Investigation of Itaconic Acid Separation by Operating a Commercialized Electrodialysis Unit with Bipolar Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
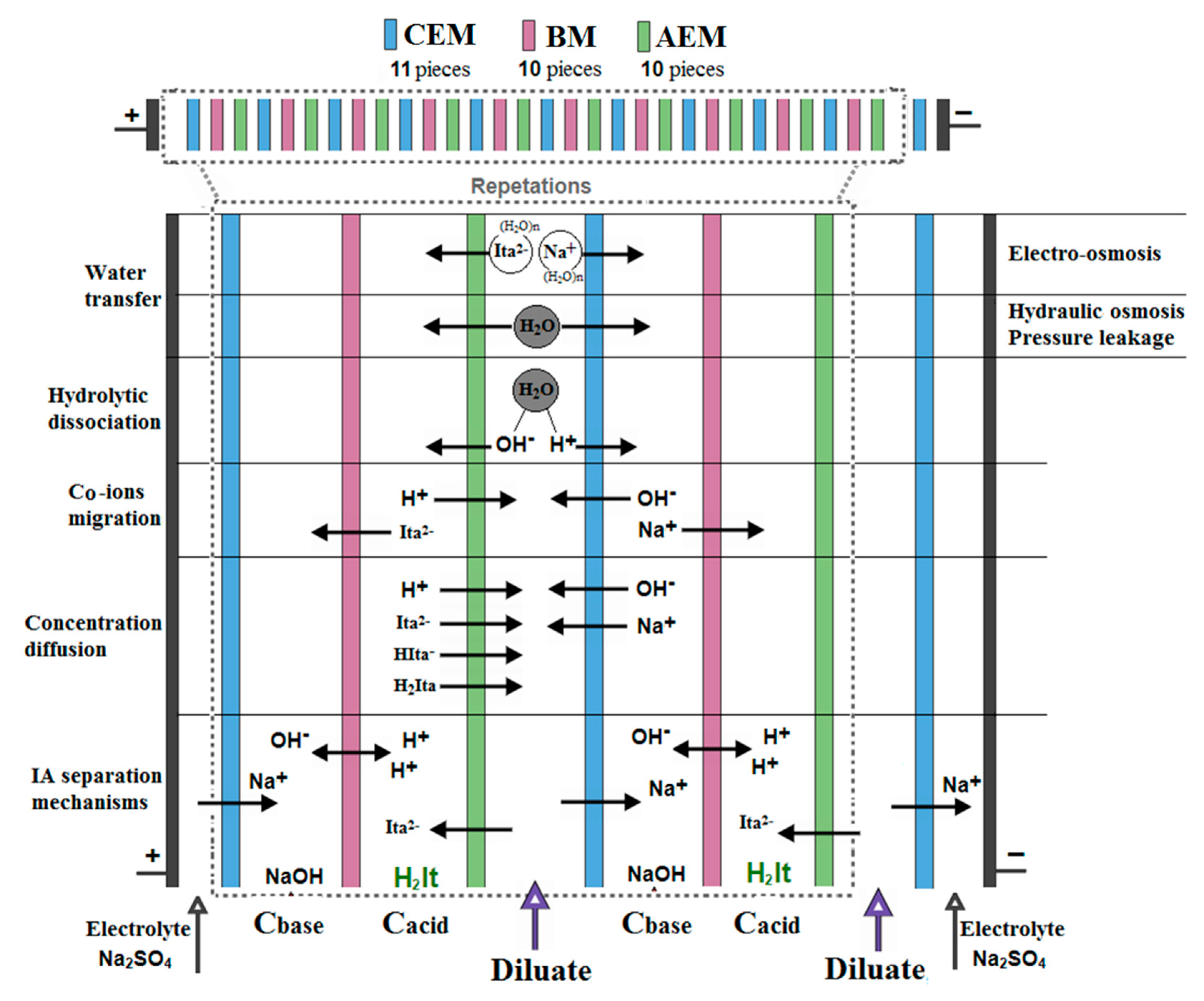
2.2. Experimental Setup
2.3. Analytical Methods
2.4. Calculations
3. Results and Discussion
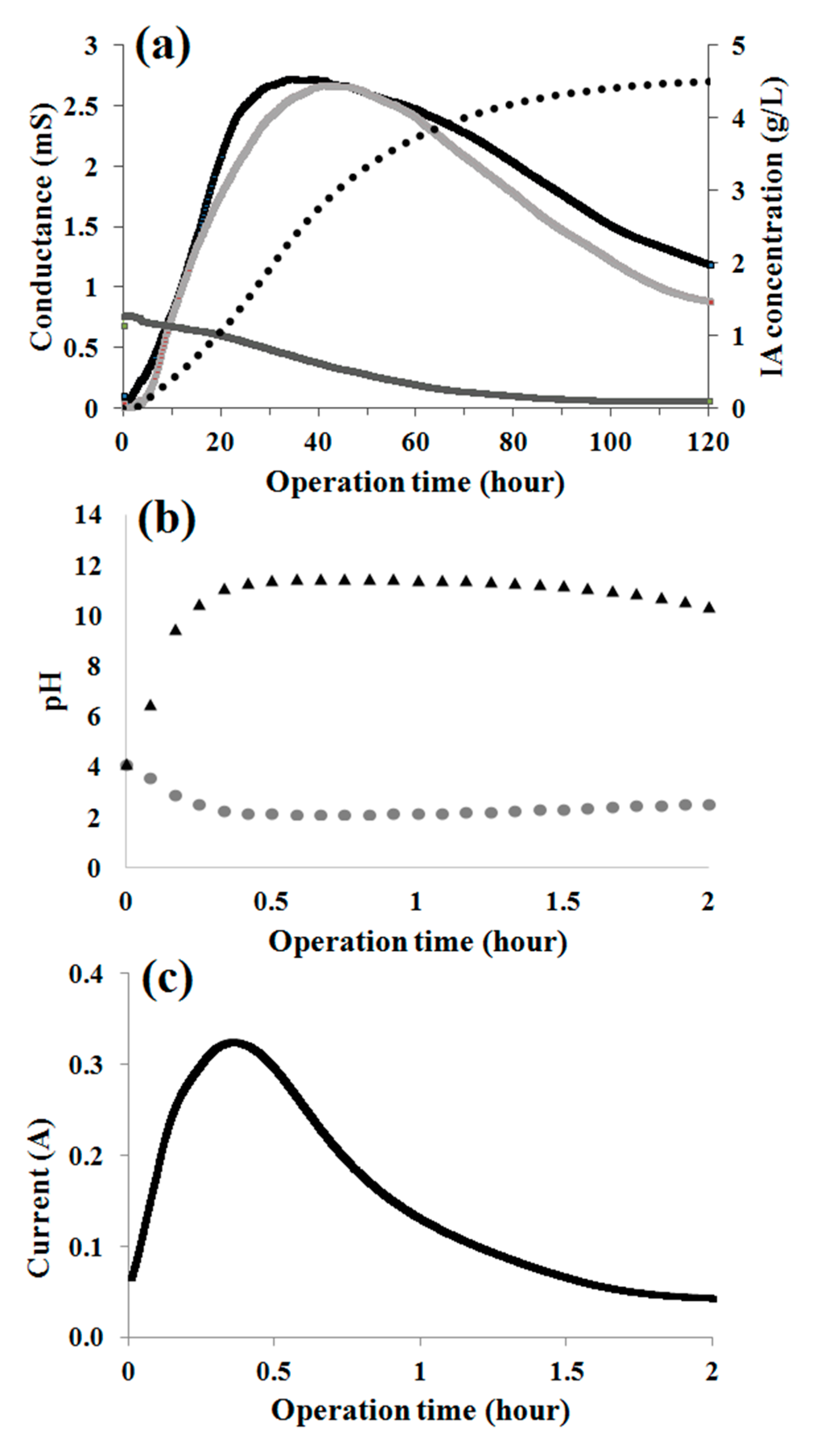
3.1. Preliminary Tests without Applied Electrical Potential (Standbymode)
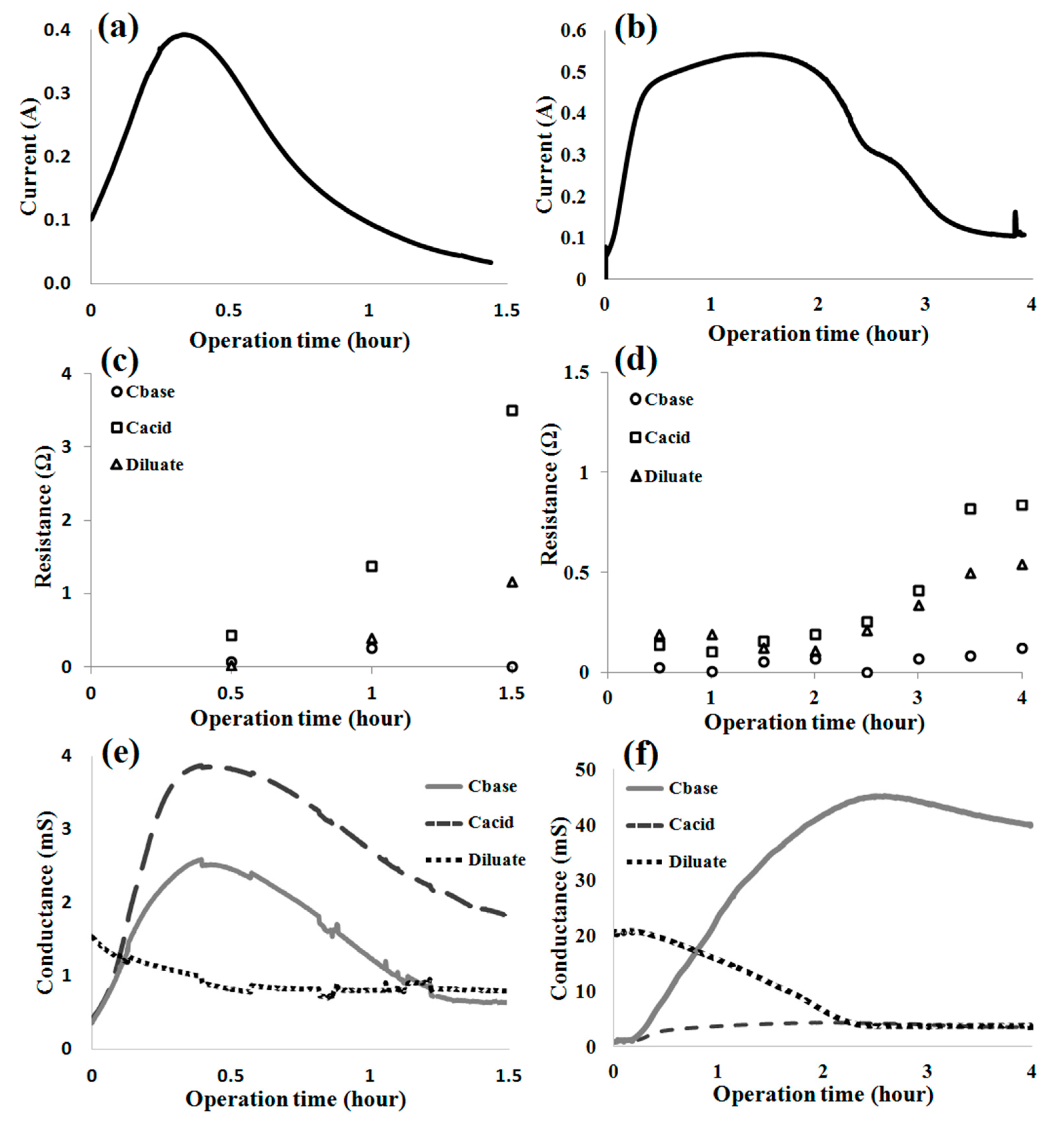
3.2. Effects of Hydroxide and Hydrogen Ions on the Separation Parameters in EDBM
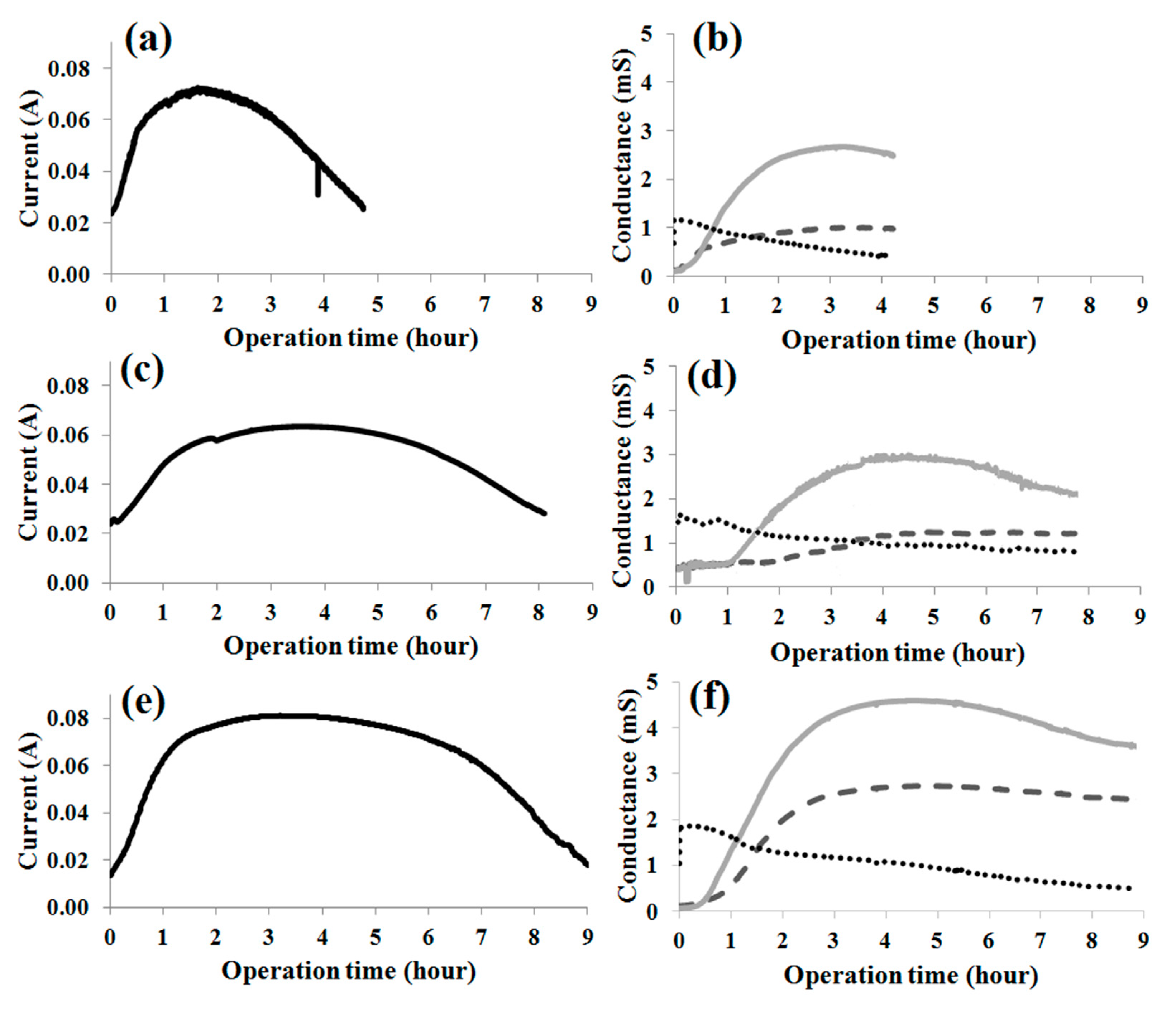
3.3. Influence of the Presence of Substrate
3.4. Effects of IA Fermentation Substrate and By-Product
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Da Cruz, J.C.; De Castro, A.M.; Sérvulo, E.F.C. World Market and Biotechnological Production of Itaconic Acid. 3 Biotech 2018, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Karaffa, L.; Kubicek, C.P. Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Sriariyanun, M.; Heitz, J.H.; Yasurin, P.; Asavasanti, S.; Tantayotai, P. Itaconic Acid: A Promising and Sustainable Platform Chemical? Appl. Sci. Eng. Prog. 2019, 12, 75–82. [Google Scholar]
- De Carvalho, J.C.; Magalhaes, A.I.; Soccol, C.R. Biobased Itaconic Acid Market and Research Trends—Is It Really a Promising Chemical? Chim. Oggi-Chem. Today 2018, 36, 56–58. [Google Scholar]
- Anouti, F.A. Concerns Regarding Food Biotechnology: An Ongoing Debate. J. Biodivers. Bioprospect. Dev. 2014, 1, 1–3. [Google Scholar] [CrossRef]
