Process of Obtaining Chromium Nitride in the Combustion Mode under Conditions of Co-Flow Filtration
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Chromium Combustion in a Nitrogen Coflow
3.2. Chromium Combustion in a Co-Flow Nitrogen-Argon Mixture
3.2.1. The Effect of the Amount of Argon in a Nitrogen-Argon Mixture on the Burning Speed and Burning Temperature of Chromium
3.2.2. The Effect of Flow Rate of Nitrogen–Argon Mixture on the Nitridation of Chromium
3.3. Phase Composition of Combustion Products
4. Discussion
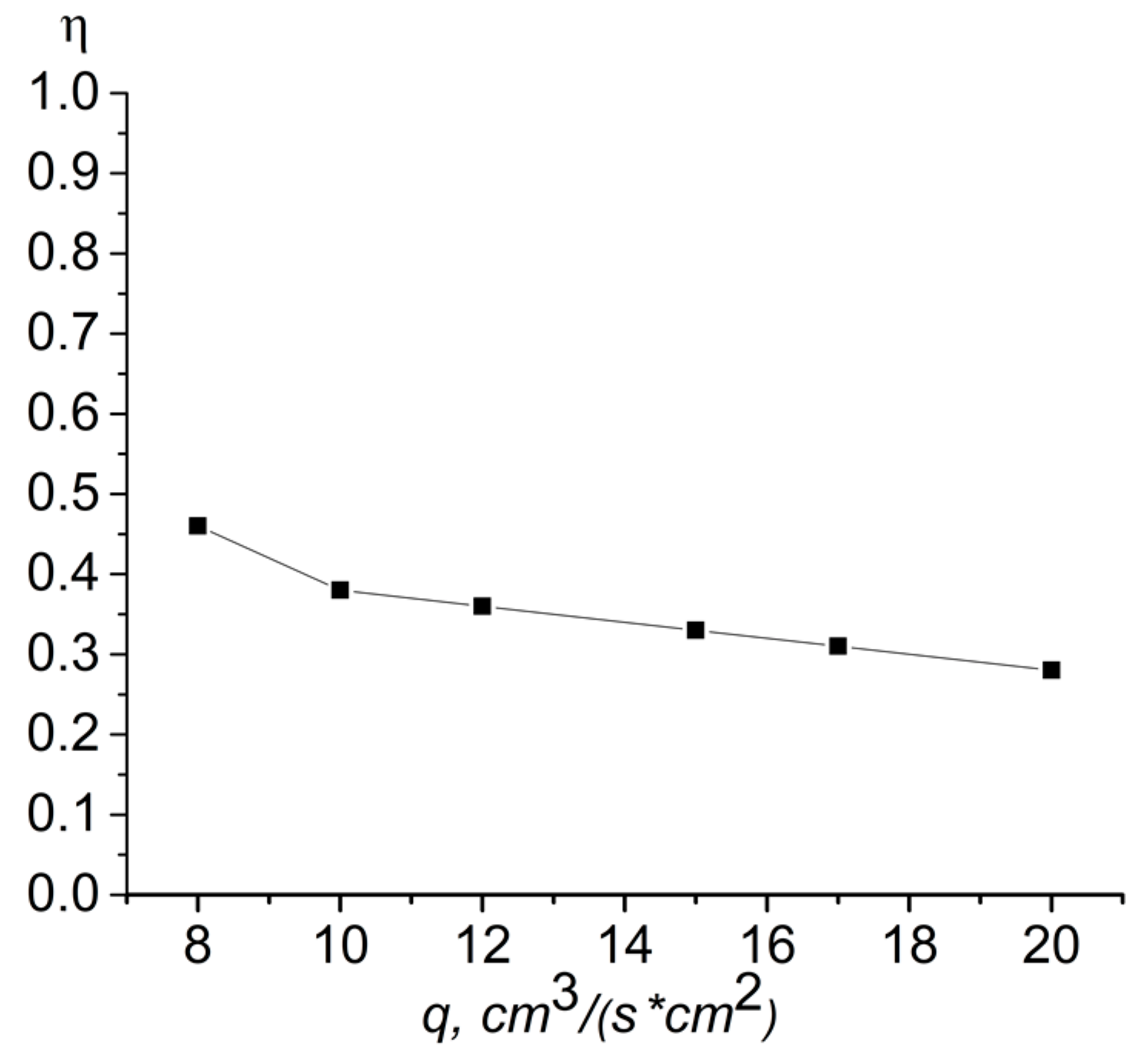
- To achieve a stable propagation of the combustion wave using chromium powder with a particle size δ = 63–80 µm when purged with pure nitrogen, it is necessary that the specific flow rate is more than 8 cm3/(s·cm2). A further decrease in specific consumption leads to a lack of fuel and a breakdown of combustion. The upper limit of the specific flow rate, when using pure nitrogen, is 20 cm3/(s·cm2). The reason for this is the rapid increase in the burning temperature with the eutectic melting point Cr–Cr2N reached. The appearance of the liquid phase violently changes the filtration conditions and leads to either non-stationary combustion, or to extinction.
