Drug Repurposing in Alternative Medicine: Sochehwan, a Polyherbal Traditional Korean Digestant, Protects against Alcoholic Steatohepatitis by Regulating Cytochrome P450 2E1 Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibodies
2.2. Preparation of SCH Sample
2.3. Animals
2.4. Serum Biochemistry
2.5. mRNA Isolation and Real-Time Polymerase Chain Reaction (qPCR)
2.6. Cell Culture and CYP2E1 Transfection
2.7. Western Blot Analysis
2.8. Statistical Analyses
3. Results
3.1. Body Weights and Serum Analysis
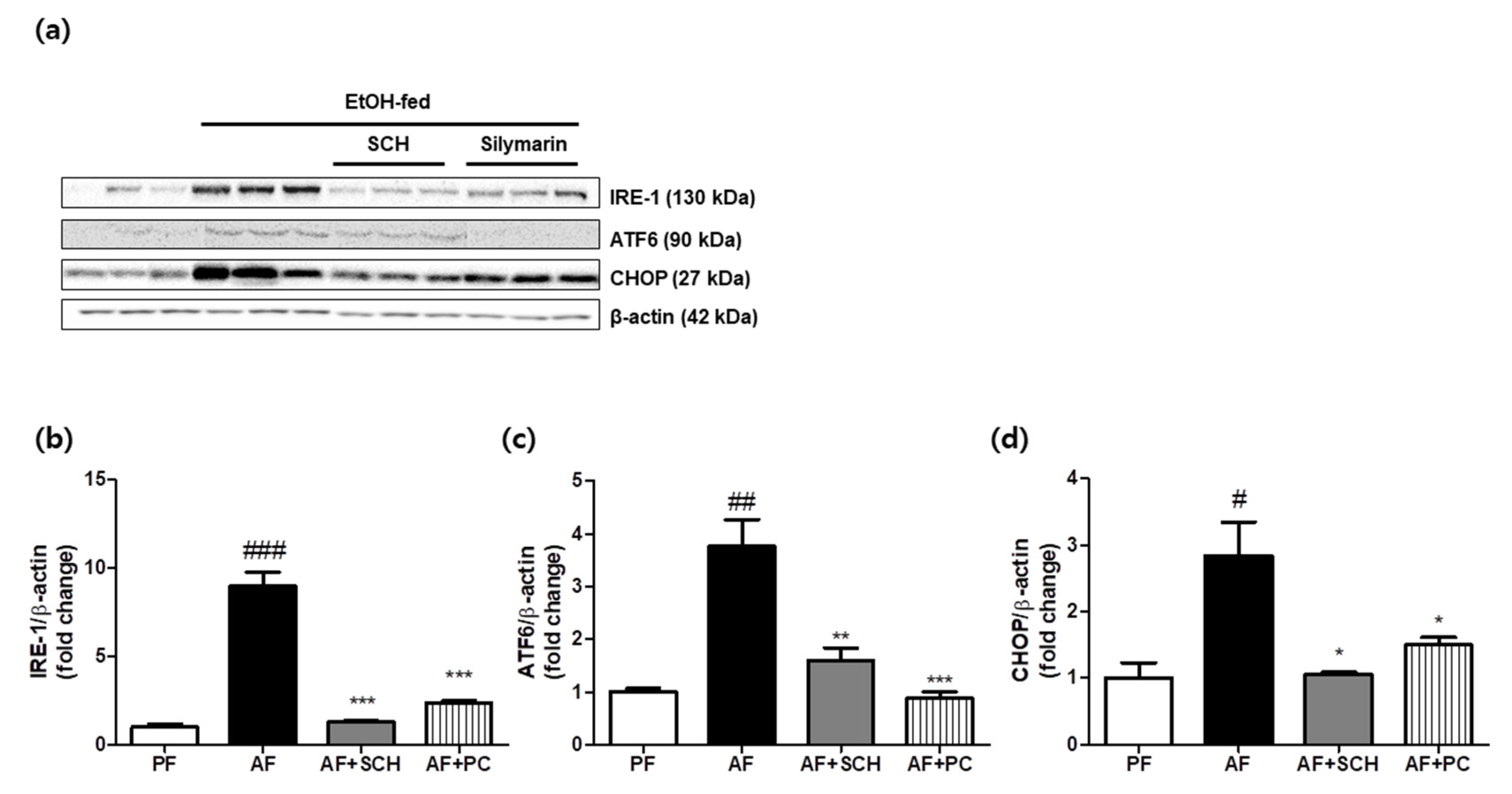
3.2. Reduction of Endoplasmic Reticulum (ER) Stress
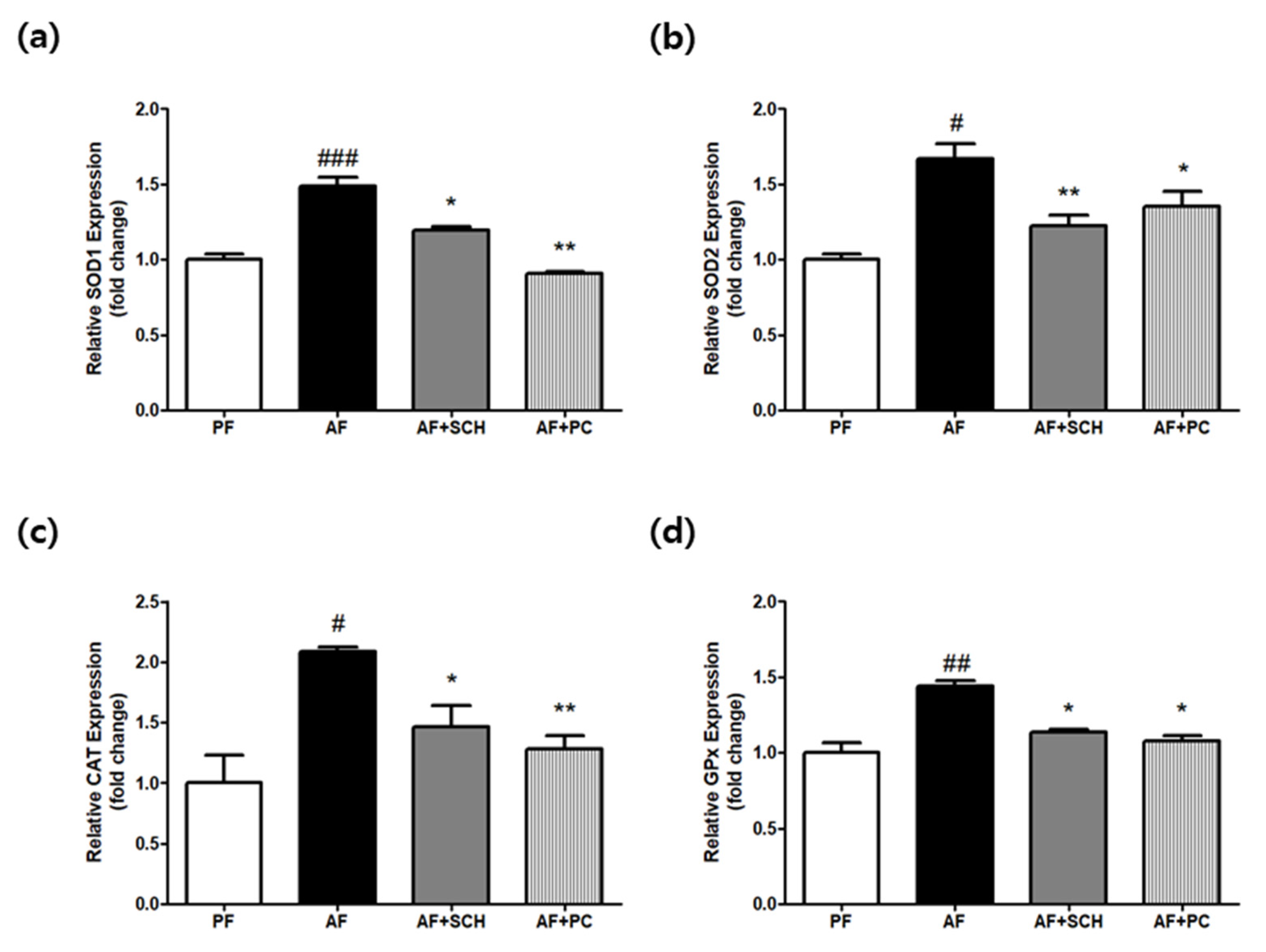
3.3. Regulation of Antioxidative Enzyme Expression
3.4. Regulation of MAPK Signals and Cytokine Production
3.5. Reduction of CYP2E1 Expression in Mouse Liver Tissue
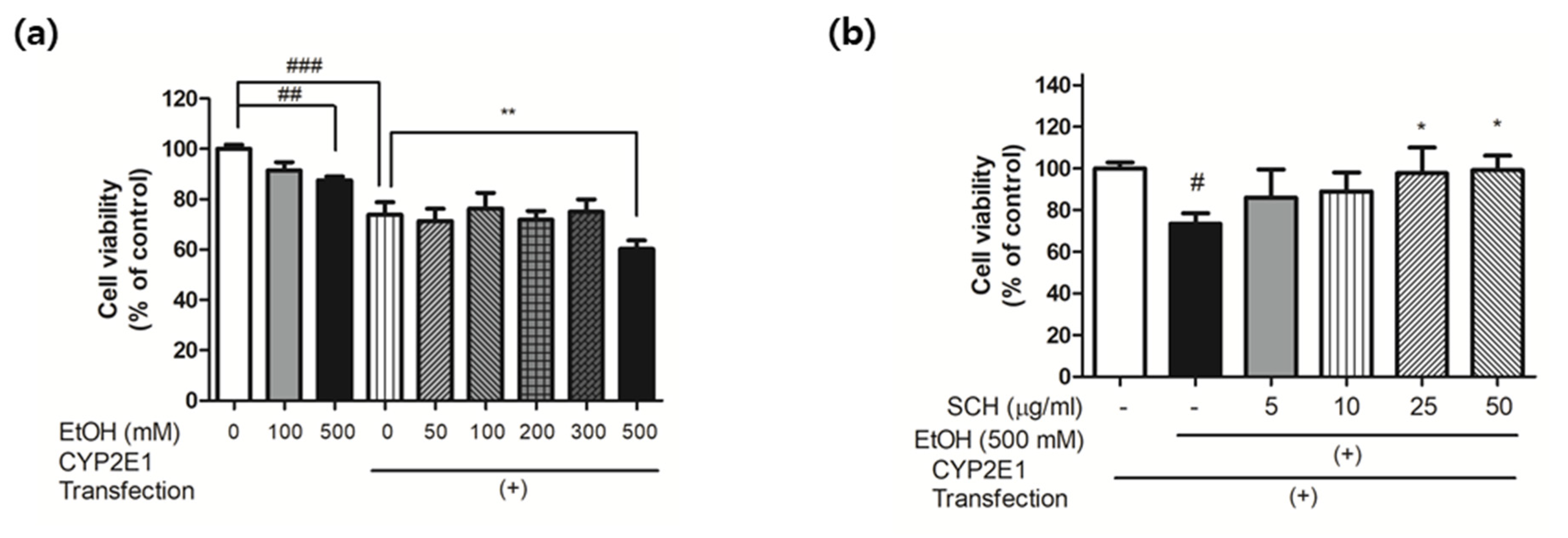
3.6. Reduction of Ethanol-Induced Injury in CYP2E1-Transfected HepG2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruha, R.; Dvořák, K.; Petrtyl, J. Alcoholic liver disease. World J. Hepatol. 2012, 4, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.; Neuman, M. The History of Alcoholic Liver Disease: From an Unrecognized Disease to One of the Most Frequent Diseases in Hepatology. J. Clin. Med. 2021, 10, 858. [Google Scholar] [CrossRef]
- Younossi, Z.; Henry, L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology 2016, 150, 1778–1785. [Google Scholar] [CrossRef]
- Gao, B.; Bataller, R. Alcoholic Liver Disease: Pathogenesis and New Therapeutic Targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef] [Green Version]
- Harjumäki, R.; Pridgeon, C.; Ingelman-Sundberg, M. CYP2E1 in Alcoholic and Non-Alcoholic Liver Injury. Roles of ROS, Reactive Intermediates and Lipid Overload. Int. J. Mol. Sci. 2021, 22, 8221. [Google Scholar] [CrossRef]