- Okabe, M.; Lies, D.; Kanamasa, S.; Park, E.Y. Biotechnological Production of Itaconic Acid and Its Biosynthesis in Aspergillus Terreus. Appl. Microbiol. Biotechnol. 2009, 84, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Moity, L.; Molinier, V.; Benazzouz, A.; Barone, R.; Marion, P.; Aubry, J.M. In Silico Design of Bio-based Commodity Chemicals: Application to Itaconic Acid Based Solvents. Green Chem. 2014, 16, 146–160. [Google Scholar] [CrossRef]
- Tomić, S.L.; Mićić, M.M.; Dobić, S.N.; Filipović, J.M.; Suljovrujić, E.H. Smart Poly (2-hydroxyethyl methacrylate/itaconic acid) Hydrogels for Biomedical Application. Radiat. Phys. Chem. 2010, 79, 643–649. [Google Scholar] [CrossRef]
- Kuenz, A.; Gallenmüller, Y.; Willke, T.; Vorlop, K.D. Microbial Production of Itaconic Acid: Developing a Stable Platform for High Product Concentrations. Appl. Microbiol. Biotechnol. 2012, 96, 1209–1216. [Google Scholar] [CrossRef]
- Klement, T.; Büchs, J. Itaconic Acid–A Biotechnological Process in Change. Bioresour. Technol. 2013, 135, 422–431. [Google Scholar] [CrossRef]
- Maassen, N.; Panakova, M.; Wierckx, N.; Geiser, E.; Zimmermann, M.; Bölker, M.; Blank, L.M.; Klinner, U. Influence of Carbon and Nitrogen Concentration on Itaconic Acid Production by the Smut Fungus Ustilago Maydis. Eng. Life Sci. 2014, 14, 129–134. [Google Scholar] [CrossRef]
- Levinson, W.E.; Kurtzman, C.P.; Kuo, T.M. Production of Itaconic Acid by Pseudozyma Antarctica NRRL Y-7808 under Nitrogen-limited Growth Conditions. Enzym. Microb. Technol. 2006, 39, 824–827. [Google Scholar] [CrossRef]
