Elements of 3D Bioprinting in Periodontal Regeneration: Frontiers and Prospects
Abstract
1. Introduction
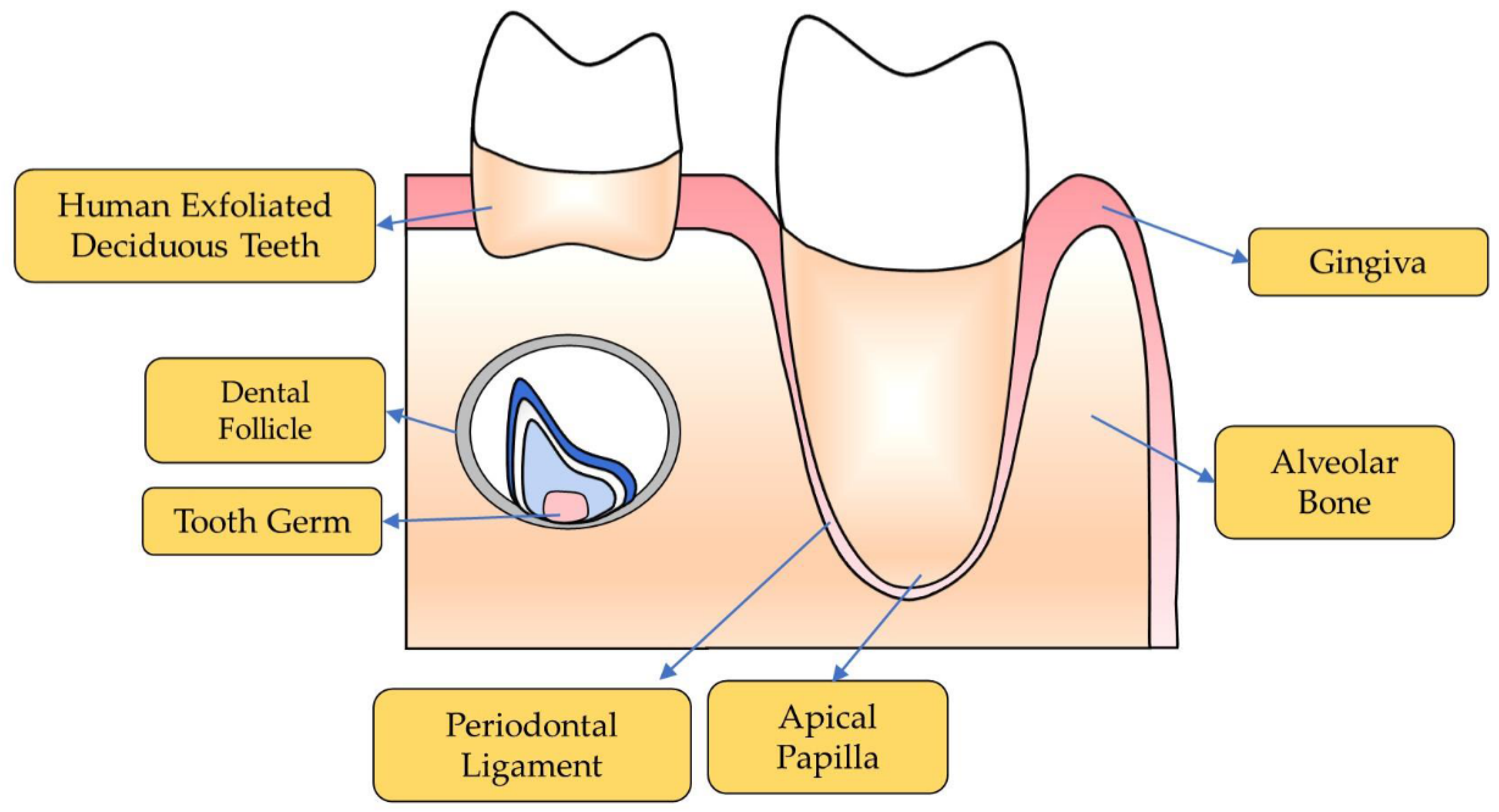
2. Oral Stem Cell
2.1. Dental Stem Cells
2.1.1. Periodontal Ligament Stem Cells
2.1.2. Stem Cells from Human Exfoliated Deciduous Teeth (SHEDs)
2.1.3. Stem Cells from Apical Papilla
2.1.4. Dental Follicle Stem Cells
2.1.5. Tooth Germ Progenitor Cells
2.2. Non-Dental Stem Cells
2.2.1. Mesenchymal Stem Cells Derived from Alveolar Bone
2.2.2. Gingival Mesenchymal Stem Cells
2.2.3. Adipose Derived Stem Cells
3. Biocompatible Materials and Scaffolds
3.1. Organic Polymers
3.2. Inorganics Materials
3.3. Biocompatible Scaffolds
3.3.1. Monophasic Scaffolds
3.3.2. Multiphasic Scaffolds
3.4. 3D Bioprinting Techniques
4. Bioactive Factors
5. Challenges and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Glossary | Abbreviations |
| Guide Tissue Regeneration | GTR |
| Bone Morphogenic Proteins | BMPs |
| Methylsulfonylmethane | MSM |
| Periodontal Ligament Stem Cells | PDLSCs |
| Stem Cells from Human Exfoliated Deciduous Teeth | SHEDs |
| Basic Fibroblast Growth Factor | bFGF |
| Stem Cells from Apical Papilla | SCAPs |
| Dental Follicle Stem Cells | DFCs |
| Tooth Germ Progenitor Cells | TGPCs |
| Mesenchymal Stem Cells Derived from Alveolar Bone | AB-MSCs |
| Gingival Mesenchymal Stem Cells | GMSCs |
| Bone Morphogenic Protein-2 | BMP-2 |
| Bone Morphogenic Protein-7 | BMP-7 |
| Polylactic Acid | PLA |
| Polycaprolactone | PCL |
| Poly (Lactic-Co-Glycolic Acid) | PLGA |
| Polyglycolic Acid | PGA |
| Fibroblast Growth Factor-2 | FGF-2 |
| Β-Glycerophosphate | BGP |
| Hydroxyapatite | HA |
| Β-Tricalcium Phosphate | TCP |
| Platelet-Derived Growth Factors | PDGFs |
| Enamel Matrix Derivatives | EMDs |
| Stromal Cell-Derived Factor-1α | SDF-1α |
References
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol. 2000 2017, 75, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Soh, Y.; Heo, S.M. Recent Advances of Therapeutic Targets for the Treatment of Periodontal Disease. Biomol. Ther. 2021, 29, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Deas, D.E.; Moritz, A.J.; Sagun, R.S., Jr.; Gruwell, S.F.; Powell, C.A. Scaling and root planing vs. conservative surgery in the treatment of chronic periodontitis. Periodontol. 2000 2016, 71, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, M.; Paduano, F. The regenerative medicine in oral and maxillofacial surgery: The most important innovations in the clinical application of mesenchymal stem cells. Int. J. Med. Sci. 2015, 12, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef]
- Nunez, J.; Vignoletti, F.; Caffesse, R.G.; Sanz, M. Cellular therapy in periodontal regeneration. Periodontol. 2000 2019, 79, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Needleman, I.; Worthington, H.V.; Giedrys-Leeper, E.; Tucker, R. WITHDRAWN: Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst. Rev. 2019, 5, CD001724. [Google Scholar] [CrossRef]
- Han, J.; Menicanin, D.; Gronthos, S.; Bartold, P.M. Stem cells, tissue engineering and periodontal regeneration. Aust. Dent. J. 2014, 59 (Suppl. 1), 117–130. [Google Scholar] [CrossRef] [PubMed]
- Abdal-Wahab, M.; Abdel Ghaffar, K.A.; Ezzatt, O.M.; Hassan, A.A.A.; El Ansary, M.M.S.; Gamal, A.Y. Regenerative potential of cultured gingival fibroblasts in treatment of periodontal intrabony defects (randomized clinical and biochemical trial). J. Periodontal. Res. 2020, 55, 441–452. [Google Scholar] [CrossRef]