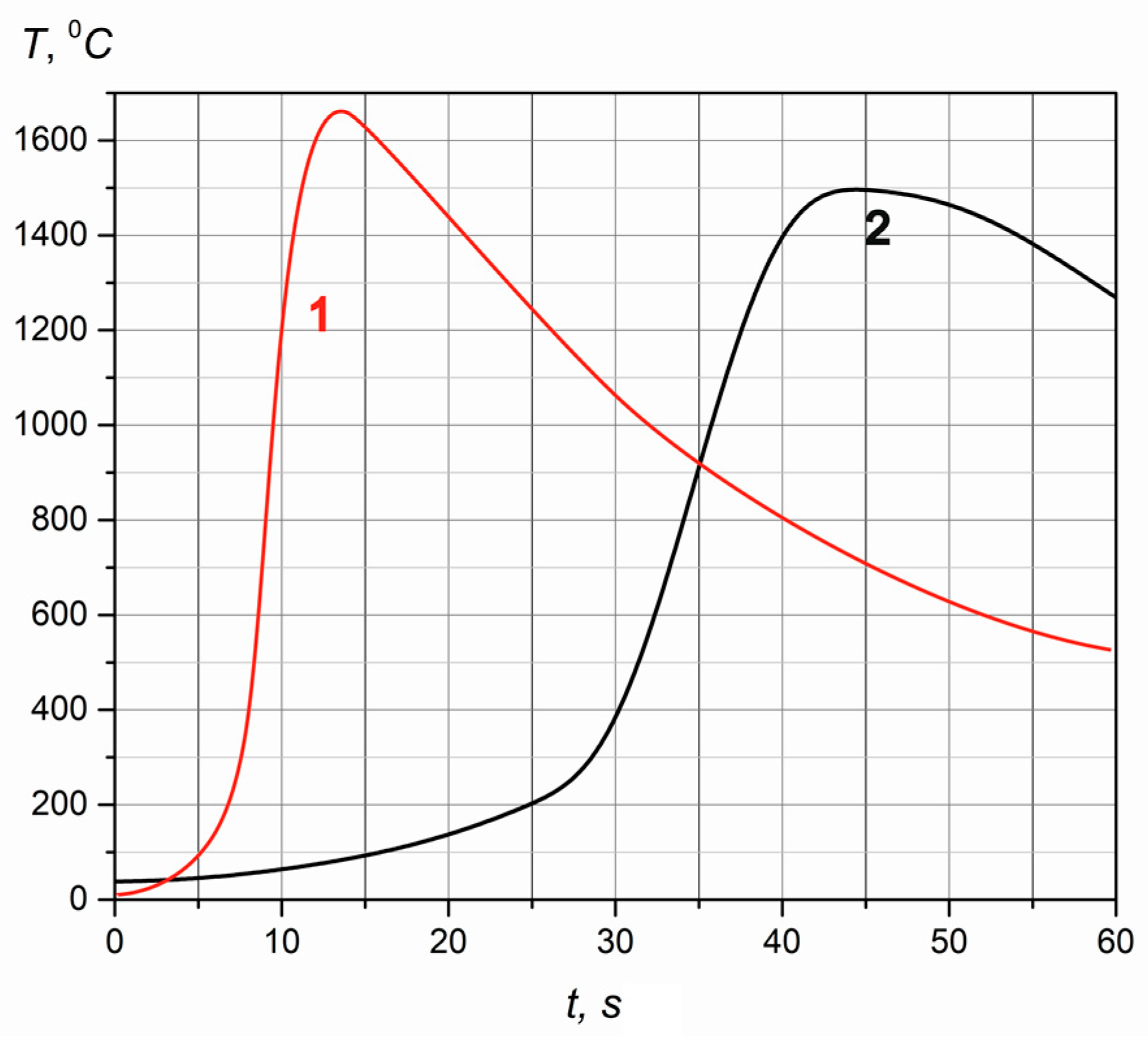
- Burning temperature decrease (Table 2), with an increase in the argon content in the mixture, is obviously related to the following fact. The reaction zone receives a large amount of cold gas ballast (argon), and a larger share of energy is spent on heating it. Further increase in the share of argon (more than 24%) in the nitrogen-containing gas leads to a breakdown of combustion and the impossibility of further propagation of the front. Reducing burning temperature by increasing the size of the particles of the initial powder is due to the increase in free space between particles and by reducing the area of contact. Thus, the heat transfer from particle to particle, due to the thermal conductivity mechanism, is reduced. This is clearly seen with the combustion of granulated chromium powder. Comparison of the adiabatic burning temperature Tad with the obtained experimental data showed that the actual burning temperature is higher than the calculated one Tad (Tad = 1280 °C for Cr2N and Tad = 2060 °C for CrN), with stoichiometric nitrogen content (11.8%—Cr2N, 21.21%—CrN). Thus, combustion was carried out in the co-flow reactor in the superadiabatic heating mode. The experimental existence of superadiabatic heating was proven in [27] when a metal chromium powder was combusted under natural filtration conditions in a high-pressure reactor.
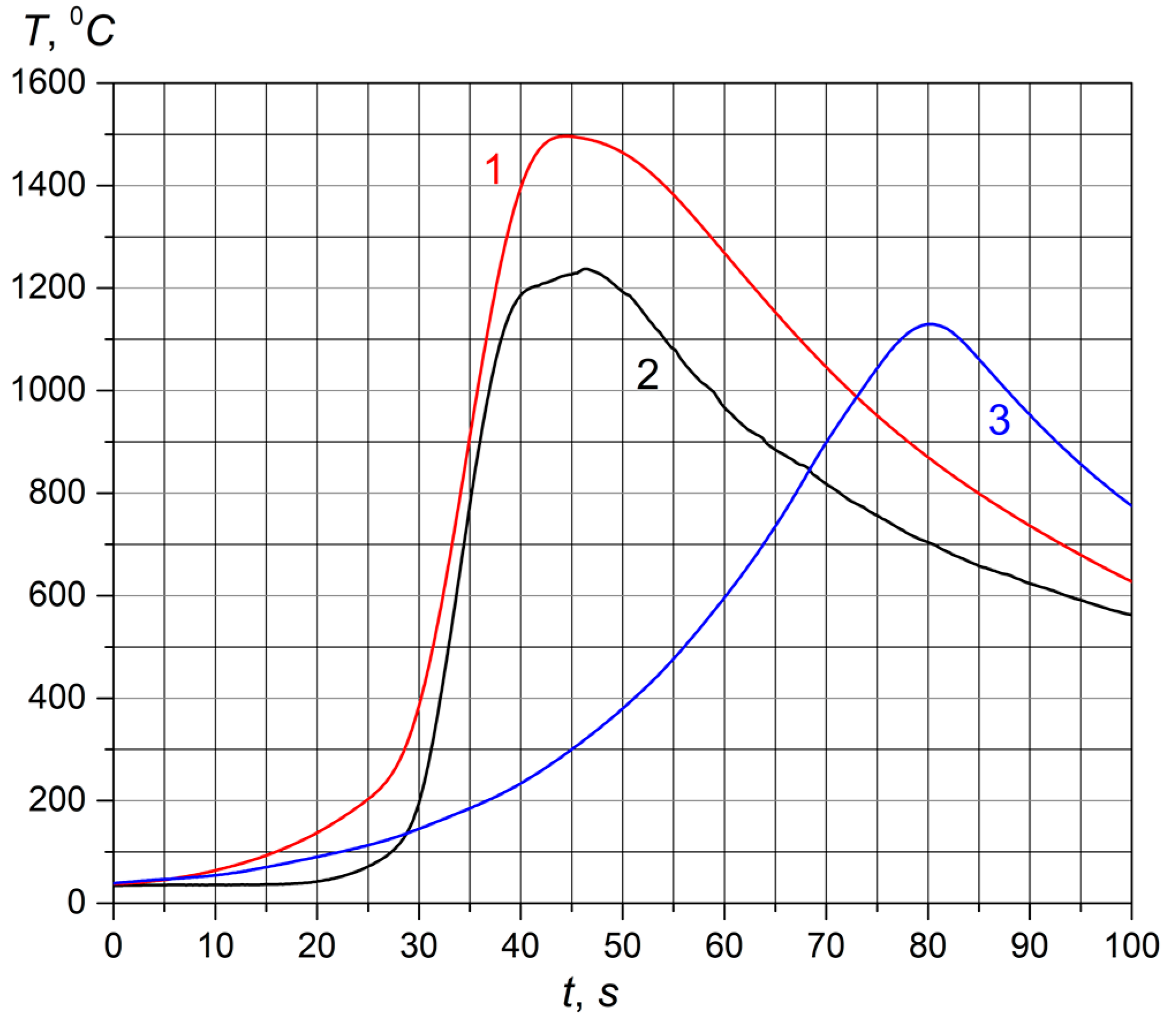