- Li, A.; Pfelzer, N.; Zuijderwijk, R.; Brickwedde, A.; Van Zeijl, C.; Punt, P. Reduced By-product Formation and Modified Oxygen Availability Improve Itaconic Acid Production in Aspergillus Niger. Appl. Microbiol. Biotechnol. 2013, 97, 3901–3911. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C. Emerging Biotechnologies for Production of Itaconic Acid and its applications As a Platform Chemical. J. Ind. Microbiol. Biotechnol. 2017, 44, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Hevekerl, A.; Kuenz, A.; Vorlop, K.D. Influence of the pH on the Itaconic Acid Production with Aspergillus Terreus. Appl. Microbiol. Biotechnol. 2014, 98, 10005–10012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Ma, F.; Lee, D.J. Recovery of Itaconic acid from Supersaturated Waste Fermentation Liquor. J. Taiwan Inst. Chem. Eng. 2009, 40, 583–585. [Google Scholar] [CrossRef]
- Komáromy, P.; Bakonyi, P.; Kucska, A.; Tóth, G.; Gubicza, L.; Bélafi-Bakó, K.; Nemestóthy, N. Optimized pH and its control strategy lead to enhanced itaconic acid fermentation by Aspergillus terreus on glucose substrate. Fermentation 2019, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, A.I.; De Carvalho, J.C.; Medina, J.D.C.; Soccol, C.R. Downstream Process Development in Biotechnological Itaconic Acid Manufacturing. Appl. Microbiol. Biotechnol. 2017, 101, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Varga, V.; Bélafi-Bakó, K.; Vozik, D.; Nemestóthy, N. Recovery of Itaconic Acid by Electrodialysis. Hung. J. Ind. Chem. 2018, 46, 43–46. [Google Scholar] [CrossRef]
