Vis-NIR Hyperspectral Imaging for Online Quality Evaluation during Food Processing: A Case Study of Hot Air Drying of Purple-Speckled Cocoyam (Colocasia esculenta (L.) Schott)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Experimental Design and Drying Experiments
2.3. Quality Attributes
2.3.1. Moisture Attributes
2.3.2. Colour Attributes
2.3.3. Chemical Attributes
2.3.4. Structural Attributes
- (a)
- Rehydration ratio
- (b)
- Volumetric shrinkage
- (c)
- Structural morphology
2.4. Hyperspectral Image Acquisition, Processing and Analysis
2.4.1. Overview
2.4.2. HSI Acquisition
2.4.3. HSI Processing
2.4.4. Multivariate Modelling
2.4.5. Selection of Optimal Wavelengths
- (a)
- PLS-BETA
- (b)
- PLS-VIP
2.5. Validation and Method Comparison
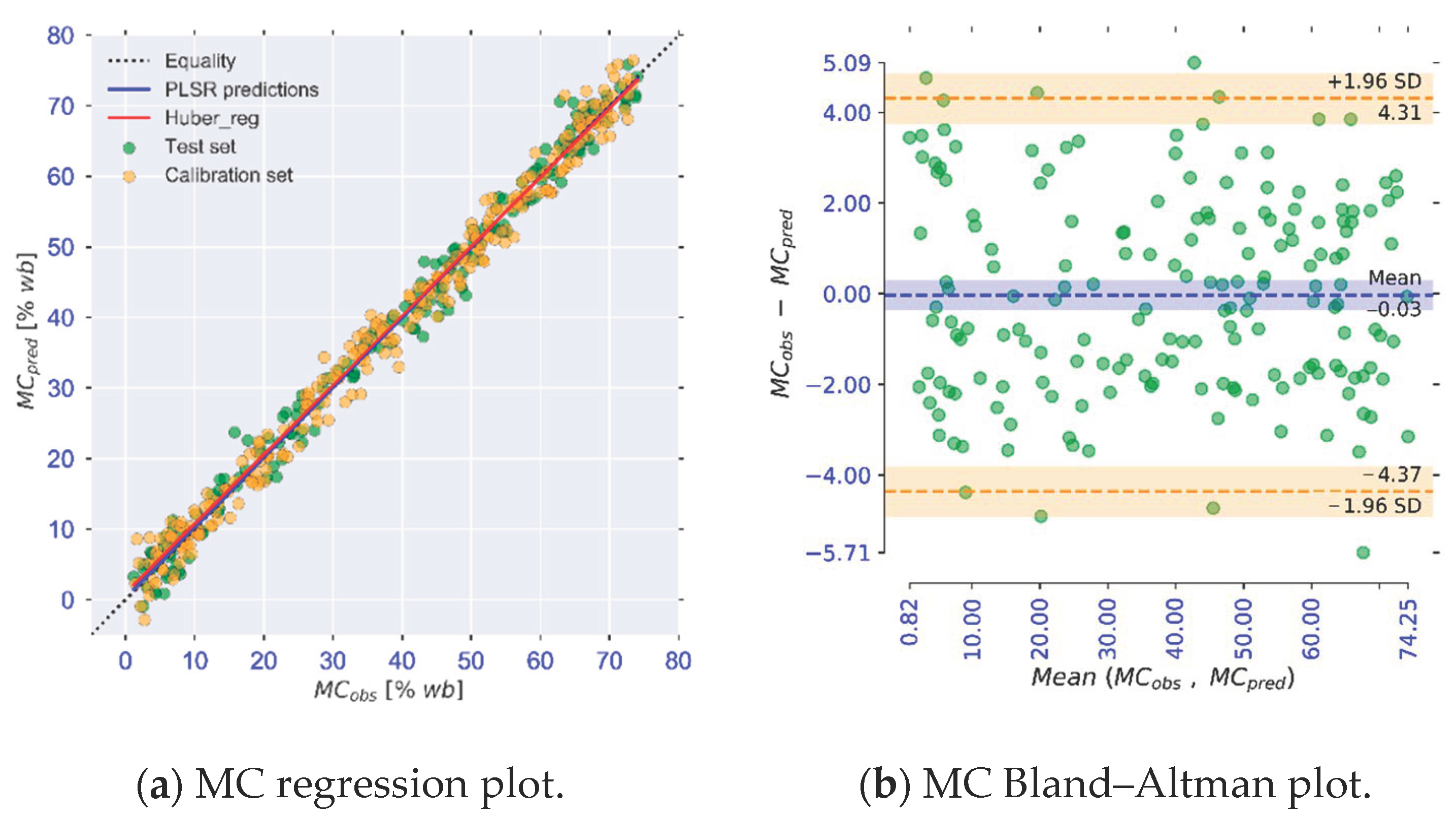
2.5.1. Huber Regression
2.5.2. Bland–Altman Plots and Analysis
2.5.3. Concordance Correlation Coefficient
2.5.4. Statistical Analysis
3. Results
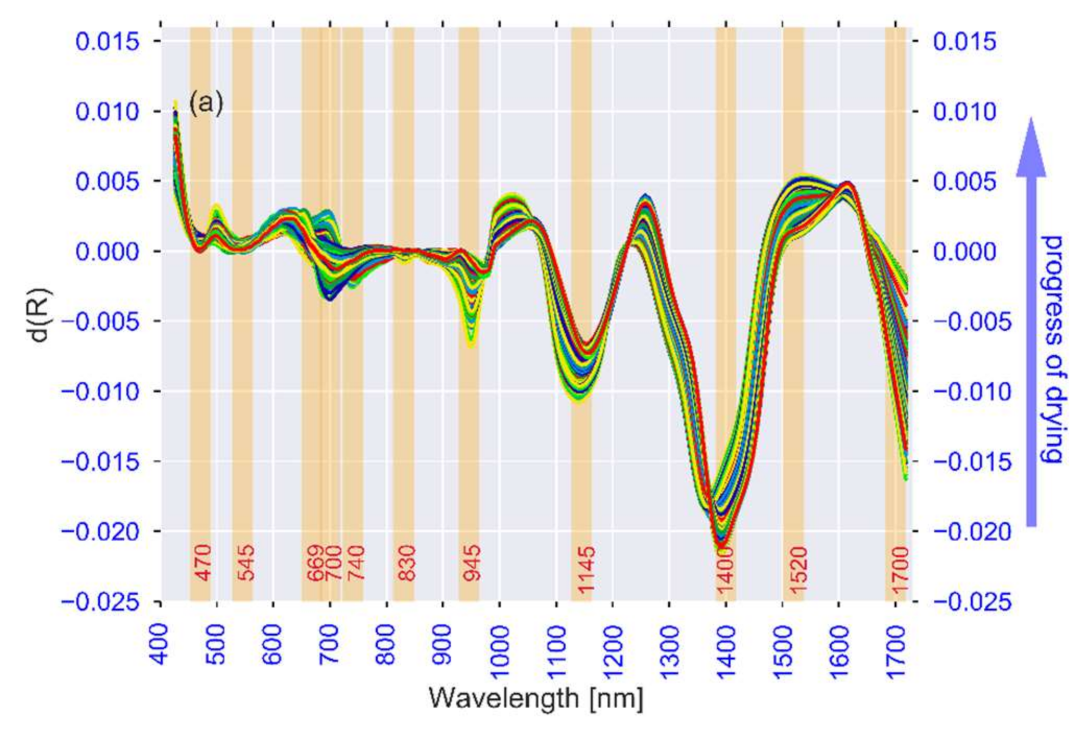
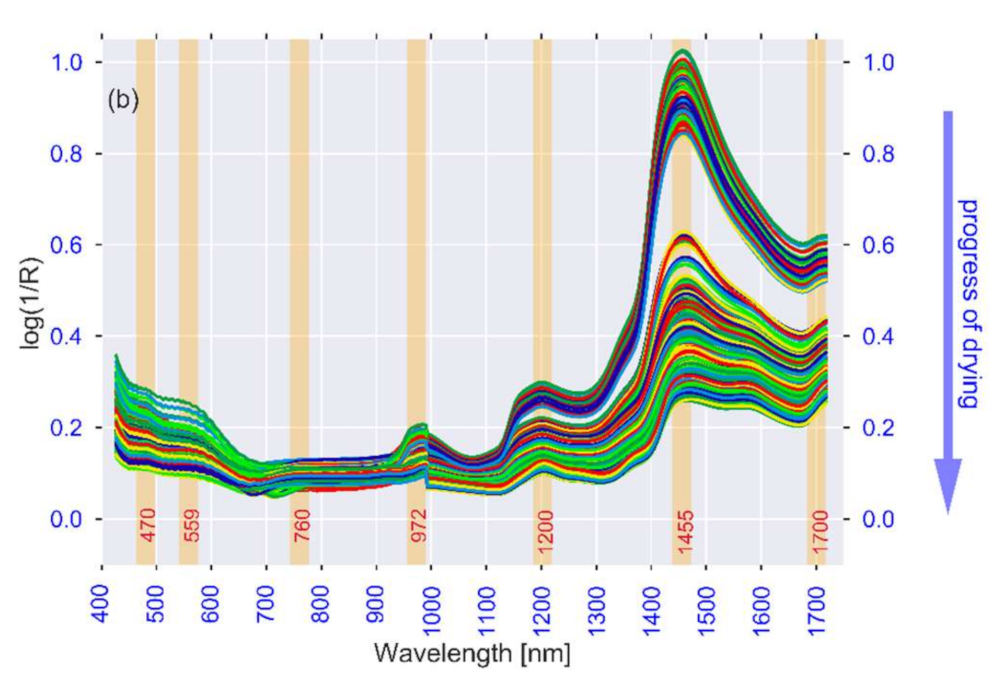
3.1. Spectral Analysis
3.2. Development of Calibration Models
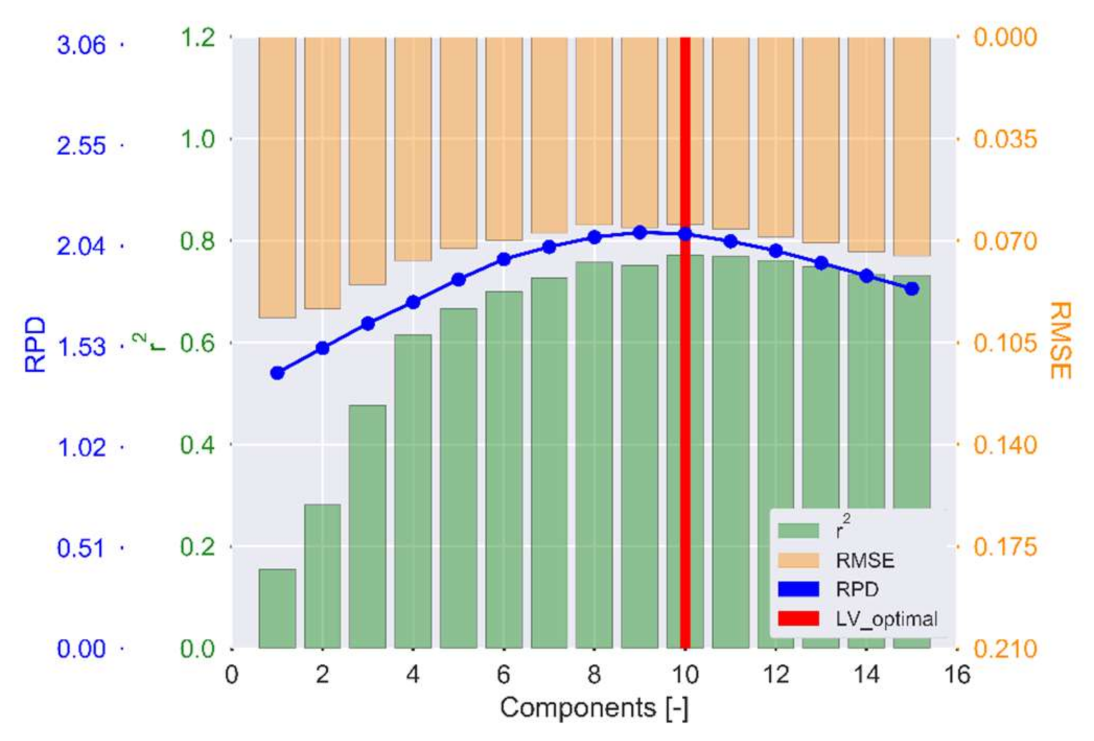
3.2.1. Selection of Optimal PLS Latent Variables
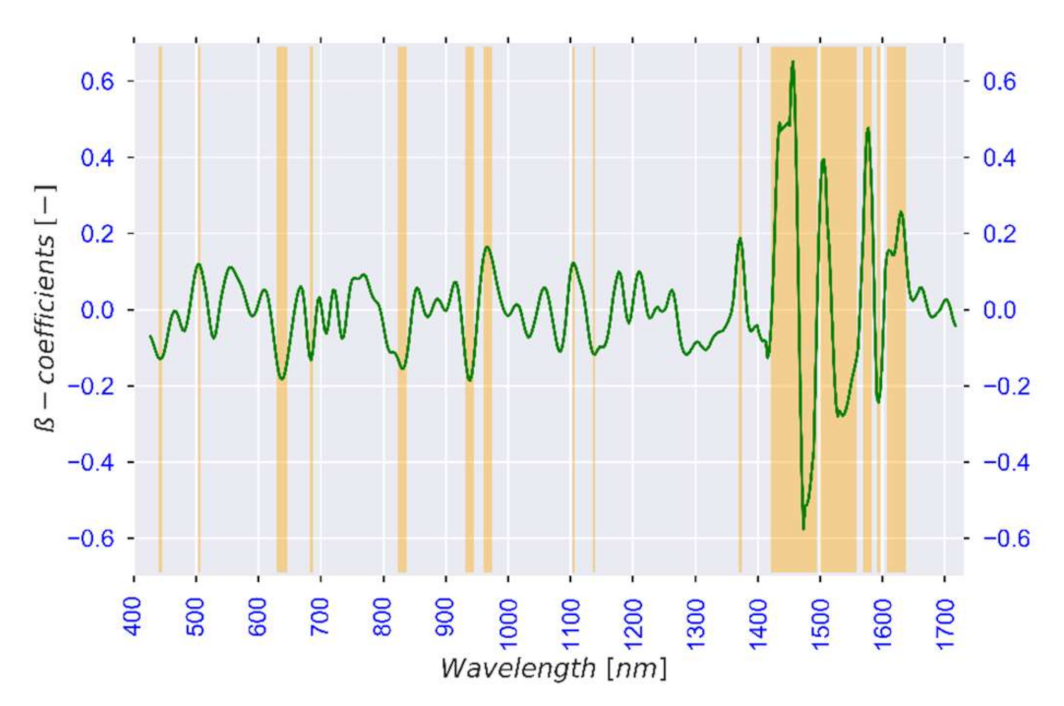
3.2.2. Selection of Optimal Wavelengths
3.3. Modelling and Method Comparison
3.3.1. Moisture Attributes
3.3.2. Colour Attributes
3.3.3. Chemical Attributes
3.3.4. Structural Attributes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviation | Meaning |
| AOAC | Association of Official Analytical Chemists |
| BI | Browning Index |
| CCC | Concordance Correlation Coefficient |
| CIE | International Commission on Illumination |
| DM | Dry Matter |
| kNN | k-Nearest Neighbours |
| LOA | Limits of Agreement |
| LV | Latent Variable |
| MA | Moving Average |
| MC | Moisture Content |
| MR | Moisture Ratio |
| MSC | Mean Scatter Correction |
| PC | Pore circularity |
| PCA | Principal Component Analysis |
| PCR | Principal Component Regression |
| PLSR | Partial Least Squares Regression |
| PPA | Percentage pore area |
| RF | Random Forests |
| RMSE | Root Mean Squared Error |
| RPD | Residual Prediction Deviation |
| RSA | Radical Scavenging Activity |
| SDG | Sustainable Development Goal |
| SEM | Scanning Electron Microscopy |
| SVM | Support Vector Machines |
| RR | Rehydration Ratio |
| TAA | Total Antioxidant Activity |
| TFC | Total Flavonoid Content |
| TPC | Total Phenolic Content |
| aw | Water Activity |
| WI | Whiteness Index |
| VIP | Variable Importance in Projection |
| Vis-NIR | Visible to Near-Infrared |
| Vs | Volumetric Shrinkage |
References
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Okop, K.J.; Ndayi, K.; Tsolekile, L.; Sanders, D.; Puoane, T. Low intake of commonly available fruits and vegetables in socio-economically disadvantaged communities of South Africa: Influence of affordability and sugary drinks intake. BMC Public Health 2019, 19, 1–14. [Google Scholar] [CrossRef] [Green Version]
- United Nations Department of Economic and Social Affairs. The Sustainable Development Goals Report 2019; United Nations Department of Economic and Social Affairs: New York, NY, USA, 2019. [Google Scholar]
- Sibhatu, K.T.; Qaim, M. Rural food security, subsistence agriculture, and seasonality. PLoS ONE 2017, 12, e0186406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, L.; Crichton, S.O.; Kulig, B.; Kiesel, B.; Hensel, O.; Sturm, B. Comparative analysis of methods and model prediction performance evaluation for continuous online non-invasive quality assessment during drying of apples from two cultivars. Therm. Sci. Eng. Prog. 2020, 18, 100461. [Google Scholar] [CrossRef]
- Satterthwaite, D.; McGranahan, G.; Tacoli, C. Urbanization and its implications for food and farming. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2809–2820. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, D.R.; Raghu, N.; Gopenath, T.S.; Palanisamy, P.; Bakthavatchalam, P.; Karthikeyan, M.; Gnanasekaran, A.; Ranjith, M.S.; Chandrashekrappa, G.K.; Basalingappa, K.M. Taro (Colocasia esculenta): An overview. J. Med. Plants Stud. 2018, 6, 156–161. [Google Scholar]