- Increasing the argon content in the mixture allows a change in the limits of specific flow rate, which allows for obtaining a stable combustion without the formation of a liquid phase. Thus, for a mixture of nitrogen with 12.8% and 24% argon, combustion was able to be carried out at a specific flow rate of 40 cm3/(s·cm2). However, the lower limit of flow rate also increased and was 12 cm3/(s·cm2). We assume that this is due to a decrease in the amount of fuel when diluted with an inert gas. At 12.8% [Ar], an inverse combustion wave was recorded.It was found that, during the combustion of chromium powder with a particle size 63–80 μm in a co-flow of nitrogen with an increase in the specific flow rate from 8 cm3/(s·cm2) to 12 cm3/(s·cm2), the chromium content increased (from 0 to 2.9 wt.%) with a decrease in the Cr2N content (from 100 to 97.1 wt.%). Thus, at a specific flow rate of 8 cm3/(s·cm2) during the combustion of chromium powder in a co-flow nitrogen, the combustion product was represented only by the Cr2N phase.It was found that, during the combustion of chromium powder with a particle size of 63–80 μm in a co-flow of a nitrogen–argon mixture (argon content 24 wt.%), with an increase in the specific flow rate from 12 to 20 cm3/(s·cm2), there was an increase in the chromium content (from 24.8 to 26.7 wt.%) and a decrease in the Cr2N content (from 75.2 to 73.3 wt.%). Thus, the argon content in the mixture, as well as the value of the specific flow rate, affects the composition of the combustion products.Stoichiometric nitrogen content in the chromium mononitride is 21.2% and in the nitride Cr2N is −11.8%. According to elemental analysis, the samples obtained contain only about 6% to 9.8% N2. Combustion occurs in the mode of incomplete transformation. At the same time, all nitrogen entering the reaction zone fully interacts with chromium. This is supported by data from flow rate sensors at the inlet and outlet of the reactor. In obtained SHS products, as a result of the combustion of both powder and granules of chromium, a slight decrease in nitridation is observed with an increase in the flow rate of the gas mixture (Table 3). Thus, the nitridation of products during the combustion of chromium powder decreased from 8.4% to 8.0% and from 9.5% to 8.7% during the combustion of granular chromium. We assumed that the reason for the decrease in nitridation is the following factor. At the forced filtration mode using a nitrogen–argon mixture, in contrast to natural filtration, there is no “post-nitridation” stage. The reaction cannot continue due to the rapid cooling of the synthesis products by the co-flow of the initial cold gas. Thus, there is a so-called quenching mode. This quenching of burnt materials records the composition of products formed in the high-temperature region.