- Gorski, B.; Jalowski, S.; Gorska, R.; Zaremba, M. Treatment of intrabony defects with modified perforated membranes in aggressive periodontitis: A 12-month randomized controlled trial. Clin. Oral. Investig. 2018, 22, 2819–2828. [Google Scholar] [CrossRef]
- Raveau, S.; Jordana, F. Tissue Engineering and Three-Dimensional Printing in Periodontal Regeneration: A Literature Review. J. Clin. Med. 2020, 9, 4008. [Google Scholar] [CrossRef]
- Hong, N.; Yang, G.H.; Lee, J.; Kim, G. 3D bioprinting and its in vivo applications. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 444–459. [Google Scholar] [CrossRef] [PubMed]
- D’Avanzo, N.; Bruno, M.C.; Giudice, A.; Mancuso, A.; Gaetano, F.; Cristiano, M.C.; Paolino, D.; Fresta, M. Influence of Materials Properties on Bio-Physical Features and Effectiveness of 3D-Scaffolds for Periodontal Regeneration. Molecules 2021, 26, 1643. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental stem cell and dental tissue regeneration. Front. Med. 2019, 13, 152–159. [Google Scholar] [CrossRef]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.T.; Liang, A.; Liu, S. Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem. Cells 2015, 33, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liu, Y.; Wang, S. Stem cell-based tooth and periodontal regeneration. Oral Dis. 2018, 24, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Dave, J.R.; Tomar, G.B. Dental Tissue-Derived Mesenchymal Stem Cells: Applications in Tissue Engineering. Crit. Rev. Biomed. Eng. 2018, 46, 429–468. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.G.; Maghaireh, H.; Mangano, F.G. Stem Cells in Dentistry: Types of Intra- and Extraoral Tissue-Derived Stem Cells and Clinical Applications. Stem Cells Int. 2018, 2018, 4313610. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.H.; Choung, P.H. MSM promotes human periodontal ligament stem cells differentiation to osteoblast and bone regeneration. Biochem. Biophys. Res. Commun. 2020, 528, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Tomokiyo, A.; Wada, N.; Maeda, H. Periodontal Ligament Stem Cells: Regenerative Potency in Periodontium. Stem Cells Dev. 2019, 28, 974–985. [Google Scholar] [CrossRef]
- Li, X.; He, X.T.; Kong, D.Q.; Xu, X.Y.; Wu, R.X.; Sun, L.J.; Tian, B.M.; Chen, F.M. M2 Macrophages Enhance the Cementoblastic Differentiation of Periodontal Ligament Stem Cells via the Akt and JNK Pathways. Stem Cells 2019, 37, 1567–1580. [Google Scholar] [CrossRef]
- Ma, Y.; Ji, Y.; Zhong, T.; Wan, W.; Yang, Q.; Li, A.; Zhang, X.; Lin, M. Bioprinting-Based PDLSC-ECM Screening for in Vivo Repair of Alveolar Bone Defect Using Cell-Laden, Injectable and Photocrosslinkable Hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 3534–3545. [Google Scholar] [CrossRef]
- Hyun, S.Y.; Lee, J.H.; Kang, K.J.; Jang, Y.J. Effect of FGF-2, TGF-beta-1, and BMPs on Teno/Ligamentogenesis and Osteo/Cementogenesis of Human Periodontal Ligament Stem Cells. Mol. Cells 2017, 40, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Dubey, N.; Ribeiro, J.S.; Bordini, E.A.F.; Ferreira, J.A.; Xu, J.; Castilho, R.M.; Bottino, M.C. Metformin-loaded nanospheres-laden photocrosslinkable gelatin hydrogel for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2021, 116, 104293. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Yanagi, Y.; Yoshimaru, K.; Zhang, X.Y.; Matsuura, T.; Nakayama, K.; Kobayashi, E.; Yamaza, H.; Nonaka, K.; Ohga, S.; et al. Regenerative medicine using stem cells from human exfoliated deciduous teeth (SHED): A promising new treatment in pediatric surgery. Surg. Today 2019, 49, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Shen, Z.; Guan, M.; Huang, Q.; Chen, L.; Qin, W.; Ge, X.; Chen, H.; Xiao, Y.; Lin, Z. Immunomodulatory Role of Stem Cells from Human Exfoliated Deciduous Teeth on Periodontal Regeneration. Tissue Eng. Part. A 2018, 24, 1341–1353. [Google Scholar] [CrossRef]
- Osathanon, T.; Nowwarote, N.; Manokawinchoke, J.; Pavasant, P. bFGF and JAGGED1 regulate alkaline phosphatase expression and mineralization in dental tissue-derived mesenchymal stem cells. J. Cell Biochem. 2013, 114, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Fan, W.; Deng, Q.; He, H.; Huang, F. Stem Cells from the Apical Papilla: A Promising Source for Stem Cell-Based Therapy. Biomed. Res. Int. 2019, 2019, 6104738. [Google Scholar] [CrossRef] [PubMed]
- Diao, S.; Yang, D.M.; Dong, R.; Wang, L.P.; Wang, J.S.; Du, J.; Wang, S.L.; Fan, Z. Enriched trimethylation of lysine 4 of histone H3 of WDR63 enhanced osteogenic differentiation potentials of stem cells from apical papilla. J. Endod. 2015, 41, 205–211. [Google Scholar] [CrossRef]
- Zhou, T.; Pan, J.; Wu, P.; Huang, R.; Du, W.; Zhou, Y.; Wan, M.; Fan, Y.; Xu, X.; Zhou, X.; et al. Dental Follicle Cells: Roles in Development and Beyond. Stem Cells Int. 2019, 2019, 9159605. [Google Scholar] [CrossRef] [PubMed]
- Tasli, P.N.; Dogan, A.; Demirci, S.; Sahin, F. Myogenic and neurogenic differentiation of human tooth germ stem cells (hTGSCs) are regulated by pluronic block copolymers. Cytotechnology 2016, 68, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, E.; Yagi, K.; Kojima, M.; Yagyuu, T.; Ohshima, A.; Sobajima, S.; Tadokoro, M.; Katsube, Y.; Isoda, K.; Kondoh, M.; et al. Multipotent cells from the human third molar: Feasibility of cell-based therapy for liver disease. Differentiation 2008, 76, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Tarle, S.; Kaigler, D. Characterization of the immunomodulatory properties of alveolar bone-derived mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 102. [Google Scholar] [CrossRef]
- Redondo, L.M.; Garcia, V.; Peral, B.; Verrier, A.; Becerra, J.; Sanchez, A.; Garcia-Sancho, J. Repair of maxillary cystic bone defects with mesenchymal stem cells seeded on a cross-linked serum scaffold. J. Craniomaxillofac. Surg. 2018, 46, 222–229. [Google Scholar] [CrossRef]
- Li, Q.; Yang, G.; Li, J.; Ding, M.; Zhou, N.; Dong, H.; Mou, Y. Stem cell therapies for periodontal tissue regeneration: A network meta-analysis of preclinical studies. Stem. Cell Res. Ther. 2020, 11, 427. [Google Scholar] [CrossRef]
- Gao, X.; Cao, Z. Gingiva-derived Mesenchymal Stem Cells and Their Potential Applications in Oral and Maxillofacial Diseases. Curr. Stem Cell Res. Ther. 2020, 15, 43–53. [Google Scholar] [CrossRef]
- Cristaldi, M.; Mauceri, R.; Campisi, G.; Pizzo, G.; Alessandro, R.; Tomasello, L.; Pitrone, M.; Pizzolanti, G.; Giordano, C. Growth and Osteogenic Differentiation of Discarded Gingiva-Derived Mesenchymal Stem Cells on a Commercial Scaffold. Front. Cell Dev. Biol. 2020, 8, 292. [Google Scholar] [CrossRef]
- Mohammed, E.; Khalil, E.; Sabry, D. Effect of Adipose-Derived Stem Cells and Their Exo as Adjunctive Therapy to Nonsurgical Periodontal Treatment: A Histologic and Histomorphometric Study in Rats. Biomolecules 2018, 8, 167. [Google Scholar] [CrossRef]
