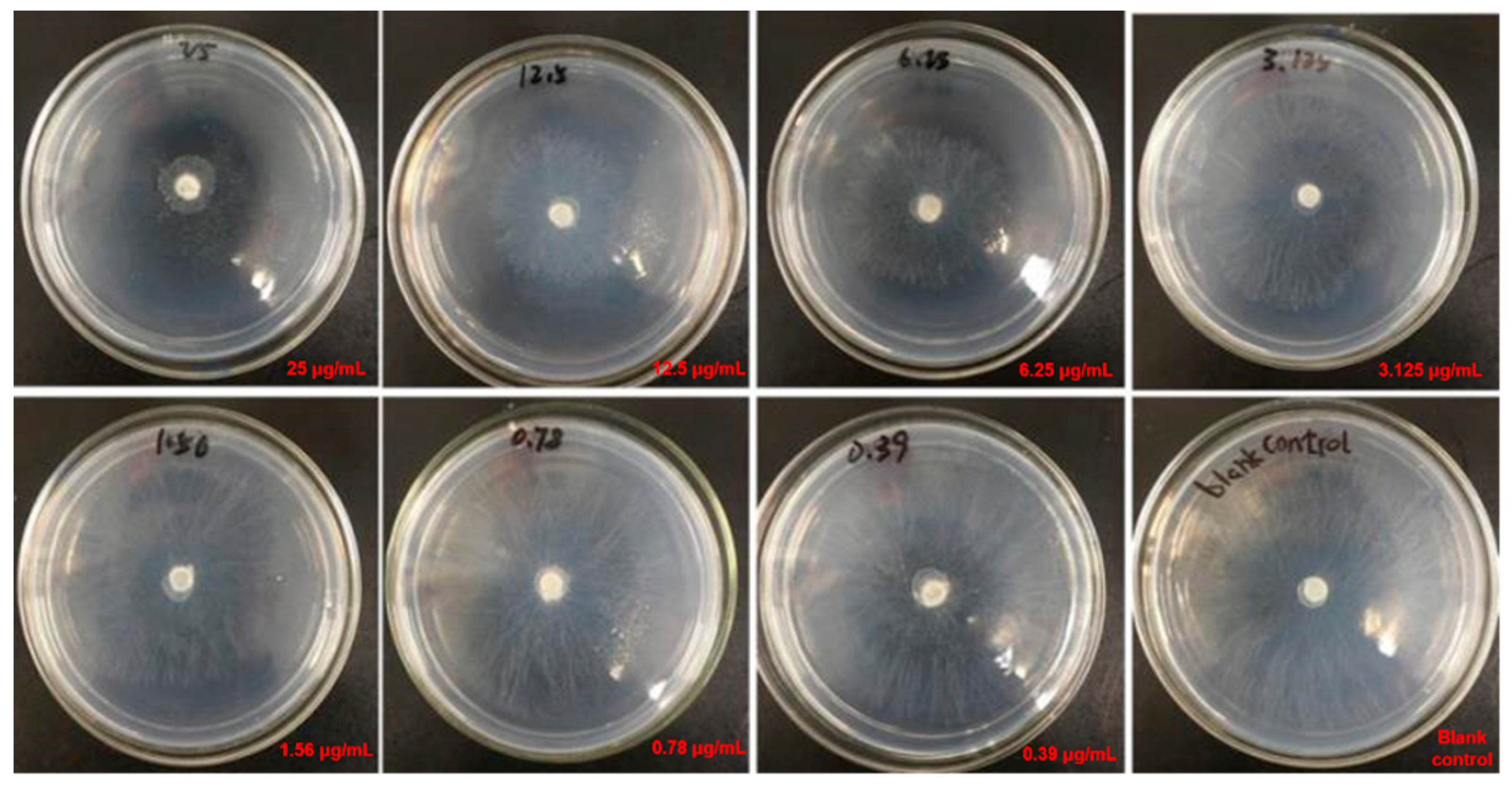
2.3.2. Antifungal Assays
3. Results
3.1. General Synthetic Procedure for Maleimides 1–20
3.1.1. N-phenyl-maleimide (1)
3.1.2. N-phenyl-3-methylmaleimide (2)
3.1.3. N-phenyl-3-phenylmaleimide (3)
3.1.4. N-phenyl-3,4-dimethylmaleimide (4)
3.1.5. N-phenyl-3,4-dichloromaleimide (5)
3.1.6. N-benzyl-maleimide (6)
3.1.7. N-benzyl-3-methylmaleimide (7)
3.1.8. N-benzyl-3-phenylmaleimide (8)
3.1.9. N-benzyl-3,4-dimethylmaleimide (9)
3.1.10. N-benzyl-3,4-dichloromaleimide (10)
3.1.11. N-phenethyl-maleimide (11)
3.1.12. N-phenethyl-3-methylmaleimide (12)
3.1.13. N-phenethyl-3-phenylmaleimide (13)
3.1.14. N-phenethyl-3,4-dimethylmaleimide (14)
3.1.15. N-phenethyl-3,4-dichloromaleimide (15)
3.1.16. N-phenylpropyl-maleimide (16)
3.1.17. N-phenylpropyl-3-methylmaleimide (17)
3.1.18. N-phenylpropyl-3-phenylmaleimide (18)
3.1.19. N-phenylpropyl-3,4-dimethylmaleimide (19)
3.1.20. N-phenylpropyl-3,4-dichloromaleimide (20)
3.2. General Synthetic Procedure for Maleimides 21–35
3.2.1. N-(4-fluorophenyl)-maleimide (21)
3.2.2. N-(4-fluorophenyl)-3-methylmaleimide (22)
3.2.3. N-(4-fluorophenyl)-3-phenylmaleimide (23)
3.2.4. N-(4-fluorophenyl)-3,4-dimethylmaleimide (24)
3.2.5. N-(4-fluorophenyl)-3,4-dichloromaleimide (25)
3.2.6. N-(4-chlorophenyl)-maleimide (26)
3.2.7. N-(4-chlorophenyl)-3-methylmaleimide (27)
3.2.8. N-(4-chlorophenyl)-3-phenylmaleimide (28)
3.2.9. N-(4-chlorophenyl)-3,4-dimethylmaleimide (29)
3.2.10. N-(4-chlorophenyl)-3,4-dichloromaleimide (30)
3.2.11. N-(4-bromophenyl)-maleimide (31)
3.2.12. N-(4-bromophenyl)-3-methylmaleimide (32)
3.2.13. N-(4-bromophenyl)-3-phenylmaleimide (33)
3.2.14. N-(4-bromophenyl)-3,4-dimethylmaleimide (34)
3.2.15. N-(4-bromophenyl)-3,4-dichloromaleimide (35)
3.3. Biological Activity
3.3.1. Chitin Synthase Inhibitory Activity
3.3.2. In Vitro Antifungal Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2013, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.P.; Wang, W.Y.; Fang, K.; Li, Z.G.; Dong, G.Q.; Miao, Z.Y.; Yao, J.Z.; Zhang, W.N.; Sheng, C.Q. Design, synthesis and antifungal activity of carbazole derivatives. Chin. Chem. Lett. 2014, 25, 229–233. [Google Scholar] [CrossRef]
- Scheffler, R.J.; Colmer, S.; Tynan, H.; Demain, A.L.; Gullo, V.P. Antimicrobials, drug discovery, and genome mining. Appl. Microbiol. Biot. 2013, 97, 969–978. [Google Scholar] [CrossRef]