- Panyoo, E.A.; Njintang, N.Y.; Hussain, R.; Gaiani, C.; Scher, J.; Mbofung, C.M.F. Physicochemical and Rheological Properties of Taro (Colocasia esculenta) Flour Affected by Cormels Weight and Method of Peeling. Food Bioprocess Technol. 2014, 7, 1354–1363. [Google Scholar] [CrossRef]
- Pereira, P.R.; Silva, J.T.; Vericimo, M.; Paschoalin, V.; Teixeira, G.A. Crude extract from taro (Colocasia esculenta) as a natural source of bioactive proteins able to stimulate haematopoietic cells in two murine models. J. Funct. Foods 2015, 18, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Alcantara, R.; Hurtada, W.; Dizon, W. The Nutritional Value and Phytochemical Components of Taro [Colocasia esculenta (L.) Schott] Powder and its Selected Processed Foods. J. Nutr. Food Sci. 2013, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Ndisya, J.; Mbuge, D.; Kulig, B.; Gitau, A.; Hensel, O.; Sturm, B. Hot air drying of purple-speckled Cocoyam (Colocasia esculenta (L.) Schott) slices: Optimisation of drying conditions for improved product quality and energy savings. Therm. Sci. Eng. Prog. 2020, 18, 100557. [Google Scholar] [CrossRef]
- Opara, L.U. CIGR Handbook of Agricultural Engineering, Volume IV Agro Processing Engineering, Chapter 2 Root Crops, Part 2.6 Storage of Edible Aroids. In CIGR Handbook of Agricultural Engineering Volume IV Agro-Processing Engineering; American Society of Agricultural and Biological Engineers (ASABE): Saint Joseph, MI, USA, 1999; pp. 214–241. [Google Scholar]
- Ndukwu, M.C.; Dirioha, C.; Abam, F.; Ihediwa, V.E. Heat and mass transfer parameters in the drying of cocoyam slice. Case Stud. Therm. Eng. 2017, 9, 62–71. [Google Scholar] [CrossRef]
- Afolabi, T.J.; Tunde-Akintunde, T.Y.; Adeyanju, J.A. Mathematical modeling of drying kinetics of untreated and pretreated cocoyam slices. J. Food Sci. Technol. 2015, 52, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Adeboyejo, F.O.; Aderibigbe, O.R.; Obarayi, M.T.; Sturm, B. Comparative evaluation of instant ’poundo’ cocoyam (Colocasia esculenta) and yam (Dioscorea rotundata) flours produced by flash and cabinet drying. Int. J. Food Sci. Technol. 2021, 56, 1482–1490. [Google Scholar] [CrossRef]
- Prabhakar, K.; Mallika, E.N. Dried Foods. In Encyclopedia of Food Microbiology; Academic Press: Cambridge, MA, USA, 2014; pp. 574–576. [Google Scholar]
- Sturm, B. Systemic Optimisation and Design Approach for Thermal Food Processes—Increase of Quality, Process- and Resource Efficiency in Dried Agricultural Products Manufacturing. Habilitation Thesis, Universität Kassel, Kassel, Germania, 2018. [Google Scholar]
- Kondakci, T.; Zhou, W. Recent Applications of Advanced Control Techniques in Food Industry. Food Bioprocess Technol. 2017, 10, 522–542. [Google Scholar] [CrossRef]
- Ilyukhin, S.V.; Haley, T.; Singh, R.K. A survey of automation practices in the food industry. Food Control 2001, 12, 285–296. [Google Scholar] [CrossRef]
- Slišković, D.; Grbić, R.; Hocenski, Ž. Methods for Plant Data-Based Process Modeling in Soft-Sensor Development. Automatika 2011, 52, 306–318. [Google Scholar] [CrossRef] [Green Version]
- Chao, K.; Kim, M.S.; Lu, R. Food process automation. Sens. Instrum. Food Qual. Saf. 2009, 3, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Raut, S.; Saleh, R.M.; Kirchhofer, P.; Kulig, B.; Hensel, O.; Sturm, B. Investigating the Effect of Different Drying Strategies on the Quality Parameters of Daucus carota L. Using Dynamic Process Control and Measurement Techniques. Food Bioprocess Technol. 2021, 14, 1067–1088. [Google Scholar] [CrossRef]
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘point’ spectroscopy in postharvest fruit and vegetable assessment: The science behind three decades of commercial use. Postharvest Biol. Technol. 2020, 168, 111246. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.-W. Advanced applications of hyperspectral imaging technology for food quality and safety analysis and assessment: A review—Part I: Fundamentals. Innov. Food Sci. Emerg. Technol. 2013, 19, 1–14. [Google Scholar] [CrossRef]
- Torres, I.; Pérez-Marín, D.; Vega-Castellote, M.; Sánchez, M.-T. Mapping of fatty acids composition in shelled almonds analysed in bulk using a Hyperspectral Imaging system. LWT 2021, 138, 110678. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Fisk, I.D. Total lipid prediction in single intact cocoa beans by hyperspectral chemical imaging. Food Chem. 2021, 344, 128663. [Google Scholar] [CrossRef]