- Eggert, A.; Maßmann, T.; Kreyenschulte, D.; Becker, M.; Heyman, B.; Büchs, J.; Jupke, A. Integrated in-situ Product Removal Process Concept for Itaconic Acid by Reactive Extraction, pH-shift back Extraction and Purification by pH-shift Crystallization. Sep. Purif. Technol. 2019, 215, 463–472. [Google Scholar] [CrossRef]
- Komáromy, P.; Rózsenberszki, T.; Bakonyi, P.; Nemestóthy, N.; Bélafi-Bakó, K. Statistical Analysis on the Variables Affecting Itaconic Acid Separation by Bipolar Membrane Electrodialysis. Desalin. Water Treat. 2020, 192, 408–414. [Google Scholar] [CrossRef]
- Nemestóthy, N.; Bakonyi, P.; Komáromy, P.; Bélafi-Bakó, K. Evaluating Aeration and Stirring Effects to Improve Itaconic Acid Production from Glucose Using Aspergillus Terreus. Biotechnol. Lett. 2019, 41, 1383–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; Jain, R.; Jain, S. Basic Techniques in Biochemistry, Microbiology and Molecular Biology; Humana: New York, NY, USA, 2020; pp. 181–183. [Google Scholar]
- Tran, A.T.; Mondal, P.; Lin, J.; Meesschaert, B.; Pinoy, L.; Van der Bruggen, B. Simultaneous Regeneration of Inorganic acid and Base from a Metal Washing Step Wastewater by Bipolar Membrane Electrodialysis after Pretreatment by Crystallization in a Fluidized Pellet Reactor. J. Memb. Sci. 2015, 473, 118–127. [Google Scholar] [CrossRef]
- Lameloise, M.L.; Lewandowski, R. Recovering l-Malic Acid from a Beverage Industry Waste Water: Experimental Study of the Conversion Stage Using Bipolar Membrane Electrodialysis. J. Memb. Sci. 2012, 403, 196–202. [Google Scholar] [CrossRef]