- Kapteyn, J.C.; Hoyer, L.L.; Hecht, J.E.; Müller, W.H.; Andel, A.; Verkleij, A.J.; Makarow, M.; Van Den Ende, H.; Klis, F.M. The cell wall architecture of candida lbicans wild-type cells and cell wall-defective mutants. Mol. Microbiol. 2000, 35, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Lenardon, M.D.; Munro, C.A.; Gow, N.A.R. Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 2010, 13, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isono, K.; Nagatsu, J.; Kawashima, Y.; Suzuki, S. Studies on polyoxins, antifungal antibiotics. J. Agric. Chem. Soc. Jpn. 1965, 29, 848–854. [Google Scholar]
- Kobinata, K.; Uramoto, M.; Nishii, M.; Kusakabe, H.; Nakamura, G.; Isono, K. Neopolyoxins A, B, and C, New chitin synthetase inhibitors. Agric. Biol. Chem. 1980, 44, 1709–1711. [Google Scholar]
- Uramoto, M.; Kobinata, K.; Jenkins, E.E.; McCloskey, J.A.; Higashijima, T.; Miyazawa, T. Neopolyoxins A, B, and C: New inhibitors of fungal cell wall chitin synthetase. Nucleic Acids Symp. Ser. 1980, 8, 69–71. [Google Scholar]
- Shubitz, L.F.; Trinh, H.T.; Perrill, R.H.; Thompson, C.M.; Hanan, N.J.; Galgiani, J.N.; Nix, D.E. Modeling nikkomycin Z dosing and pharmacology in murine pulmonary coccidioidomycosis preparatory to phase 2 clinical trials. J. Infect. Dis. 2014, 209, 1949–1954. [Google Scholar] [CrossRef] [Green Version]
- Jeong, T.S.; Hwang, E.I.; Lee, H.B.; Lee, E.S.; Kim, Y.K.; Min, B.S.; Bae, K.H.; Bok, S.H.; Kim, S.U. Chitin synthase II inhibitory activity of ursolic acid, isolated from crataegus pinnatifida. Planta Med. 1999, 65, 261–262. [Google Scholar] [CrossRef]
- Kim, C.J.; Yoo, H.R.; Yoo, M.S.; Kwon, B.E.; Hwang, K.J. Attitude, beliefs, and intentions to care for sars patients among korean clinical nurses. Taehan Kanho Hakhoe Chi 2006, 36, 596–603. [Google Scholar] [PubMed]
- Uda, J.; Obi, K.; Iwase, K.; Sugimoto, O.; Ebisu, H.; Matsuda, A. Synthesis and structure-activity relationships of novel nikkomycin analogs: Inhibitors of the fungal cell wall biosynthesis enzyme chitin synthase. Nucleic Acids Symp. Ser. 1999, 42, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Tokumura, T.; Horie, T. Kinetics of nikkomycin Z degradation in aqueous solution and in plasma. Biol. Pharm. Bull. 1997, 20, 577–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, S.; Igarashi, Y.; Yagami, K. Antimicrobial activity of some Narylalkyl maleimides. Pest. Sci. 2006, 34, 99–104. [Google Scholar] [CrossRef]
- Wattanadilok, R.; Sawangwong, P.; Rodrigues, C.; Cidade, H.; Pinto, M.; Pinto, E.; Silva, A.; Kijjoa, A. Antifungal activity evaluation of the constituents of haliclona baeri and haliclona cymaeformis, collected from the gulf of thailand. Mar. Drugs 2007, 5, 40–51. [Google Scholar] [CrossRef]