- Venkataiah, V.S.; Handa, K.; Njuguna, M.M.; Hasegawa, T.; Maruyama, K.; Nemoto, E.; Yamada, S.; Sugawara, S.; Lu, L.; Takedachi, M.; et al. Periodontal Regeneration by Allogeneic Transplantation of Adipose Tissue Derived Multi-Lineage Progenitor Stem Cells in vivo. Sci. Rep. 2019, 9, 921. [Google Scholar] [CrossRef]
- Tobita, M.; Uysal, A.C.; Ogawa, R.; Hyakusoku, H.; Mizuno, H. Periodontal tissue regeneration with adipose-derived stem cells. Tissue Eng. Part A 2008, 14, 945–953. [Google Scholar] [CrossRef]
- Kocan, B.; Maziarz, A.; Tabarkiewicz, J.; Ochiya, T.; Banas-Zabczyk, A. Trophic Activity and Phenotype of Adipose Tissue-Derived Mesenchymal Stem Cells as a Background of Their Regenerative Potential. Stem. Cells Int. 2017, 2017, 1653254. [Google Scholar] [CrossRef]
- Kang, W.; Liang, Q.; Du, L.; Shang, L.; Wang, T.; Ge, S. Sequential application of bFGF and BMP-2 facilitates osteogenic differentiation of human periodontal ligament stem cells. J. Periodontal. Res. 2019, 54, 424–434. [Google Scholar] [CrossRef]
- Panduwawala, C.P.; Zhan, X.; Dissanayaka, W.L.; Samaranayake, L.P.; Jin, L.; Zhang, C. In Vivo periodontal tissue regeneration by periodontal ligament stem cells and endothelial cells in three-dimensional cell sheet constructs. J. Periodontal. Res. 2017, 52, 408–418. [Google Scholar] [CrossRef]
- Chen, F.M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang, Y.J.; Dong, G.Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem. Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Y.; Guo, W.; Yang, B.; Tian, W. Stem cells from human exfoliated deciduous teeth as an alternative cell source in bio-root regeneration. Theranostics 2019, 9, 2694–2711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, C.; Zhang, S.; Ma, X.; Xiao, R.; Liu, H. Effects of rhBMP-2 on Bone Formation Capacity of Rat Dental Stem/Progenitor Cells from Dental Follicle and Alveolar Bone Marrow. Stem. Cells Dev. 2021, 30, 441–457. [Google Scholar] [CrossRef]
- Tasli, P.N.; Aydin, S.; Yalvac, M.E.; Sahin, F. Bmp 2 and bmp 7 induce odonto- and osteogenesis of human tooth germ stem cells. Appl. Biochem. Biotechnol. 2014, 172, 3016–3025. [Google Scholar] [CrossRef] [PubMed]
- Matichescu, A.; Ardelean, L.C.; Rusu, L.C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced Biomaterials and Techniques for Oral Tissue Engineering and Regeneration—A Review. Materials 2020, 13, 5303. [Google Scholar] [CrossRef] [PubMed]
- Maurus, P.B.; Kaeding, C.C. Bioabsorbable implant material review. Oper. Tech. Sports Med. 2004, 12, 158–160. [Google Scholar] [CrossRef]
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and biocompatibility: An historical overview. J. Biomed. Mater. Res. A 2020, 108, 1617–1633. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Ferrage, L.; Bertrand, G.; Lenormand, P.; Grossin, D.; Ben-Nissan, B. A review of the additive manufacturing (3DP) of bioceramics: Alumina, zirconia (PSZ) and hydroxyapatite. J. Aust. Ceram. Soc. 2016, 53, 11–20. [Google Scholar] [CrossRef]