- Tongwen, X.; Weihua, Y. Effect of Cell Configurations on the Performance of Citric Acid Production by a Bipolar Membrane Electrodialysis. J. Memb. Sci. 2002, 203, 145–153. [Google Scholar] [CrossRef]
- Huang, X.; Lu, X.; Li, Y.; Li, X.; Li, J.J. Improving Itaconic Acid Production through Genetic Engineering of an Industrial Aspergillus Terreus Strain. Microb. Cell Fact. 2014, 13, 119. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Y.; Zhang, X.; Feng, H.; Xu, T. In-situ Combination of Fermentation and Electrodialysis with Bipolar Membranes for the Production of Lactic Acid: Continuous Operation. Bioresour. Technol. 2013, 147, 442–448. [Google Scholar] [CrossRef]
- Tuckerman, M.E.; Marx, D.; Parrinello, M. The Nature and Transport Mechanism of Hydrated Hydroxide Ions in Aqueous Solution. Nature 2002, 417, 925–929. [Google Scholar] [CrossRef]
- Wang, D.; Meng, W.; Lei, Y.; Li, C.; Cheng, J.; Qu, W.; Li, S. The Novel Strategy for Increasing the Efficiency and Yield of the Bipolar Membrane Electrodialysis by the Double Conjugate Salts Stress. Polymers 2020, 12, 343. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Lu, H.; Wang, J. Recovery of Citric Acid from Fermented Liquid by Bipolar Membrane Electrodialysis. J. Clean. Prod. 2017, 143, 250–256. [Google Scholar] [CrossRef]
- Galier, S.; Roux-de Balmann, H. Study of the Mass Transfer Phenomena Involved in an Electrophoretic Membrane Contactor. J. Memb. Sci. 2001, 194, 117–133. [Google Scholar] [CrossRef] [Green Version]