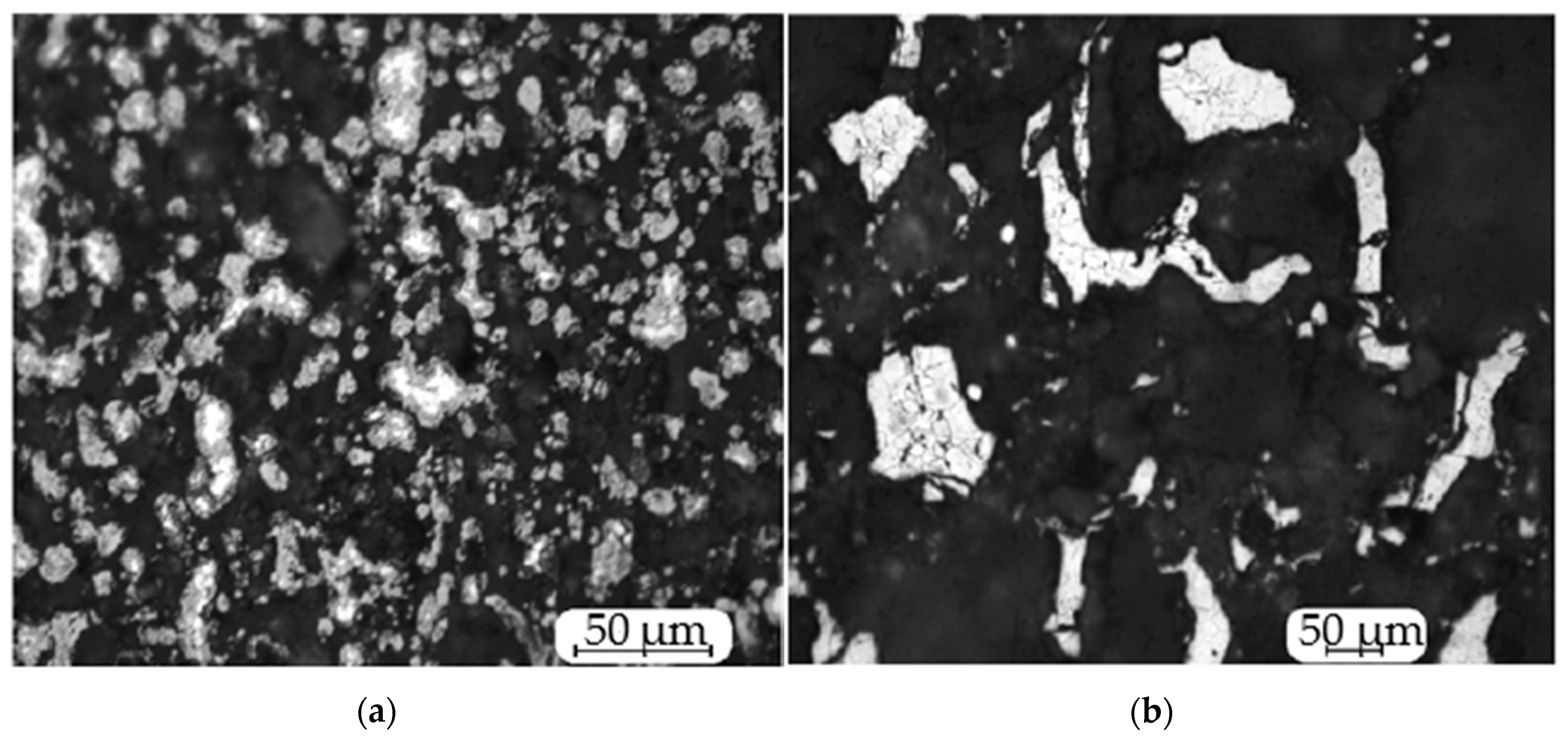
- Phase analysis has confirmed the existence of the Cr2N phase. According to XRD pattern phases (Figure 6), chromium and Cr2N were detected. Information about the region of existence of the Cr2N phase is quite contradictory [28,29]. In [29], the homogeneity range is represented in the range of 9.2–11.9% N2. In this paper we obtained the samples with nitrogen containing from 6% to 9.8%. Forced filtration mode allows for the synthesis of non-stoichiometric chromium nitride, Cr2N, of various compositions.Studies of the microstructure of synthesized chromium nitride samples showed that the samples obtained in the co-flow filtration mode retained high porosity, and the particles retained their initial cut. The result can be explained by the fact that the maximum temperature in the combustion zone (1640 °C) is significantly lower than the melting temperature in the Cr-N system. The images (Figure 8) show that the microstructure of the particles obtained in the forced filtration mode is single-phase, while the particles after combustion in the natural filtration mode are clearly two-phase. The lighter areas in the middle of the particles are the Cr2N phase, and the darker ones are CrN.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mukas’yan, A.S.; Martynenko, V.M.; Merzhanov, A.G.; Borovinskaya, I.P.; Blinov, M.Y. Mechanism and principles of silicon combustion in nitrogen. Combust. Explos. Shock Waves 1986, 22, 534–540. [Google Scholar] [CrossRef]
- Sharivker, S.Y.; Borovinskaya, I.P.; Mukas’yan, A.S.; Karpov, V.V. Mechanical activation of powders of silicon and aluminum nitrides produced by the method of self-propagating high-temperature synthesis. Powder Metall. Met. Ceram. 1993, 32, 189–193. [Google Scholar] [CrossRef]
- Sharivker, S.Y.; Borovinskaya, I.P.; Vishnyakova, G.A.; Barinov, Y.N.; Mukas’yan, A.S.; Knyazik, A.M. Morphological and technological characteristics of silicon nitride powder prepared by self-propagating high-temperature synthesis. Sov. Powder Metall. Met. Ceram. 1992, 31, 915–920. [Google Scholar]
- Mukas’yan, A.S.; Stepanov, B.V.; Gal’chenko, Y.A.; Borovinskaya, I.P. Mechanism of structure formation of silicon nitride with combustion of silicon in nitrogen. Combust. Explos. Shock Waves 1990, 26, 39–45. [Google Scholar] [CrossRef]