- Benelli, A.; Fabbri, A. Vis/NIR hyperspectral imaging technology in predicting the quality properties of three fruit cultivars during production and storage. In Proceedings of the 2020 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Trento, Italy, 4–6 November 2020; pp. 155–159. [Google Scholar]
- Meng, Q.; Shang, J.; Huang, R.; Zhang, Y. Determination of soluble solids content and firmness in plum using hyperspectral imaging and chemometric algorithms. J. Food Process. Eng. 2021, 44. [Google Scholar] [CrossRef]
- Badaró, A.T.; Amigo, J.M.; Blasco, J.; Aleixos, N.; Ferreira, A.R.; Clerici, M.T.P.S.; Barbin, D.F. Near-infrared hyperspectral imaging and spectral unmixing methods for evaluation of fibre distribution in enriched pasta. Food Chem. 2021, 343, 128517. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Aheto, J.H.; Dai, C.; Ren, Y.; Bai, J. Monitoring microstructural changes and moisture distribution of dry-cured pork: A combined confocal laser scanning microscopy and hyperspectral imaging study. J. Sci. Food Agric. 2021, 101, 2727–2735. [Google Scholar] [CrossRef] [PubMed]
- Rady, A.M.; Guyer, D.; Watson, N.J. Near-infrared Spectroscopy and Hyperspectral Imaging for Sugar Content Evaluation in Potatoes over Multiple Growing Seasons. Food Anal. Methods 2021, 14, 581–595. [Google Scholar] [CrossRef]
- Hu, N.; Li, W.; Du, C.; Zhang, Z.; Gao, Y.; Sun, Z.; Yang, L.; Yu, K.; Zhang, Y.; Wang, Z. Predicting micronutrients of wheat using hyperspectral imaging. Food Chem. 2021, 343, 128473. [Google Scholar] [CrossRef] [PubMed]
- Sturm, B.; Raut, S.; Kulig, B.; Münsterer, J.; Kammhuber, K.; Hensel, O.; Crichton, S.O. In-process investigation of the dynamics in drying behaviour and quality development of hops using visual and environmental sensors combined with chemometrics. Comput. Electron. Agric. 2020, 175, 105547. [Google Scholar] [CrossRef]
- Arefi, A.; Sturm, B.; von Gersdorff, G.; Nasirahmadi, A.; Hensel, O. Vis-NIR hyperspectral imaging along with Gaussian process regression to monitor quality attributes of apple slices during drying. LWT 2021, 152, 112297. [Google Scholar] [CrossRef]
- Zude, M.; Herold, B.; Roger, J.-M.; Bellon-Maurel, V.; Landahl, S. Non-destructive tests on the prediction of apple fruit flesh firmness and soluble solids content on tree and in shelf life. J. Food Eng. 2006, 77, 254–260. [Google Scholar] [CrossRef]
- Lebot, V.; Malapa, R.; Bourrieau, M. Rapid Estimation of Taro (Colocasia esculenta) Quality by Near-Infrared Reflectance Spectroscopy. J. Agric. Food Chem. 2011, 59, 9327–9334. [Google Scholar] [CrossRef] [PubMed]
- Areekij, S.; Ritthiruangdej, P.; Kasemsumran, S.; Therdthai, N.; Haruthaithanasan, V.; Ozaki, Y. Rapid and nondestructive analysis of deep-fried taro chip qualities using near infrared spectroscopy. J. Near Infrared Spectrosc. 2017, 25, 127–137. [Google Scholar] [CrossRef]
- Huber, P. Robust Statistics; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, H.X.; Lokhnygina, Y.; Kosinski, A.S.; Haber, M. Comparison of Concordance Correlation Coefficient and Coefficient of Individual Agreement in Assessing Agreement. J. Biopharm. Stat. 2007, 17, 721–738. [Google Scholar] [CrossRef] [PubMed]
- Hagenimana, V. Solar Drying of Sweet potato Storage Roots. Department for International Development, UK, 2001. Available online: https://assets.publishing.service.gov.uk/media/57a08d5ae5274a27b20017cd/R7036_File21d_Drying_Roots.pdf (accessed on 22 August 2019).
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gathersberg, MD, USA, 2000. [Google Scholar]
- Diamante, L.; Munro, P. Mathematical modelling of the thin layer solar drying of sweet potato slices. Sol. Energy 1993, 51, 271–276. [Google Scholar] [CrossRef]
- Luo, M.R. CIELAB. In Encyclopedia of Color Science and Technology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–7. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sturm, B.; Hofacker, W.C.; Hensel, O. Optimizing the Drying Parameters for Hot-Air–Dried Apples. Dry. Technol. 2012, 30, 1570–1582. [Google Scholar] [CrossRef]