- Pronk, W.; Biebow, M.; Boller, M. Electrodialysis for Recovering Salts from a Urine Solution Containing Micropollutants. Environ. Sci. Technol. 2006, 40, 2414–2420. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y. Ion-exchange Membrane Electrodialysis for Saline Water Desalination and Its Application to Seawater Concentration. Ind. Eng. Chem. Res. 2011, 50, 7494–7503. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Shel’deshov, N.V.; Gnusin, N.P. Dissociation of Water Molecules in Systems with Ion-exchange Membranes. Russ. Chem. Rev. 1988, 57, 801–808. [Google Scholar] [CrossRef]
- Fan, H.; Yip, N.Y. Elucidating Conductivity-permselectivity Tradeoffs in Electrodialysis and Reverse Electrodialysis by Structure-property Analysis of Ion-exchange Membranes. J. Memb. Sci. 2019, 573, 668–681. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| Effective surface of the membrane module | 1984 cm2 |
| Effective surface of one membrane in the module | 64 cm2 |
| General size of membranes | 56 × 206 mm |
| Effective size of membranes | 40 × 160 mm |
| Number of the membrane cells | 10 pieces |
| RALEX® anion exchange membrane AM(H)-PP | 10 pieces |
| RALEX® cation exchange membrane CM(H)-PP | 11 pieces |
| RALEX® bipolar membrane (PP) | 10 pieces |
| Thickness of the spacer | 0.8 mm |
| Electrode (anode, cathode), Ti/Pt | 2 pieces |
| Size of ED module | 135 × 90 × 250 mm |
| Weight of module with preservation solution | 1.9 kg |
| Operating flow-rates | 25–60 L/h |
| Min. and Max. temperature | 10–35 ℃ |
| Operating voltage | 3 V/triplet |
| Max. voltage/current | 35 V/3 A |
| Type of Membrane | Characteristics | |
|---|---|---|
| Anion-exchange membraneRALEX®AMH-PES | Permselectivity (0.5/0.1 M KCl) | >90% |