- Zakorzhevskii, V.V.; Kovalev, I.D.; Barinov, Y.N. Self-propagating high-temperature synthesis of titanium nitride with the participation of ammonium chloride. Inorg. Mater. 2017, 53, 278–286. [Google Scholar] [CrossRef]
- Sytschev, A.E.; Merzhanov, A.G. Self-propagating high-temperature synthesis of nanomaterials. Russ. Chem. Rev. 2004, 73, 147–159. [Google Scholar] [CrossRef]
- Titova, Y.V.; Shiganova, L.A.; Maidan, D.A.; Bichurov, G.V. Self-propagating high-temperature synthesis of nanostructured aluminum nitride powder with the use of aluminum fluoride and sodium azide. Russ. J. Non-Ferr. Met. 2014, 55, 177–181. [Google Scholar] [CrossRef]
- Shoji, Y.; Yoshinaka, M.; Hirota, K.; Yamaguchi, O. Fabrication of bulk chromium nitrides using self-propagating high-temperature synthesis and hot isostatic pressing. J. Jpn. Soc. Powder Powder Metall. 2002, 49, 323–326. [Google Scholar] [CrossRef]
- Hirota, K.; Takano, Y.; Yoshinaka, M.; Yamaguchi, O. Hot Isostatic pressing of chromium nitrides (Cr2N and CrN) prepared by self-propagating high-temperature synthesis. J. Am. Ceram. Soc. 2001, 84, 2120–2122. [Google Scholar] [CrossRef]
- Yeh, C.L.; Liu, E.W. Combustion synthesis of chromium nitrides by SHS of Cr powder compacts under nitrogen pressures. J. Alloys Compd. 2006, 426, 131–135. [Google Scholar] [CrossRef]
- Aldushin, A.P.; Seplyarskii, B.S.; Shkadinskii, K.G. Theory of filtrational combustion. Combust. Explos. Shock Waves 1980, 16, 33–40. [Google Scholar] [CrossRef]
- Aldushin, A.P.; Merzhanov, A.G.; Seplyarskii, B.S. Theory of filtration combustion of metals. Combust. Explos. Shock Waves 1976, 12, 285–294. [Google Scholar] [CrossRef]
- Aldushin, A.P.; Ivleva, T.P. Coflow filtration combustion in a slit-like reactor: Simultaneous manifestation of fluid-dynamic and thermal instability. Int. J. Self Propag. High Temp. Synth. 2014, 23, 125–132. [Google Scholar] [CrossRef]
- Aldushin, A.P. New results in the theory of filtration combustion. Combust. Flame 1993, 94, 308–320. [Google Scholar] [CrossRef]
- Aldushin, A.P.; Ivleva, T.P. Hydrodynamic instability of the coflow filtration combustion: Numerical simulation. Dokl. Phys. Chem. 2013, 451, 157–160. [Google Scholar] [CrossRef]