- Heit, C.; Dong, H.; Chen, Y.; Thompson, D.C.; Deitrich, R.A.; Vasiliou, V.K. The Role of CYP2E1 in Alcohol Metabolism and Sensitivity in the Central Nervous System. Subcell. Biochem. 2013, 67, 235–247. [Google Scholar] [CrossRef] [Green Version]
- Koop, D.R. Alcohol metabolism’s damaging effects on the cell: A focus on reactive oxygen generation by the enzyme cy-tochrome P450 2E1. Alcohol Res. Health 2006, 29, 274–280. [Google Scholar] [PubMed]
- Abdelmegeed, A.M.; Ha, S.-K.; Choi, Y.; Akbar, M.; Song, B.-J. Role of CYP2E1 in mitochondrial dysfunction and hepat-ic injury by alcohol and non-alcoholic substances. Curr. Mol. Pharmacol. 2017, 10, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Millonig, G.; Nair, J.; Patsenker, E.; Stickel, F.; Mueller, S.; Bartsch, H.; Seitz, H.K. Ethanol-induced cytochrome P4502E1 causes carcinogenic etheno-DNA lesions in alcoholic liver disease. Hepatology 2009, 50, 453–461. [Google Scholar] [CrossRef]
- Aubert, J.; Begriche, K.; Knockaert, L.; Robin, M.; Fromenty, B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: Mechanisms and pathophysiological role. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.E.; Mezey, E.; Hardwick, J.P.; Salem, N., Jr.; Clemens, D.L.; Song, B.J. Increased ethanol-inducible cytochrome P450-2E1 and cytochrome P450 isoforms in exosomes of alcohol-exposed rodents and patients with alcoholism through oxidative and endoplasmic reticulum stress. Hepatol. Commun. 2017, 1, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Farha, M.A.; Brown, E.D. Drug repurposing for antimicrobial discovery. Nat. Microbiol. 2019, 4, 565–577. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Repurposing drugs to target nonalcoholic steatohepatitis. World J. Gastroenterol. 2019, 25, 1783–1796. [Google Scholar] [CrossRef]