| Resistance in 0.5 M NaCl, DC current | <7.5 Ωcm2 | |
| Stability | Acid, base, thermal (max 50 ℃) | |
| Thickness of swollen membrane | <0.75 mm | |
| Transport number (0.5/0.1 M KCl) | >0.95% | |
| Cation-exchange membraneRALEX®CMH-PES | Permselectivity (0.5/0.1 M KCl) | >90% |
| Resistance in 0.5 M NaCl, DC current | <8 Ωcm2 | |
| Stability | Acid, base, thermal (max 50 ℃) | |
| Thickness of swollen membrane | <0.70 mm | |
| Transport number (0.5/0.1 M KCl) | <0.95% | |
| ID of the Experiment | IA Conc. (g/L) | Glucose Conc. (g/L) | MA Conc. (g/L) | Potential (V) | pHinitial |
|---|---|---|---|---|---|
| A. | 5 | 15 | 0 | 30 | 2 |
| B. | 33 | 33 | 0 | 20 | 5 |
| C. | 5 | 15 | 0.5 | 10 | 3 |
| D. | 10 | 25 | 0.75 | 10 | 3 |
| E. | 15 | 33 | 1.0 | 10 | 3 |
| ID: A. | ID: B. | ID: C. | ID: D. | ID: E. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Segment | Diluate | Cbase | Cacid | Diluate | Cbase | Cacid | Diluate | Cbase | Cacid | Diluate | Cbase | Cacid | Diluate | Cbase | Cacid |
| Initial volume (mL) | 1000 | 1000 | 1000 | 1000 | 1000 | ||||||||||
| Final volume (mL) | 980 | 1000 | 960 | 960 | 980 | 1000 | 850 | 960 | 1120 | 960 | 990 | 980 | 960 | 970 | 970 |
| Initial conc. of glucose (g/L) | 15 | 0 | 0 | 33 | 0 | 0 | 15 | 0 | 0 | 25 | 0 | 0 | 33 | 0 | 0 |
| Final conc. of glucose (g/L) | 15 | <0.1 | <0.1 | 32 | <0.1 | <0.1 | 15 | <0.1 | <0.1 | 25 | <0.1 | <0.1 | 32 | <0.1 | <0.1 |
| Initial conc. of IA (g/L) | 5 | 0 | 0 | 33 | 0 | 0 | 5 | 0 | 0 | 10 | 0 | 0 | 15 | 0 | 0 |
| Final conc. of IA (g/L) | <0.1 | 0.5 | 4.5 | <0.1 | <0.1 | 32 | <0.1 | 0.4 | 4.5 | <0.1 | 1 | 9 | <0.1 | 1 | 14 |