- Ogolla, J.A.; Kulig, B.; Badulescu, L.A.; Okoth, M.W.; Esper, G.; Breitenbach, J.; Hensel, O.; Sturm, B. Influence of Inlet Drying Air Temperature and Milk Flow Rate on the Physical, Optical and Thermal Properties of Spray-Dried Camel Milk Powders. Food Bioprocess Technol. 2019, 12, 751–768. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Cardona, A. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Lee, C. Minimum cross entropy thresholding. Pattern Recognit. 1993, 26, 617–625. [Google Scholar] [CrossRef]
- Rasband, W. Circularity. ImajeJ. 2000. Available online: https://imagej.nih.gov/ij/plugins/circularity.html (accessed on 29 December 2020).
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Duchesnay, E. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Mariani, N.C.T.; Da Costa, R.C.; De Lima, K.M.G.; Nardini, V.; Junior, L.C.C.; Teixeira, G. Predicting soluble solid content in intact jaboticaba [Myrciaria jaboticaba (Vell.) O. Berg] fruit using near-infrared spectroscopy and chemometrics. Food Chem. 2014, 159, 458–462. [Google Scholar] [CrossRef]
- Williams, P.C.; Sobering, D. Comparison of Commercial near Infrared Transmittance and Reflectance Instruments for Analysis of Whole Grains and Seeds. J. Near Infrared Spectrosc. 1993, 1, 25–32. [Google Scholar] [CrossRef]
- Chandrashekar, G.; Sahin, F. A survey on feature selection methods. Comput. Electr. Eng. 2014, 40, 16–28. [Google Scholar] [CrossRef]
- Alma, O.G.; Bulut, E. Genetic Algorithm Based Variable Selection for Partial Least Squares Regression Using ICOMP Criterion. Asian J. Math. Stat. 2012, 5, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Rong, M.; Gong, D.; Gao, X. Feature Selection and Its Use in Big Data: Challenges, Methods, and Trends. IEEE Access 2019, 7, 19709–19725. [Google Scholar] [CrossRef]
- Wang, Z.X.; He, Q.P.; Wang, J. Comparison of variable selection methods for PLS-based soft sensor modeling. J. Process Control 2015, 26, 56–72. [Google Scholar] [CrossRef]
- Qin, J.; Chao, K.; Kim, M.S.; Lu, R.; Burks, T.F. Hyperspectral and multispectral imaging for evaluating food safety and quality. J. Food Eng. 2013, 118, 157–171. [Google Scholar] [CrossRef]
- Pirouz, D.M. An Overview of Partial Least Squares. SSRN Electron. J. 2006, 2006, 1631359. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, M.E.; Honarvar, M.; Ansari, K.; Jamshidi, B. Measurement of quality parameters of sugar beet juices using near-infrared spectroscopy and chemometrics. J. Food Eng. 2020, 271, 109775. [Google Scholar] [CrossRef]
- Afanador, N.; Tran, T.; Buydens, L. An assessment of the jackknife and bootstrap procedures on uncertainty estimation in the variable importance in the projection metric. Chemom. Intell. Lab. Syst. 2014, 137, 162–172. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Van Mulbregt, P. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, L.; Kulig, B.; Moscetti, R.; Massantini, R.; Pawelzik, E.; Hensel, O.; Sturm, B. Comparison between Hyperspectral Imaging and Chemical Analysis of Polyphenol Oxidase Activity on Fresh-Cut Apple Slices. J. Spectrosc. 2020, 2020, 7012525. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Measurement in Medicine: The Analysis of Method Comparison Studies. J. R. Stat. Soc. Ser. D (Stat.) 1983, 32, 307. [Google Scholar] [CrossRef]
- Ungerer, J.P.J.; Pretorius, C. Method comparison—A practical approach based on error identification. Clin. Chem. Lab. Med. 2017, 56, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Medica 2015, 25, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.I.-K. A Concordance Correlation Coefficient to Evaluate Reproducibility. Biometrics 1989, 45, 255. [Google Scholar] [CrossRef]
- Lin, L.; Hedayat, A.S.; Sinha, B.; Yang, M. Statistical Methods in Assessing Agreement. J. Am. Stat. Assoc. 2002, 97, 257–270. [Google Scholar] [CrossRef]
- Morley, S.K.; Brito, T.V.; Welling, D.T. Measures of Model Performance Based on the Log Accuracy Ratio. Space Weather 2018, 16, 69–88. [Google Scholar] [CrossRef]
- Golbraikh, A.; Tropsha, A. Beware of q2! J. Mol. Graph. Model. 2002, 20, 269–276. [Google Scholar] [CrossRef]
- Fox, J. Robust Regression: Appendix to An R and S-PLUS Companion to Applied Regression. 2002. Available online: https://www.saedsayad.com/docs/RobustRegression.pdf (accessed on 6 October 2021).