- Tobinick, E.L. The value of drug repositioning in the current pharmaceutical market. Drug News Perspect. 2009, 22, 119–125. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef]
- Sucher, N.J. The application of Chinese medicine to novel drug discovery. Expert Opin. Drug Discov. 2012, 8, 21–34. [Google Scholar] [CrossRef]
- Lim, D.; Kim, H.; Kim, Y.-M.; Chin, Y.-W.; Park, W.-H.; Kim, J.-E. Drug repurposing in alternative medicine: Herbal digestive Sochehwan exerts multifaceted effects against metabolic syndrome. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Heo, J. Dongeuibogam; Bupin Publisher: Seoul, Korea, 1999. [Google Scholar]
- Lim, D.-W.; Kim, H.; Lee, S.-J.; Yu, G.-R.; Kim, J.-E.; Park, W.-H. Jwa Kum Whan Attenuates Nonalcoholic Fatty Liver Disease by Modulating Glucose Metabolism and the Insulin Signaling Pathway. Evid.-Based Complement. Altern. Med. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-W.; Kim, H.; Park, J.-Y.; Kim, J.-E.; Moon, J.-Y.; Park, S.-D.; Park, W.-H. Amomum cardamomum L. ethyl acetate fraction protects against carbon tetrachloride-induced liver injury via an antioxidant mechanism in rats. BMC Complement. Altern. Med. 2016, 16, 155. [Google Scholar] [CrossRef] [Green Version]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Karimi-Khouzani, O.; Heidarian, E.; Amini, S.A. Anti-inflammatory and ameliorative effects of gallic acid on fluoxetine-induced oxidative stress and liver damage in rats. Pharmacol. Rep. 2017, 69, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Vogle, A.; Qian, T.; Zhu, S.; Burnett, E.; Fey, H.; Zhu, Z.; Keshavarzian, A.; Shaikh, M.; Hoshida, Y.; Kim, M.; et al. Restricted immunological and cellular pathways are shared by murine models of chronic alcohol consumption. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Hou, S.; Zhang, D.; Xia, H.; Wang, Y.-C.; Jiang, J.; Yin, H.; Ying, H. Regulation of fatty acid composition and lipid storage by thyroid hormone in mouse liver. Cell Biosci. 2014, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- Simon, G.; Heckmann, V.; Tóth, D.; Pauka, D.; Petrus, K.; Molnár, T.F. The effect of hepatic steatosis and fibrosis on liver weight and dimensions. Leg. Med. 2020, 47, 101781. [Google Scholar] [CrossRef]
- You, M.; Arteel, G.E. Effect of ethanol on lipid metabolism. J. Hepatol. 2019, 70, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Green, R.M. Endoplasmic reticulum stress and liver diseases. Liver Res. 2019, 3, 55–64. [Google Scholar] [CrossRef]
- Verfaillie, T.; Salazar, M.; Velasco, G.; Agostinis, P. Linking ER Stress to Autophagy: Potential Implications for Cancer Therapy. Int. J. Cell Biol. 2010, 2010, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szegezdi, E.; Logue, S.; Gorman, A.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef] [Green Version]
- Madkour, L.H. Reactive Oxygen Species (ROS), Nanoparticles, and Endoplasmic Reticulum(er) Stress-Induced Cell Death Mechanisms; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Whitmarsh, A.J. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 1773, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, P.; Szabo, G. Signalling pathways in alcohol-induced liver inflammation. J. Hepatol. 2009, 50, 1258–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albano, E. Alcohol, oxidative stress and free radical damage. Proc. Nutr. Soc. 2006, 65, 278–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaitantzi, H.; Meyer, C.; Rakoczy, P.; Thomas, M.; Wahl, K.; Wandrer, F.; Bantel, H.; Alborzinia, H.; Wölfl, S.; Ehnert, S.; et al. Ethanol sensitizes hepatocytes for TGF-β-triggered