- Aldushin, A.P.; Ivleva, T.P. Continuous infiltration-mediated SHS process in a coflow reactor: Numerical modeling. Int. J. Self Propag. High Temp. Synth. 2013, 22, 77–83. [Google Scholar] [CrossRef]
- Babkin, V.S.; Drobyshevich, V.I.; Laevskii, Y.M.; Potytnyakov, S.I. Filtration combustion of gases. Combust. Explos. Shock Waves 1983, 19, 147–155. [Google Scholar] [CrossRef]
- Seplyarskii, B.S.; Vadchenko, S.G.; Kostin, S.V.; Brauer, G.B. Combustion of Ti+0.5C and Ti+C mixtures of bulk density in inert gas coflow. Combust. Explos. Shock Waves 2009, 45, 30–37. [Google Scholar] [CrossRef]
- Seplyarskii, B.S.; Brauer, G.B.; Tarasov, A.G. Combustion of the gasless system Ti + 0.5C in a nitrogen coflow. Combust. Explos. Shock Waves 2011, 47, 294–301. [Google Scholar] [CrossRef]
- Seplyarskii, B.S.; Kostin, S.V.; Brauer, G.B. Dynamic combustion regimes of the Ti-(Ti+0.5C) layered system in a concurrent nitrogen flow. Combust. Explos. Shock Waves 2008, 44, 655–661. [Google Scholar] [CrossRef]
- Seplyarskii, B.S.; Kochetkov, R.A.; Lisina, T.G. Convective Combustion of a Ti + 0.5C Granulated Mixture. Domain of Existence and Fundamental Phenomena. Combust. Explos. Shock Waves 2019, 55, 295–299. [Google Scholar] [CrossRef]
- Seplyarskii, B.S.; Kochetkov, R.A. A Study of the Characteristics of the Combustion of Ti + xC (x > 0.5) Powder and Granular Compositions in a Gas Coflow. Russ. J. Phys. Chem. B 2017, 11, 798–807. [Google Scholar] [CrossRef]
- Salgansky, E.A.; Kislov, V.M.; Glazov, S.V.; Zholudev, A.F.; Manelis, G.B. Filtration combustion of a carbon-inert material system in the regime with superadiabatic heating. Combust. Explos. Shock Waves 2008, 44, 273–280. [Google Scholar] [CrossRef]
- Salgansky, E.A.; Kislov, V.M.; Glazov, S.V.; Zholudev, A.F.; Manelis, G.B. Specific features of filtration combustion of a pyrolized solid fuel. Combust. Explos. Shock Waves 2010, 46, 528–532. [Google Scholar] [CrossRef]
- Glazov, S.V.; Salganskii, E.A.; Kislov, V.M.; Salganskaya, M.V.; Zholudev, A.F. Change in the structure of the filtration combustion wave due to a fuel composition change. Combust. Explos. Shock Waves 2010, 46, 286–292. [Google Scholar] [CrossRef]