- Jake, T.M.; Tirrell, L. pyCompare v1.5.1. Zenodo, 26 August 2020. [Google Scholar]
- Davidson-Pilon, C.; Kalderstam, J.; Jacobson, N.; Reed, S.; Kuhn, B.; Zivich, P.; Williamson, M.; Abdeali, J.K.; Datta, D.; Fiore-Gartland, A.; et al. Lifelines: v0.25.4. Zenodo, August 2020. [Google Scholar]
- Quinn, C.; Haber, M.J.; Pan, Y. Use of the Concordance Correlation Coefficient When Examining Agreement in Dyadic Research. Nurs. Res. 2009, 58, 368–373. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.; Pearson, E.S. Tests for Departure from Normality. Empirical Results for the Distributions of b2 and √b1. Biometrika 1973, 60, 613. [Google Scholar] [CrossRef]
- Levene, H. Robust tests for equality of variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Olkin, I., Hotelling, H., Eds.; Stanford University Press: Palo Alto, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Rongtong, B.; Suwonsichon, T.; Ritthiruangdej, P.; Kasemsumran, S. Determination of water activity, total soluble solids and moisture, sucrose, glucose and fructose contents in osmotically dehydrated papaya using near-infrared spectroscopy. Agric. Nat. Resour. 2018, 52, 557–564. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L.; Nieuwoudt, H.; Cronje, P.J.R.; Saeys, W.; Nicolaï, B. NIR Spectroscopy Applications for Internal and External Quality Analysis of Citrus Fruit—A Review. Food Bioprocess Technol. 2012, 5, 425–444. [Google Scholar] [CrossRef]
- Zude-Sasse, M.; Pflanz, M.; Kaprielian, C.; Aivazian, B.L. NIRS as a tool for precision horticulture in the citrus industry. Biosyst. Eng. 2008, 99, 455–459. [Google Scholar] [CrossRef]
- Clément, A.; Dorais, M.; Vernon, M. Nondestructive Measurement of Fresh Tomato Lycopene Content and Other Physicochemical Characteristics Using Visible−NIR Spectroscopy. J. Agric. Food Chem. 2008, 56, 9813–9818. [Google Scholar] [CrossRef]
- Jun, Q.; Ning, W.; Ngadi, M.; Singh, B. Water Content and Weight Estimation for Potatoes Using Hyperspectral Imaging. In Proceedings of the in 2005 ASAE Annual Meeting, Tampa, FL, USA, 17–20 July 2005. [Google Scholar]
- Amjad, W.; Crichton, S.O.; Munir, A.; Hensel, O.; Sturm, B. Hyperspectral imaging for the determination of potato slice moisture content and chromaticity during the convective hot air drying process. Biosyst. Eng. 2018, 166, 170–183. [Google Scholar] [CrossRef]
- Pu, R.; Ge, S.; Kelly, N.M.; Gong, P. Spectral absorption features as indicators of water status in coast live oak (Quercus agrifolia) leaves. Int. J. Remote Sens. 2003, 24, 1799–1810. [Google Scholar] [CrossRef]
- Workman, J.; Weyer, L. Practical Guide and Spectral Atlas for Interpretive Near-Infrared Spectroscopy, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Velazquez, G.; Herrera-Gómez, A.; Martίn-Polo, M. Identification of bound water through infrared spectroscopy in methylcellulose. J. Food Eng. 2003, 59, 79–84. [Google Scholar] [CrossRef]
- Gowen, A.A. Water and Food Quality. Contemp. Mater. 2012, 1, 31–37. [Google Scholar] [CrossRef]
- Caurie, M. Bound water: Its definition, estimation and characteristics. Int. J. Food Sci. Technol. 2011, 46, 930–934. [Google Scholar] [CrossRef]
- Gowen, A.A.; Tsenkova, R.; Esquerre, C.; Downey, G.; O’Donnell, C.P. Use of near Infrared Hyperspectral Imaging to Identify Water Matrix Co-Ordinates in Mushrooms (Agaricus Bisporus) Subjected to Mechanical Vibration. J. Near Infrared Spectrosc. 2009, 17, 363–371. [Google Scholar] [CrossRef]
- Khan, I.H.; Wellard, R.M.; Nagy, S.A.; Joardder, M.U.H.; Karim, A. Investigation of bound and free water in plant-based food material using NMR T 2 relaxometry. Innov. Food Sci. Emerg. Technol. 2016, 38, 252–261. [Google Scholar] [CrossRef]
- Prothon, F.; Ahrné, L.; Sjöholm, I. Mechanisms and Prevention of Plant Tissue Collapse during Dehydration: A Critical Review. Crit. Rev. Food Sci. Nutr. 2003, 43, 447–479. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Lewicki, P.P. Some remarks on rehydration of dried foods. J. Food Eng. 1998, 36, 81–87. [Google Scholar] [CrossRef]
- Ashtiani, S.-H.M.; Sturm, B.; Nasirahmadi, A. Effects of hot-air and hybrid hot air-microwave drying on drying kinetics and textural quality of nectarine slices. Heat Mass Transf. 2018, 54, 915–927. [Google Scholar] [CrossRef]
- Tsenkova, R.N.; Iordanova, I.K.; Toyoda, K.; Brown, D.R. Prion protein fate governed by metal binding. Biochem. Biophys. Res. Commun. 2004, 325, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Segtnan, V.H.; Šašić, Š.; Isaksson, T.; Ozaki, Y. Studies on the Structure of Water Using Two-Dimensional Near-Infrared Correlation Spectroscopy and Principal Component Analysis. Anal. Chem. 2001, 73, 3153–3161. [Google Scholar] [CrossRef]
- Ndabikunze, B.K.; Talwana, H.A.; Issa-Zacharia, A.; Palapala, V. Proximate and mineral composition of cocoyam (Colocasia esculenta L. and Xanthosoma sagittifolium L.) grown along the Lake Victoria basin in Tanzania and Uganda. Afr. J. Food Sci. 2011, 5, 248–254. [Google Scholar]