- Mansour, A.; Abu Nada, L.; El-Hadad, A.A.; Mezour, M.A.; Ersheidat, A.; Al-Subaie, A.; Moussa, H.; Laurenti, M.; Kaartinen, M.T.; Tamimi, F. Biomimetic trace metals improve bone regenerative properties of calcium phosphate bioceramics. J. Biomed. Mater. Res. A 2021, 109, 666–681. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Sareethammanuwat, M.; Apinyauppatham, K.; Boonyuen, S. Characteristics and biologic effects of thermosensitive quercetin-chitosan/collagen hydrogel on human periodontal ligament stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1656–1670. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.N.; Ma, B.; Shao, J.; Liu, H.; Ge, S. Gingipain-Responsive Thermosensitive Hydrogel Loaded with SDF-1 Facilitates In Situ Periodontal Tissue Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 36880–36893. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, S.J.; Jung, T.G.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Surface modification of a three-dimensional polycaprolactone scaffold by polydopamine, biomineralization, and BMP-2 immobilization for potential bone tissue applications. Colloids Surf. B Biointerfaces 2021, 199, 111528. [Google Scholar] [CrossRef] [PubMed]
- Verardi, S.; Lombardi, T.; Stacchi, C. Clinical and Radiographic Evaluation of Nanohydroxyapatite Powder in Combination with Polylactic Acid/Polyglycolic Acid Copolymer as Bone Replacement Graft in the Surgical Treatment of Intrabony Periodontal Defects: A Retrospective Case Series Study. Materials 2020, 13, 269. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, Z.; Xia, S.; Liu, Y.; Lei, S.; Zhong, M.; Chen, X. Poly lactic-co-glycolic acid scaffold loaded with plasmid DNA encoding fibroblast growth factor-2 promotes periodontal ligament regeneration of replanted teeth. J. Periodontal. Res. 2020, 55, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Chapple, I.L.C.; Giannobile, W.V. Wound models for periodontal and bone regeneration: The role of biologic research. Periodontol. 2000 2015, 68, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.N.; Cho, Y.J.; Tarafder, S.; Lee, C.H. The recent advances in scaffolds for integrated periodontal regeneration. Bioact Mater. 2021, 6, 3328–3342. [Google Scholar] [CrossRef] [PubMed]
- Carrel, J.P.; Wiskott, A.; Moussa, M.; Rieder, P.; Scherrer, S.; Durual, S. A 3D printed TCP/HA structure as a new osteoconductive scaffold for vertical bone augmentation. Clin. Oral Implant. Res. 2016, 27, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Pilipchuk, S.P.; Monje, A.; Jiao, Y.; Hao, J.; Kruger, L.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Integration of 3D Printed and Micropatterned Polycaprolactone Scaffolds for Guidance of Oriented Collagenous Tissue Formation In Vivo. Adv. Healthc. Mater. 2016, 5, 676–687. [Google Scholar] [CrossRef]
- Cho, H.; Tarafder, S.; Fogge, M.; Kao, K.; Lee, C.H. Periodontal ligament stem/progenitor cells with protein-releasing scaffolds for cementum formation and integration on dentin surface. Connect. Tissue Res. 2016, 57, 488–495. [Google Scholar] [CrossRef]
- Sheikh, Z.; Javaid, M.A.; Hamdan, N.; Hashmi, R. Bone Regeneration Using Bone Morphogenetic Proteins and Various Biomaterial Carriers. Materials 2015, 8, 1778–1816. [Google Scholar] [CrossRef]
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.W.; Bartold, P.M. Multiphasic scaffolds for periodontal tissue engineering. J. Dent. Res. 2014, 93, 1212–1221. [Google Scholar] [CrossRef]
- Zeng, W.Y.; Ning, Y.; Huang, X. Advanced technologies in periodontal tissue regeneration based on stem cells: Current status and future perspectives. J. Dent. Sci. 2021, 16, 501–507. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chiu, Y.C.; Lee, A.K.; Lin, Y.A.; Lin, P.Y.; Shie, M.Y. Biofabrication of Gingival Fibroblast Cell-Laden Collagen/Strontium-Doped Calcium Silicate 3D-Printed Bi-Layered Scaffold for Osteoporotic Periodontal Regeneration. Biomedicines 2021, 9, 431. [Google Scholar] [CrossRef]