- Braverman, B.; Ziatdinov, M.; Maksimov, Y. Chromium combustion in nitrogen. Combust. Explos. Shock Waves 1999, 35, 501–505. [Google Scholar] [CrossRef]
- Braverman, B.; Ziatdinov, M.; Maksimov, Y. Superadiabatic heating in combustion chromium in nitrogen. Combust. Explos. Shock Waves 1999, 35, 645–647. [Google Scholar] [CrossRef]
- Mills, T. The thermodinamic relations in the chromium-nitrogen system. J. Less-Common Met. 1972, 26, 223–234. [Google Scholar] [CrossRef]
- Lyakishev, N.P. (Ed.) State Diagrams of Double Metal Systems: A Reference Book; Mashinostroyeniye: Moscow, Russia, 1997; Volume 2, pp. 142–144. [Google Scholar]




| δ, μm | ||
|---|---|---|
| 63–80 | 500–1000 | |
| u, mm/s | 2.4 | 0.82 |
| Tmax, °C | 1640 | 1488 |
| [N], % | 7.5 | 7.8 |
| N2 | N2+7% Ar | N2+12.8% Ar | N2+24% Ar | |||||
|---|---|---|---|---|---|---|---|---|
| δ, μm | 63–80 | 500–1000 | 63–80 | 500–1000 | 63–80 | 500–1000 | 63–80 | 500–1000 |
| u, mm/s | 2.4 | 0.82 | 1.3 | – | 1.2 | 0.77 | 0.67 | 0.53 |
| Tmax, °C | 1640 | 1488 | 1440 | – | 1460 | 1478 | 1240 | 1143 |
| Initial Powder Size δ, μm | q = 12 cm3/(s·cm2) | q = 40 cm3/(s·cm2) |
|---|---|---|
| 63–80 | 8.4% | 8.0% |
| 500–1000 | 9.5% | 8.7% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evseev, N.; Ziatdinov, M.; Romandin, V.; Zhukov, A.; Tolynbekov, A.; Ryzhikh, Y. Process of Obtaining Chromium Nitride in the Combustion Mode under Conditions of Co-Flow Filtration. Processes 2020, 8, 1056. https://doi.org/10.3390/pr8091056
Evseev N, Ziatdinov M, Romandin V, Zhukov A, Tolynbekov A, Ryzhikh Y. Process of Obtaining Chromium Nitride in the Combustion Mode under Conditions of Co-Flow Filtration. Processes. 2020; 8(9):1056. https://doi.org/10.3390/pr8091056
Chicago/Turabian StyleEvseev, Nikolay, Mansur Ziatdinov, Vladimir Romandin, Alexander Zhukov, Aidos Tolynbekov, and Yuliya Ryzhikh. 2020. "Process of Obtaining Chromium Nitride in the Combustion Mode under Conditions of Co-Flow Filtration" Processes 8, no. 9: 1056. https://doi.org/10.3390/pr8091056
APA StyleEvseev, N., Ziatdinov, M., Romandin, V., Zhukov, A., Tolynbekov, A., & Ryzhikh, Y. (2020). Process of Obtaining Chromium Nitride in the Combustion Mode under Conditions of Co-Flow Filtration. Processes, 8(9), 1056. https://doi.org/10.3390/pr8091056