- Temesgen, M.; Retta, N. Nutritional Potential, Health and Food Security Benefits of Taro Colocasia esculenta (L.): A Review. Food Sci. Qual. Manag. 2015, 36, 23–30. [Google Scholar]
- Wang, W.; Heitschmidt, G.W.; Windham, W.R.; Feldner, P.; Ni, X.; Chu, X. Feasibility of Detecting Aflatoxin B 1 on Inoculated Maize Kernels Surface using Vis/NIR Hyperspectral Imaging. J. Food Sci. 2014, 80, 116. [Google Scholar] [CrossRef]
- Malegori, C.; Marques, E.J.N.; de Freitas, S.T.; Pimentel, M.F.; Pasquini, C.; Casiraghi, E. Comparing the analytical performances of Micro-NIR and FT-NIR spectrometers in the evaluation of acerola fruit quality, using PLS and SVM regression algorithms. Talanta 2017, 165, 112–116. [Google Scholar] [CrossRef]
- Miyamoto, K.; Kitano, Y. Non-Destructive Determination of Sugar Content in Satsuma Mandarin Fruit by near Infrared Transmittance Spectroscopy. J. Near Infrared Spectrosc. 1995, 3, 227–237. [Google Scholar] [CrossRef]
- Roggo, Y.; Duponchel, L.; Huvenne, J.-P. Quality Evaluation of Sugar Beet (Beta vulgaris) by Near-Infrared Spectroscopy. J. Agric. Food Chem. 2004, 52, 1055–1061. [Google Scholar] [CrossRef]
- Delwiche, S.R.; Mekwatanakarn, W.; Wang, C.Y. Soluble Solids and Simple Sugars Measurement in Intact Mango Using Near Infrared Spectroscopy. HortTechnology 2008, 18, 410–416. [Google Scholar] [CrossRef]
- Eleazu, C.O. Characterization of the natural products in cocoyam (Colocasia esculenta) using GC–MS. Pharm. Biol. 2016, 54, 2880–2885. [Google Scholar] [CrossRef] [Green Version]
- Ferreres, F.; Gonçalves, R.F.; Gil-Izquierdo, A.; Valentão, P.; Silva, A.M.S.; Silva, J.B.; Santos, D.; Andrade, P.B. Further Knowledge on the Phenolic Profile of Colocasia esculenta (L.) Shott. J. Agric. Food Chem. 2012, 60, 7005–7015. [Google Scholar] [CrossRef]
- Gonçalves, R.; Silva, A.; Silva, A.M.; Valentão, P.; Ferreres, F.; Gil-Izquierdo, A.; Silva, J.; Santos, D.; Andrade, P.B. Influence of taro (Colocasia esculenta L. Shott) growth conditions on the phenolic composition and biological properties. Food Chem. 2013, 141, 3480–3485. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Coloured pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Champagne, A.; Hilbert, G.; Legendre, L.; Lebot, V. Diversity of anthocyanins and other phenolic compounds among tropical root crops from Vanuatu, South Pacific. J. Food Compos. Anal. 2011, 24, 315–325. [Google Scholar] [CrossRef]
- Prasad, K.; Jacob, S.; Siddiqui, M.W. Fruit Maturity, Harvesting, and Quality Standards. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 41–69. [Google Scholar]
- Kumar, V.; Sharma, H.K. Process optimization for extraction of bioactive compounds from taro (Colocasia esculenta), using RSM and ANFIS modeling. J. Food Meas. Charact. 2017, 11, 704–718. [Google Scholar] [CrossRef]
- Cozzolino, D.; Cynkar, W.U.; Dambergs, R.G.; Mercurio, M.D.; Smith, P.A. Measurement of Condensed Tannins and Dry Matter in Red Grape Homogenates Using Near Infrared Spectroscopy and Partial Least Squares. J. Agric. Food Chem. 2008, 56, 7631–7636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shen, Y.; Chen, J.; Xiao, P.; Bao, J. Nondestructive Prediction of Total Phenolics, Flavonoid Contents, and Antioxidant Capacity of Rice Grain Using Near-Infrared Spectroscopy. J. Agric. Food Chem. 2008, 56, 8268–8272. [Google Scholar] [CrossRef] [PubMed]
- Dykes, L.; Hoffmann, L.; Rodríguez, O.P.; Rooney, W.L.; Rooney, L.W. Prediction of total phenols, condensed tannins, and 3-deoxyanthocyanidins in sorghum grain using near-infrared (NIR) spectroscopy. J. Cereal Sci. 2014, 60, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Albanell, E.; Martínez, M.; de Marchi, M.; Manuelian, C.L. Prediction of bioactive compounds in barley by near-infrared reflectance spectroscopy (NIRS). J. Food Compos. Anal. 2021, 97, 103763. [Google Scholar] [CrossRef]
- Kusumiyati; Sutari, W.; Farida; Mubarok, S.; Hamdani, J.S. Prediction of surface colour of ‘crystal’ guava using UV-Vis-NIR spectroscopy and multivariate analysis. IOP Conf. Ser. Earth Environ. Sci. 2019, 365, 012026. [Google Scholar] [CrossRef]
- Xie, C.; Chu, B.; He, Y. Prediction of banana colour and firmness using a novel wavelengths selection method of hyperspectral imaging. Food Chem. 2018, 245, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Munera, S.; Besada, C.; Aleixos, N.; Talens, P.; Salvador, A.; Sun, D.-W.; Cubero, S.; Blasco, J. Non-destructive assessment of the internal quality of intact persimmon using colour and VIS/NIR hyperspectral imaging. LWT 2017, 77, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Kohl, S.K.; Landmark, J.D.; Stickle, D.F. Demonstration of Absorbance Using Digital Color Image Analysis and Colored Solutions. J. Chem. Educ. 2006, 83, 644. [Google Scholar] [CrossRef]