- Costa, P.F.; Vaquette, C.; Zhang, Q.; Reis, R.L.; Ivanovski, S.; Hutmacher, D.W. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J. Clin. Periodontol. 2014, 41, 283–294. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Miao, L.; Wang, Y.; Ren, S.; Yang, X.; Hu, Y.; Sun, W. Controlled release of recombinant human cementum protein 1 from electrospun multiphasic scaffold for cementum regeneration. Int. J. Nanomedicine. 2016, 11, 3145–3158. [Google Scholar] [CrossRef]
- Yu, N.; Nguyen, T.; Cho, Y.D.; Kavanagh, N.M.; Ghassib, I.; Giannobile, W.V. Personalized scaffolding technologies for alveolar bone regenerative medicine. Orthod. Craniofac. Res. 2019, 22 (Suppl. 1), 69–75. [Google Scholar] [CrossRef]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef]
- Trivedi, P.; Liu, R.; Bi, H.; Xu, C.; Rosenholm, J.M.; Akerfelt, M. 3D Modeling of Epithelial Tumors-The Synergy between Materials Engineering, 3D Bioprinting, High-Content Imaging, and Nanotechnology. Int. J. Mol. Sci. 2021, 22, 6225. [Google Scholar] [CrossRef]
- Della Bona, A.; Cantelli, V.; Britto, V.T.; Collares, K.F.; Stansbury, J.W. 3D printing restorative materials using a stereolithographic technique: A systematic review. Dent. Mater. 2021, 37, 336–350. [Google Scholar] [CrossRef]
- Hong, J.; Yeo, M.; Yang, G.H.; Kim, G. Cell-Electrospinning and Its Application for Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 6208. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, L.; Yang, B.; Tian, W. Three-dimensional printing biotechnology for the regeneration of the tooth and tooth-supporting tissues. Biotechnol. Bioeng. 2019, 116, 452–468. [Google Scholar] [CrossRef]
- Qu, M.; Wang, C.; Zhou, X.; Libanori, A.; Jiang, X.; Xu, W.; Zhu, S.; Chen, Q.; Sun, W.; Khademhosseini, A. Multi-Dimensional Printing for Bone Tissue Engineering. Adv. Healthc. Mater. 2021, 10, e2001986. [Google Scholar] [CrossRef]
- Zhuang, Y.; Lin, K.; Yu, H. Advance of Nano-Composite Electrospun Fibers in Periodontal Regeneration. Front. Chem. 2019, 7, 495. [Google Scholar] [CrossRef]
- Liu, J.; Chen, B.; Bao, J.; Zhang, Y.; Lei, L.; Yan, F. Macrophage polarization in periodontal ligament stem cells enhanced periodontal regeneration. Stem. Cell Res. Ther. 2019, 10, 320. [Google Scholar] [CrossRef]
- Chien, K.H.; Chang, Y.L.; Wang, M.L.; Chuang, J.H.; Yang, Y.C.; Tai, M.C.; Wang, C.Y.; Liu, Y.Y.; Li, H.Y.; Chen, J.T.; et al. Promoting Induced Pluripotent Stem Cell-driven Biomineralization and Periodontal Regeneration in Rats with Maxillary-Molar Defects using Injectable BMP-6 Hydrogel. Sci. Rep. 2018, 8, 114. [Google Scholar] [CrossRef]
- Nagai, K.; Ideguchi, H.; Kajikawa, T.; Li, X.; Chavakis, T.; Cheng, J.; Messersmith, P.B.; Heber-Katz, E.; Hajishengallis, G. An injectable hydrogel-formulated inhibitor of prolyl-4-hydroxylase promotes T regulatory cell recruitment and enhances alveolar bone regeneration during resolution of experimental periodontitis. FASEB J. 2020, 34, 13726–13740. [Google Scholar] [CrossRef]
- Mancini, L.; Romandini, M.; Fratini, A.; Americo, L.M.; Panda, S.; Marchetti, E. Biomaterials for Periodontal and Peri-Implant Regeneration. Materials 2021, 14, 3319. [Google Scholar] [CrossRef]