- Bradsher, C.K.; Harvan, D.J. Stereochemistry of the addition of N-arylmaleimides to the acridizinium ion. J. Org. Chem. 1971, 36, 67–70. [Google Scholar] [CrossRef]
- Uehara, Y.I.; Fisher, J.M.; Rabinovitz, M. Showdomycin and its reactive moiety, maleimide. A comparison in selective toxicity and mechanism of action in vitro. Biochem. Pharmacol. 1980, 29, 2199–2204. [Google Scholar] [CrossRef]
- Kar, S.; Wang, M.F.; Yao, W.; Michejda, C.J.; Carr, B.I. PM-20, a novel inhibitor of Cdc25A, induces extracellular signal-regulated kinase 1/2 phosphorylation and inhibits hepatocellular carcinoma growth in vitro and in vivo. Mol. Cancer Ther. 2006, 5, 1511–1519. [Google Scholar] [CrossRef] [Green Version]
- Hwang, E.I.; Lee, Y.M.; Lee, S.M.; Yeo, W.H.; Moon, J.S.; Kang, T.H.; Park, K.D.; Kim, S.U. Inhibition of chitin synthase 2 and antifungal activity of lignans from the stem bark of Lindera erythrocarpa. Planta Med. 2007, 73, 679–682. [Google Scholar] [CrossRef]
- Gholap, A.R.; Toti, K.S.; Shirazi, F.; Kumari, R.; Bhat, M.K.; Deshpande, M.V.; Srinivasan, K.V. Synthesis and evaluation of antifungal properties of a series of the novel 2-amino-5-oxo-4-phenyl-5,6,7,8-tetrahydroquinoline-3-carbonitrile and its analogues. Bioorg. Med. Chem. 2007, 15, 6705–6715. [Google Scholar] [CrossRef]
- Chen, X.L.; Zhu, X.H.; Ding, Y.C.; Shen, Y.C. Antifungal activity of tautomycin and related compounds against Sclerotinia sclerotiorum. J. Antibiot. 2011, 64, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.L.; Zheng, Y.G.; Shen, Y.C. Natural products with maleic anhydride structure: Nonadrides, tautomycin, chaetomellic anhydride, and other compounds. Chem. Rev. 2007, 107, 1777–1830. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fan, Y.X.; Shen, Z.Z.; Shen, Y.C.; Chen, X.L. Antifungal activity of simple compounds with maleic anhydride or dimethylmaleimide structure against botrytis cinerea. J. Pestic. Sci. 2012, 37, 247–251. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.Z.; Fan, Y.X.; Li, F.G.; Chen, X.L.; Shen, Y.C. Synthesis of N-substituted dimethylmaleimides and their antifungal activities against Sclerotinia sclerotiorum. J. Pest. Sci. 2013, 86, 353–360. [Google Scholar] [CrossRef]
- Ke, S.Y.; Liu, F.Y.; Wang, N.; Yang, Q.; Qian, X.H. 1,3,4-Oxadiazoline derivatives as novel potential inhibitors targeting chitin biosynthesis: Design, synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2009, 19, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Li, C.F.; Wang, F.; Ma, H.; Luo, M.; Yu, Y.H. Study on antibacterial activity of Arnebia euchroma (Royle) johnst hairy roots extract. J. Agric. Univ. Hebei 2010, 33, 92–96. [Google Scholar]