- Macdougall, D. Colour measurement of food: Principles and practice. In Colour Measurement; Woodhead Publishing: Sawston, UK, 2010; pp. 312–342. [Google Scholar]
- Rustioni, L.; di Meo, F.; Guillaume, M.; Failla, O.; Trouillas, P. Tuning colour variation in grape anthocyanins at the molecular scale. Food Chem. 2013, 141, 4349–4357. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, D.; Wu, Y.; Wang, D.; Wei, Y.; Wu, J.; Ji, B. Stability and absorption of anthocyanins from blueberries subjected to a simulated digestion process. Int. J. Food Sci. Nutr. 2013, 65, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Shao, Y.; Xuan, G.; Wang, Y.; Liu, Y.; Han, X. Real-time hyperspectral imaging for the in-field estimation of strawberry ripeness with deep learning. Artif. Intell. Agric. 2020, 4, 31–38. [Google Scholar] [CrossRef]
- Dai, Q.; Cheng, J.-H.; Sun, D.-W.; Zeng, X.-A. Advances in Feature Selection Methods for Hyperspectral Image Processing in Food Industry Applications: A Review. Crit. Rev. Food Sci. Nutr. 2013, 55, 1368–1382. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Prieto, B.; Eriksson, L.; Trygg, J. Variable influence on projection (VIP) for OPLS models and its applicability in multivariate time series analysis. Chemom. Intell. Lab. Syst. 2015, 146, 297–304. [Google Scholar] [CrossRef]
- Chong, I.-G.; Jun, C.-H. Performance of some variable selection methods when multicollinearity is present. Chemom. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Jun, C.-H.; Lee, S.-H.; Park, H.-S.; Lee, J.-H. Use of partial least squares regression for variable selection and quality prediction. In Proceedings of the 2009 International Conference on Computers & Industrial Engineering, Troyes, France, 6–9 July 2009; pp. 1302–1307. [Google Scholar]
- Joardder, M.U.H.; Mourshed, M.; Masud, M.H. Characteristics of Bound Water. In State of Bound Water: Measurement and Significance in Food Processing; Springer: Cham, Switzerland; Manhattan, NY, USA, 2019; pp. 29–45. [Google Scholar]
- Büning-Pfaue, H. Analysis of water in food by near infrared spectroscopy. Food Chem. 2003, 82, 107–115. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, L.; Cheng, F. Classification of Frozen Corn Seeds Using Hyperspectral VIS/NIR Reflectance Imaging. Molecules 2019, 24, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berezhnoy, I.; Postma, E.; Herik, J.V.D. Computer analysis of Van Gogh’s complementary colours. Pattern Recognit. Lett. 2007, 28, 703–709. [Google Scholar] [CrossRef]
- Deylami, M.Z.; Rahman, R.A.; Tan, C.P.; Bakar, J.; Olusegun, L. Effect of blanching on enzyme activity, color changes, anthocyanin stability and extractability of mangosteen pericarp: A kinetic study. J. Food Eng. 2016, 178, 12–19. [Google Scholar] [CrossRef]
- Francis, F.J.; Markakis, P.C. Food colourants: Anthocyanins. Crit. Rev. Food Sci. Nutr. 1989, 28, 273–314. [Google Scholar] [CrossRef]
- Von Gersdorff, G.J.E.; Kulig, B.; Hensel, O.; Sturm, B. Method comparison between real-time spectral and laboratory-based measurements of moisture content and CIELAB colour pattern during dehydration of beef slices. J. Food Eng. 2021, 294, 110419. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Piñeiro, G.; Perelman, S.; Guerschman, J.P.; Paruelo, J.M. How to evaluate models: Observed vs. predicted or predicted vs. observed? Ecol. Model. 2008, 216, 316–322. [Google Scholar] [CrossRef]

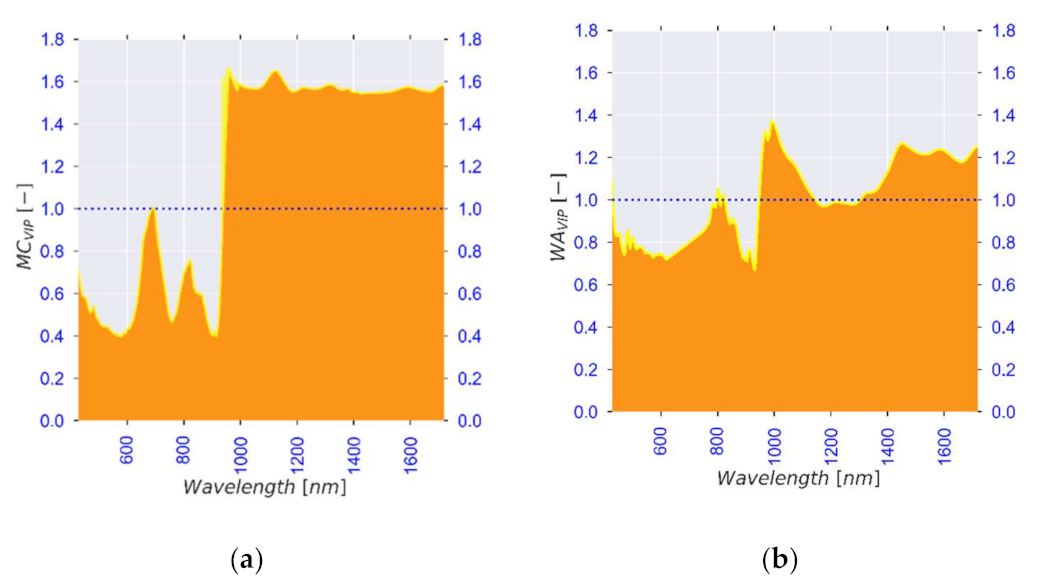
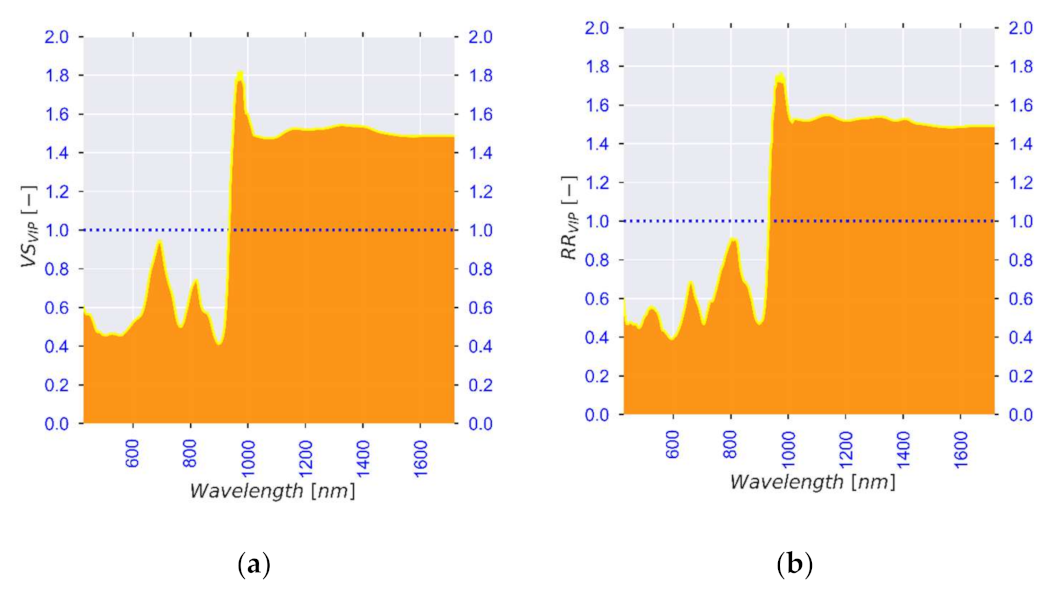
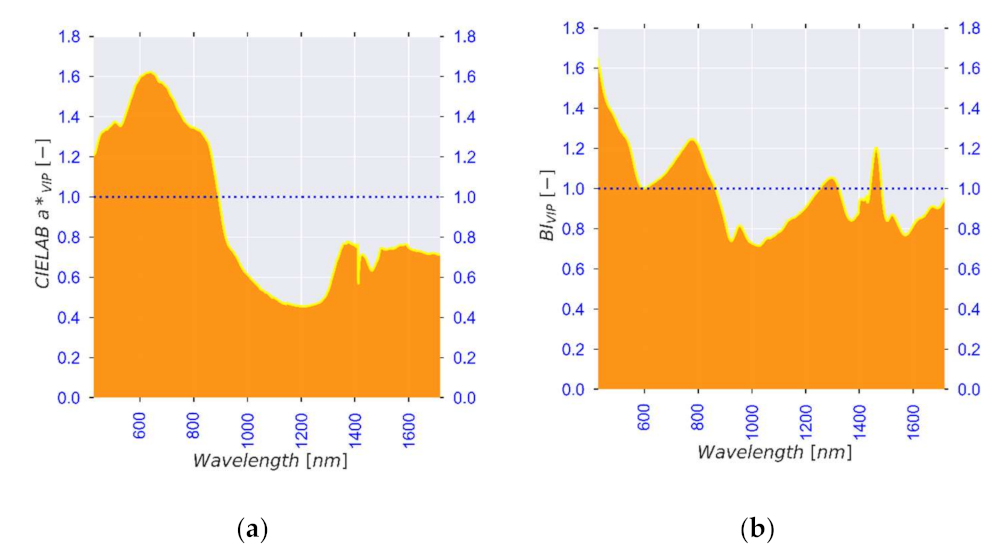



| Standard No. | 1 | 2 | 3 | 4 | 5 | 6 |
| Concentration (µg/mL) | 5.97 | 11.94 | 17.91 | 23.88 | 29.85 | 35.82 |
| Log (1/R) at 735.8 nm | 0.286 | 0.445 | 0.646 | 0.924 | 1.316 | 1.695 |
| Standard No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Concentration (mg/L) | 0.0 | 5.0 | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 |
| Log (1/R) at 425.0 nm | 0.071 | 0.180 | 0.281 | 0.502 | 0.707 | 0.921 | 1.138 | 1.328 |
| Response | Pre-Processing | No. of LVs | Calibration [n = 362] | Prediction [n = 241] | ||||
|---|---|---|---|---|---|---|---|---|
| RMSEC | r2C | RPDC | RMSEP | r2P | RPDP | |||
| Moisture Attributes | ||||||||
| MC | MSC + d2(R) | 13 | 1.698 | 0.99 | 13.1 | 1.978 | 0.99 | 11.2 |
| MR | MSC + d2(R) | 10 | 0.026 | 0.99 | 12.6 | 0.030 | 0.99 | 10.8 |
| aw | MSC+R | 11 | 0.059 | 0.93 | 3.8 | 0.065 | 0.92 | 3.5 |
| Colour Attributes | ||||||||
| CIELAB L* | MSC + d(R) | 15 | 0.821 | 0.64 | 1.8 | 0.907 | 0.53 | 1.6 |
| CIELAB a* | MSC + d2(R) | 6 | 0.303 | 0.75 | 2.1 | 0.460 | 0.50 | 1.4 |
| CIELAB b* | MSC + d(R) | 13 | 0.335 | 0.79 | 2.3 | 0.343 | 0.78 | 2.3 |
| BI | log (1/R) | 14 | 0.489 | 0.81 | 2.5 | 0.491 | 0.78 | 2.4 |
| WI | MSC + d.(R) | 15 | 0.109 | 0.76 | 2.1 | 0.134 | 0.65 | 1.7 |
| Chroma | log (1/R) | 11 | 0.313 | 0.80 | 2.4 | 0.343 | 0.76 | 2.2 |
| Hue angle | MSC+d.(R) | 7 | 0.043 | 0.75 | 2.2 | 0.049 | 0.72 | 1.9 |