- Pan, J.; Deng, J.; Luo, Y.; Yu, L.; Zhang, W.; Han, X.; You, Z.; Liu, Y. Thermosensitive Hydrogel Delivery of Human Periodontal Stem Cells Overexpressing Platelet-Derived Growth Factor-BB Enhances Alveolar Bone Defect Repair. Stem. Cells Dev. 2019, 28, 1620–1631. [Google Scholar] [CrossRef]
- Mihaylova, Z.; Tsikandelova, R.; Sanimirov, P.; Gateva, N.; Mitev, V.; Ishkitiev, N. Role of PDGF-BB in proliferation, differentiation and maintaining stem cell properties of PDL cells in vitro. Arch. Oral. Biol. 2018, 85, 1–9. [Google Scholar] [CrossRef]
- Hisanaga, Y.; Suzuki, E.; Aoki, H.; Sato, M.; Saito, A.; Saito, A.; Azuma, T. Effect of the combined use of enamel matrix derivative and atelocollagen sponge scaffold on osteoblastic differentiation of mouse induced pluripotent stem cells in vitro. J. Periodontal. Res. 2018, 53, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hu, J.; Chen, H.; Chen, L.; Zhang, N.; Zhao, L.; Wen, N.; Yang, Y. Enamel matrix derivative enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells on the titanium implant surface. Organogenesis 2017, 13, 103–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Wang, P.; Wang, Y.; Li, J.; Qiao, D.; Chen, R.; Yang, W.; Yan, F. Gold Nanoparticles Promote the Bone Regeneration of Periodontal Ligament Stem Cell Sheets Through Activation of Autophagy. Int. J. Nanomed. 2021, 16, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ji, Y.; Huang, G.; Ling, K.; Zhang, X.; Xu, F. Bioprinting 3D cell-laden hydrogel microarray for screening human periodontal ligament stem cell response to extracellular matrix. Biofabrication 2015, 7, 044105. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Li, X.; Wang, J.; He, X.T.; Sun, H.H.; Chen, F.M. Concise Review: Periodontal Tissue Regeneration Using Stem Cells: Strategies and Translational Considerations. Stem. Cells Transl. Med. 2019, 8, 392–403. [Google Scholar] [CrossRef]
- Liang, Q.; Du, L.; Zhang, R.; Kang, W.; Ge, S. Stromal cell-derived factor-1/Exendin-4 cotherapy facilitates the proliferation, migration and osteogenic differentiation of human periodontal ligament stem cells in vitro and promotes periodontal bone regeneration in vivo. Cell Prolif. 2021, 54, e12997. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Sheng, L.; Chen, G.; Guo, S.; Xie, L.; Yang, B.; Guo, W.; Tian, W. The combination use of platelet-rich fibrin and treated dentin matrix for tooth root regeneration by cell homing. Tissue Eng. Part A 2015, 21, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Funda, G.; Taschieri, S.; Bruno, G.A.; Grecchi, E.; Paolo, S.; Girolamo, D.; Del Fabbro, M. Nanotechnology Scaffolds for Alveolar Bone Regeneration. Materials 2020, 13, 201. [Google Scholar] [CrossRef] [PubMed]
| Types | Sources | Differentiation and Functions | References | |
|---|---|---|---|---|
| Dental | Periodontal Ligament Stem Cells | Periodontal ligament | Osteoblasts Adipocytes Chondrocytes Immunomodulation Periodontal regeneration | [20,21,22,23,24] |
| Stem Cells from Human Exfoliated Deciduous Teeth | Pulp of the human exfoliated deciduous teeth | Adipocytes Osteoblasts Odontoblasts Nerve cells Hepatocytes Endothelial cells | [20,21,22,23,24,25,26,27,28] | |
| Stem Cells from Apical Papilla | Apical papilla | Odontoblasts Nerve cells Hepatocyte-like cells Periodontal tissue | [25,26,27,28,29,30] | |