apoptosis. Cell Death Dis. 2018, 9, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schattenberg, J.M.; Czaja, M.J. Regulation of the effects of CYP2E1-induced oxidative stress by JNK signaling. Redox Biol. 2014, 3, 7–15. [Google Scholar] [CrossRef] [Green Version]
- McChesney, J.D. Natural products in drug discovery—Organizing for success. P. R. Health Sci. J. 2002, 21. [Google Scholar]
- Shen, B. A New Golden Age of Natural Products Drug Discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.-R.; Lee, S.-J.; Lim, D.-W.; Kim, H.; Kim, J.-E.; Park, W.-H. Drug Repurposing in Alternative Medicine: Sochehwan, a Polyherbal Traditional Korean Digestant, Protects against Alcoholic Steatohepatitis by Regulating Cytochrome P450 2E1 Expression. Processes 2021, 9, 1760. https://doi.org/10.3390/pr9101760
Yu G-R, Lee S-J, Lim D-W, Kim H, Kim J-E, Park W-H. Drug Repurposing in Alternative Medicine: Sochehwan, a Polyherbal Traditional Korean Digestant, Protects against Alcoholic Steatohepatitis by Regulating Cytochrome P450 2E1 Expression. Processes. 2021; 9(10):1760. https://doi.org/10.3390/pr9101760
Chicago/Turabian StyleYu, Ga-Ram, Seung-Jun Lee, Dong-Woo Lim, Hyuck Kim, Jai-Eun Kim, and Won-Hwan Park. 2021. "Drug Repurposing in Alternative Medicine: Sochehwan, a Polyherbal Traditional Korean Digestant, Protects against Alcoholic Steatohepatitis by Regulating Cytochrome P450 2E1 Expression" Processes 9, no. 10: 1760. https://doi.org/10.3390/pr9101760
APA StyleYu, G.-R., Lee, S.-J., Lim, D.-W., Kim, H., Kim, J.-E., & Park, W.-H. (2021). Drug Repurposing in Alternative Medicine: Sochehwan, a Polyherbal Traditional Korean Digestant, Protects against Alcoholic Steatohepatitis by Regulating Cytochrome P450 2E1 Expression. Processes, 9(10), 1760. https://doi.org/10.3390/pr9101760