- Alexander, B.; Browse, D.J.; Reading, S.J.; Benjamin, I.S. A simple and accurate mathematical method for calculation of the EC50. J. Pharmacol. Toxicol. Meth. 1999, 41, 55–58. [Google Scholar] [CrossRef]



| Compound | n | R1 | R2 |
| 1 | 0 | H | H |
| 2 | 0 | H | CH3 |
| 3 | 0 | H | Phenyl |
| 4 | 0 | CH3 | CH3 |
| 5 | 0 | Cl | Cl |
| 6 | 1 | H | H |
| 7 | 1 | H | CH3 |
| 8 | 1 | H | Phenyl |
| 9 | 1 | CH3 | CH3 |
| 10 | 1 | Cl | Cl |
| 11 | 2 | H | H |
| 12 | 2 | H | CH3 |
| 13 | 2 | H | Phenyl |
| 14 | 2 | CH3 | CH3 |
| 15 | 2 | Cl | Cl |
| 16 | 3 | H | H |
| 17 | 3 | H | CH3 |
| 18 | 3 | H | Phenyl |
| 19 | 3 | CH3 | CH3 |
| 20 | 3 | Cl | Cl |
| Compound | R | R1 | R2 |
| 21 | F | H | H |
| 22 | F | H | CH3 |
| 23 | F | H | Phenyl |
| 24 | F | CH3 | CH3 |
| 25 | F | Cl | Cl |
| 26 | Cl | H | H |
| 27 | Cl | H | CH3 |
| 28 | Cl | H | Phenyl |
| 29 | Cl | CH3 | CH3 |
| 30 | Cl | Cl | Cl |
| 31 | Br | H | H |
| 32 | Br | H | CH3 |
| 33 | Br | H | Phenyl |
| 34 | Br | CH3 | CH3 |
| 35 | Br | Cl | Cl |
| Compound | IC50 (mM) | Compound | IC50 (mM) | Compound | IC50 (mM) |
| 4 | 0.26 | 20 | 0.12 | 31 | 0.29 |
| 6 | 0.26 | 21 | 0.34 | 32 | 0.37 |
| 7 | 0.32 | 24 | 0.38 | 33 | 0.30 |
| 11 | 0.21 | 26 | 0.17 | 35 | 0.23 |
| 19 | 0.20 | 30 | 0.23 | Polyoxin B | 0.19 |
| Compound | EC50 (μg/mL) | Compound | EC50 (μg/mL) | Compound | EC50 (μg/mL) |
| 1 | 15.82 | 14 | 17.92 | 27 | 75.83 |
| 2 | 28.95 | 15 | 145.43 | 28 | 52.27 |
| 3 | 30.17 | 16 | 15.08 | 29 | 72.95 |
| 4 | 12.73 | 17 | 54.64 | 30 | 19.95 |
| 5 | 48.47 | 18 | 39.95 | 31 | 5.78 |
| 6 | 6.95 | 19 | 9.13 | 32 | 7.83 |
| 7 | 28.65 | 20 | 8.47 | 33 | 9.52 |
| 8 | 48.25 | 21 | 16.23 | 34 | 42.13 |
| 9 | 14.59 | 22 | 112.35 | 35 | 11.28 |
| 10 | 31.07 | 23 | 195.68 | Pyrimethanil | 5.62 |
| 11 | 9.23 | 24 | 75.38 | Polyoxin B | 8.95 |
| 12 | 45.38 | 25 | 25.92 | ||
| 13 | 37.63 | 26 | 13.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Qiu, S.; Bao, Y.; Chen, H.; Lu, Y.; Chen, X. Screening and Application of Chitin Synthase Inhibitors. Processes 2020, 8, 1029. https://doi.org/10.3390/pr8091029
Shi X, Qiu S, Bao Y, Chen H, Lu Y, Chen X. Screening and Application of Chitin Synthase Inhibitors. Processes. 2020; 8(9):1029. https://doi.org/10.3390/pr8091029
Chicago/Turabian StyleShi, Xiaozai, Shuo Qiu, Yingling Bao, Hanchi Chen, Yuele Lu, and Xiaolong Chen. 2020. "Screening and Application of Chitin Synthase Inhibitors" Processes 8, no. 9: 1029. https://doi.org/10.3390/pr8091029