| Initial conc. of MA (g/L) | No MA added | 0.5 | 0 | 0 | 0.75 | 0 | 0 | 1.0 | 0 | 0 | |||||
| Final conc. of MA (g/L) | No MA added | <0.1 | <0.2 | <0.3 | <0.2 | <0.2 | 0.3 | <0.3 | <0.3 | 0.3 | |||||
| Operation time (hour) | 1.5 | 4.0 | 5.0 | 8.0 | 9.0 | ||||||||||
| CE (%) | 74.3 | 98.5 | 78.3 | 97.9 | 98.5 | ||||||||||
| Product recovery (%) | 90 | 97 | 90 | 90 | 93 | ||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rózsenberszki, T.; Komáromy, P.; Kőrösi, E.; Bakonyi, P.; Nemestóthy, N.; Bélafi-Bakó, K. Investigation of Itaconic Acid Separation by Operating a Commercialized Electrodialysis Unit with Bipolar Membranes. Processes 2020, 8, 1031. https://doi.org/10.3390/pr8091031
Rózsenberszki T, Komáromy P, Kőrösi E, Bakonyi P, Nemestóthy N, Bélafi-Bakó K. Investigation of Itaconic Acid Separation by Operating a Commercialized Electrodialysis Unit with Bipolar Membranes. Processes. 2020; 8(9):1031. https://doi.org/10.3390/pr8091031
Chicago/Turabian StyleRózsenberszki, Tamás, Péter Komáromy, Enikő Kőrösi, Péter Bakonyi, Nándor Nemestóthy, and Katalin Bélafi-Bakó. 2020. "Investigation of Itaconic Acid Separation by Operating a Commercialized Electrodialysis Unit with Bipolar Membranes" Processes 8, no. 9: 1031. https://doi.org/10.3390/pr8091031
APA StyleRózsenberszki, T., Komáromy, P., Kőrösi, E., Bakonyi, P., Nemestóthy, N., & Bélafi-Bakó, K. (2020). Investigation of Itaconic Acid Separation by Operating a Commercialized Electrodialysis Unit with Bipolar Membranes. Processes, 8(9), 1031. https://doi.org/10.3390/pr8091031