| Dental Follicle Stem Cells | Dental follicle | Osteoblasts Odontoblasts Periodontal ligament tissue Adipocytes Chondrocytes | [29,30,31] | |
| Tooth Germ Progenitor Cells | Tooth germ | Adipocytes Hepatocytes Osteoblasts Neurogenic tissues | [18,31,32,33] | |
| Mesenchymal Stem Cells Derived from Alveolar Bone | Alveolar bone | Osteoblasts Adipocytes Chondrocytes Immunomodulation | [18,32,33,34,35] | |
| Non-dental | Gingival Mesenchymal Stem Cells | Gingiva | Adipocytes Osteoblasts Chondrocytes | [18,34,35,36,37,38] |
| Adipose Derived Stem Cells | Adipose tissue | Osteoblasts Periodontal ligament-like tissue Immunomodulation | [36,37,38,39,40,41,42] |
| Types | Advantages | Disadvantages | Ref. | ||
|---|---|---|---|---|---|
| Organic polymers | Natural materials | chitosan, alginate, polypeptide and collagen | Excellent hydrophilicity | Poor mechanical property High degrading rate | [52,56,57] |
| Preferable biocompatibility | |||||
| Weak cytotoxicity | |||||
| Synthetic materials | PLA, PCL, PLGA and PGA | Proper degrading rate | Lack of bio-conductivity | [52,58,59] | |
| Good physicochemical and mechanical property | |||||
| Inorganic polymers | Bio-ceramics | HA TCP | Comparable structure with bone Good osteoinductivity | Hard to degrade Brittle | [49,54] |
| 3D Bioprinting Techniques | Strengths | Limitations | Ref. | |
|---|---|---|---|---|
| Photocuring-based bioprinting | high speed high printing resolution good structural integrity and mechanical property excellent cell viability | hard to operate required photosensitive material with certain viscosity | [73,74,75] | |
| Extrusion | sufficient mechanical property | low cell viability caused by inevitable shear force | [73,77] | |
| Multi-choices of biomaterials | limited printing accuracy (100 μm) | |||
| able to print high concentration cell fluid | Bio-ink with certain curing and shear thinning properties | |||
| Droplet-jet bioprinting | Inkjet | simple to operate | narrow range of bio-active materials | [73,74] |
| fairly affordable excellent resolution and precision fast speed | potential mechanical or thermal damage to cells low cellular concentration lack of structural strength | |||
| laser assisted bioprinting | high speed | costly long preparing stage limited choice in bio-ink possibility in containing metal residue | [73,77,78] | |
| up to single cell accuracy | ||||
| able to print different materials to regenerate native structure | ||||
| Non-contacting and nozzle-free | ||||
| Cell-Electrospinning | good cellular activities similar to the structure of extracellular matrix homogeneous cell density in structure | poor mechanical strength hard to develop to 3D structure poor accuracy of fiber deposition | [12,13,76,79] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Huang, X. Elements of 3D Bioprinting in Periodontal Regeneration: Frontiers and Prospects. Processes 2021, 9, 1724. https://doi.org/10.3390/pr9101724
Wang Z, Huang X. Elements of 3D Bioprinting in Periodontal Regeneration: Frontiers and Prospects. Processes. 2021; 9(10):1724. https://doi.org/10.3390/pr9101724
Chicago/Turabian StyleWang, Ziyi, and Xin Huang. 2021. "Elements of 3D Bioprinting in Periodontal Regeneration: Frontiers and Prospects" Processes 9, no. 10: 1724. https://doi.org/10.3390/pr9101724
APA StyleWang, Z., & Huang, X. (2021). Elements of 3D Bioprinting in Periodontal Regeneration: Frontiers and Prospects. Processes, 9(10), 1724. https://doi.org/10.3390/pr9101724