| Chemical Attributes | ||||||||
| TAA | MSC + d2(R) | 13 | 7.100 | 0.70 | 2.0 | 7.600 | 0.69 | 1.9 |
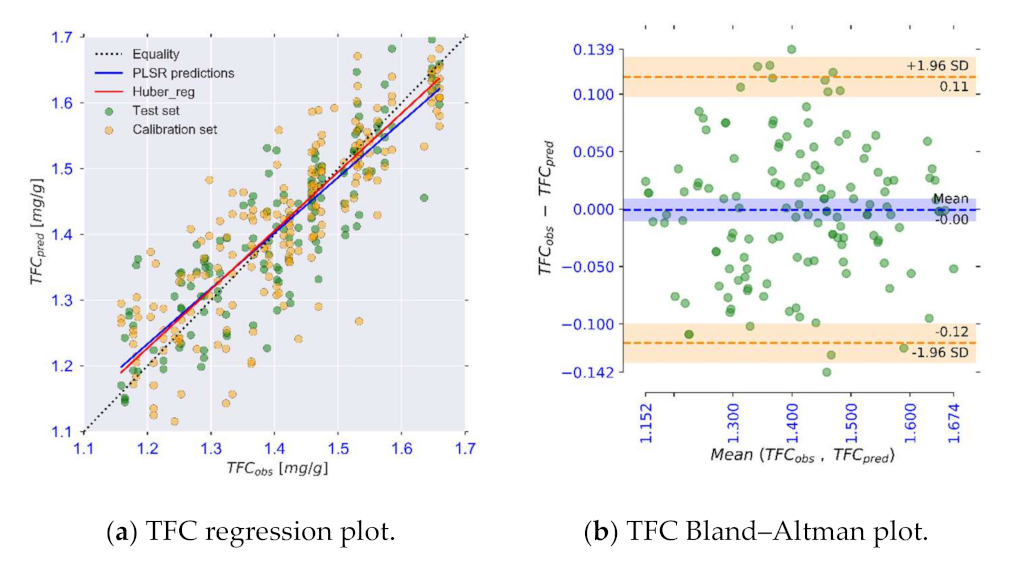
| TFC | MSC + d2(R) | 12 | 0.063 | 0.77 | 2.2 | 0.063 | 0.76 | 2.2 |
| TPC | MSC + d2(R) | 9 | 0.239 | 0.46 | 1.5 | 0.280 | 0.45 | 1.3 |
| Structural Attributes | ||||||||
| Vs | MSC + d2(R) | 9 | 0.039 | 0.97 | 5.5 | 0.042 | 0.96 | 5.2 |
| RR | MSC + d(R) | 15 | 0.017 | 0.99 | 8.4 | 0.021 | 0.98 | 7.0 |
| PPA | MSC + d2(R) | 9 | 1.774 | 0.82 | 2.4 | 2.347 | 0.64 | 1.8 |
| PC | MSC + d2(R) | 5 | 0.020 | 0.85 | 2.7 | 0.022 | 0.84 | 2.4 |
| Response | Huber Regression | CCC | Bland–Altman | |||||
|---|---|---|---|---|---|---|---|---|
| β1 | β1—95% C.I [LCL, UCL] | β0 | Β0—95% C.I [LCL, UCL] | Mean Diff. | LOAu | LOAl | ||
| MC [% w.b] | 0.99 | [0.98, 1.01] b | 0.12 | [−0.56, 0.80] b | 0.96 | −0.02 | 4.31 | −4.37 |
| aw [-] | 0.87 | [0.82, 0.92] a | 0.13 | [0.08, 0.17] a | 0.73 | 0.00 | 0.15 | −0.15 |
| MR [-] | 0.99 | [0.97, 1.01] b | 0.01 | [−0.01, 0.02] b | 0.96 | 0.00 | 0.08 | −0.08 |
| Response | Huber Regression | CCC | Bland–Altman | |||||
|---|---|---|---|---|---|---|---|---|
| β1 | β1—95% C.I [LCL, UCL] | β0 | Β0—95% C.I [LCL, UCL] | Mean Diff. | LOAu | LOAl | ||
| BI [-] | 0.99 | [0.89, 1.08] b | 0.14 | [−0.90, 1.18] b | 0.86 | 0.00 | 0.96 | −0.96 |
| WI [-] | 0.85 | [0.64, 1.05] b | 14.41 | [−5.06, 33.87] b | 0.83 | 0.01 | 0.23 | −0.22 |
| CIELAB L* [-] | 0.84 | [0.73, 0.96] a | 13.33 | [3.73, 22.93] a | 0.80 | 0.01 | 1.84 | −1.82 |
| CIELAB a* [-] | 0.84 | [0.73, 0.95] a | 0.34 | [0.06, 0.63] a | 0.81 | −0.01 | 0.75 | −0.78 |
| CIELAB b* [-] | 0.92 | [0.81, 1.04] b | 0.56 | [−0.26, 1.38] b | 0.84 | −0.01 | 0.70 | −0.71 |
| Chroma [-] | 0.95 | [0.84, 1.05] b | 0.40 | [−0.41, 1.22] b | 0.84 | 0.00 | 0.67 | −0.67 |
| Hue angle [°] | 0.93 | [0.74, 1.12] b | 0.09 | [−0.14, 0.33] b | 0.85 | 0.00 | 0.09 | −0.09 |
| Response | Huber Regression | CCC | Bland–Altman | |||||
|---|---|---|---|---|---|---|---|---|
| β1 | β1—95% C.I [LCL, UCL] | β0 | Β0—95% C.I [LCL, UCL] | Mean Diff. | LOAu | LOAl | ||
| TAA [% RSA] | 0.92 | [0.83, 1.01] b | 5.19 | [−0.04, 10.42] b | 0.84 | 0.17 | 13.97 | −13.62 |
| TFC [mg/g] | 0.95 | [0.88, 1.01] b | 0.08 | [−0.02, 0.17] b | 0.85 | 0.00 | 0.11 | −0.12 |
| TPC [µg/g] | 0.77 | [0.60, 0.95] a | 0.81 | [0.17, 1.45] a | 0.77 | −0.01 | 0.50 | −0.52 |
| Response | Huber Regression | CCC | Bland–Altman | |||||
|---|---|---|---|---|---|---|---|---|
| β1 | β1—95% C.I [LCL, UCL] | β0 | Β0—95% C.I [LCL, UCL] | Mean diff. | LOAu | LOAl | ||
| Vs [-] | 0.98 | [0.93, 1.04] b | 0.01 | [−0.02, 0.05] b | 0.92 | 0.00 | 0.08 | −0.09 |
| RR [-] | 0.98 | [0.93, 1.03] b | 0.02 | [−0.04, 0.09] b | 0.80 | 0.00 | 0.05 | −0.05 |
| PPA [%] | 0.85 | [0.67, 1.03] b | 2.31 | [−0.79, 5.42] b | 0.84 | −0.17 | 4.82 | −5.15 |
| PC [-] | 0.86 | [0.69, 1.02] b | 0.09 | [−0.01, 0.18] b | 0.87 | 0.00 | 0.05 | −0.05 |
| Observed λ [nm] | Literature λ [nm] | Association | Reference |
|---|---|---|---|
| 972, 1400 | 950–1000, 1400 | Moisture content (Free water, water activity) | [84,85,86] |
| 1200, 1455, 1520 | 1174, 1454, 1496 | Moisture content (Bound water, structure) | [91,97,98] |
| 740, 760, 830, 900, 1590 | 720–920, 1593 | Carbohydrates (Starch and sugars) | [102,103,104,136] |
| 700, 740, 760, 830, 945, 1455, 1700 | 700–1000, 1415–1512, 1650–1750 | Bioactive compounds | [33,113,114,115] |
| 545, 559 | 516–560 | Colour (browning and anthocyanins) | [122,123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndisya, J.; Gitau, A.; Mbuge, D.; Arefi, A.; Bădulescu, L.; Pawelzik, E.; Hensel, O.; Sturm, B. Vis-NIR Hyperspectral Imaging for Online Quality Evaluation during Food Processing: A Case Study of Hot Air Drying of Purple-Speckled Cocoyam (Colocasia esculenta (L.) Schott). Processes 2021, 9, 1804. https://doi.org/10.3390/pr9101804
Ndisya J, Gitau A, Mbuge D, Arefi A, Bădulescu L, Pawelzik E, Hensel O, Sturm B. Vis-NIR Hyperspectral Imaging for Online Quality Evaluation during Food Processing: A Case Study of Hot Air Drying of Purple-Speckled Cocoyam (Colocasia esculenta (L.) Schott). Processes. 2021; 9(10):1804. https://doi.org/10.3390/pr9101804
Chicago/Turabian StyleNdisya, John, Ayub Gitau, Duncan Mbuge, Arman Arefi, Liliana Bădulescu, Elke Pawelzik, Oliver Hensel, and Barbara Sturm. 2021. "Vis-NIR Hyperspectral Imaging for Online Quality Evaluation during Food Processing: A Case Study of Hot Air Drying of Purple-Speckled Cocoyam (Colocasia esculenta (L.) Schott)" Processes 9, no. 10: 1804. https://doi.org/10.3390/pr9101804
APA StyleNdisya, J., Gitau, A., Mbuge, D., Arefi, A., Bădulescu, L., Pawelzik, E., Hensel, O., & Sturm, B. (2021). Vis-NIR Hyperspectral Imaging for Online Quality Evaluation during Food Processing: A Case Study of Hot Air Drying of Purple-Speckled Cocoyam (Colocasia esculenta (L.) Schott). Processes, 9(10), 1804. https://doi.org/10.3390/pr9101804







