Computational Study of the Adsorption of Phosphates as Wastewater Pollutant Molecules on Faujasites
Abstract
:1. Introduction
2. Materials and Methods
Adsorption Energy
3. Results and Discussion
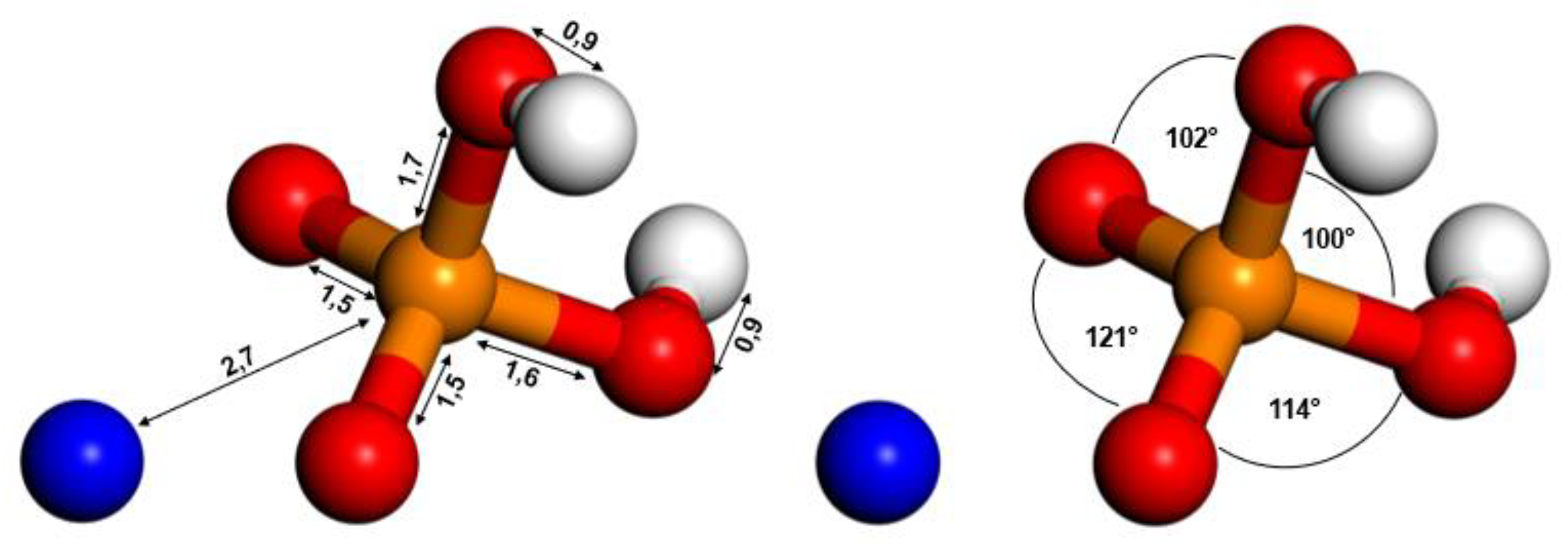
3.1. Molecular Optimization
3.2. Optimized X- and Y-Type Faujasite Zeolites
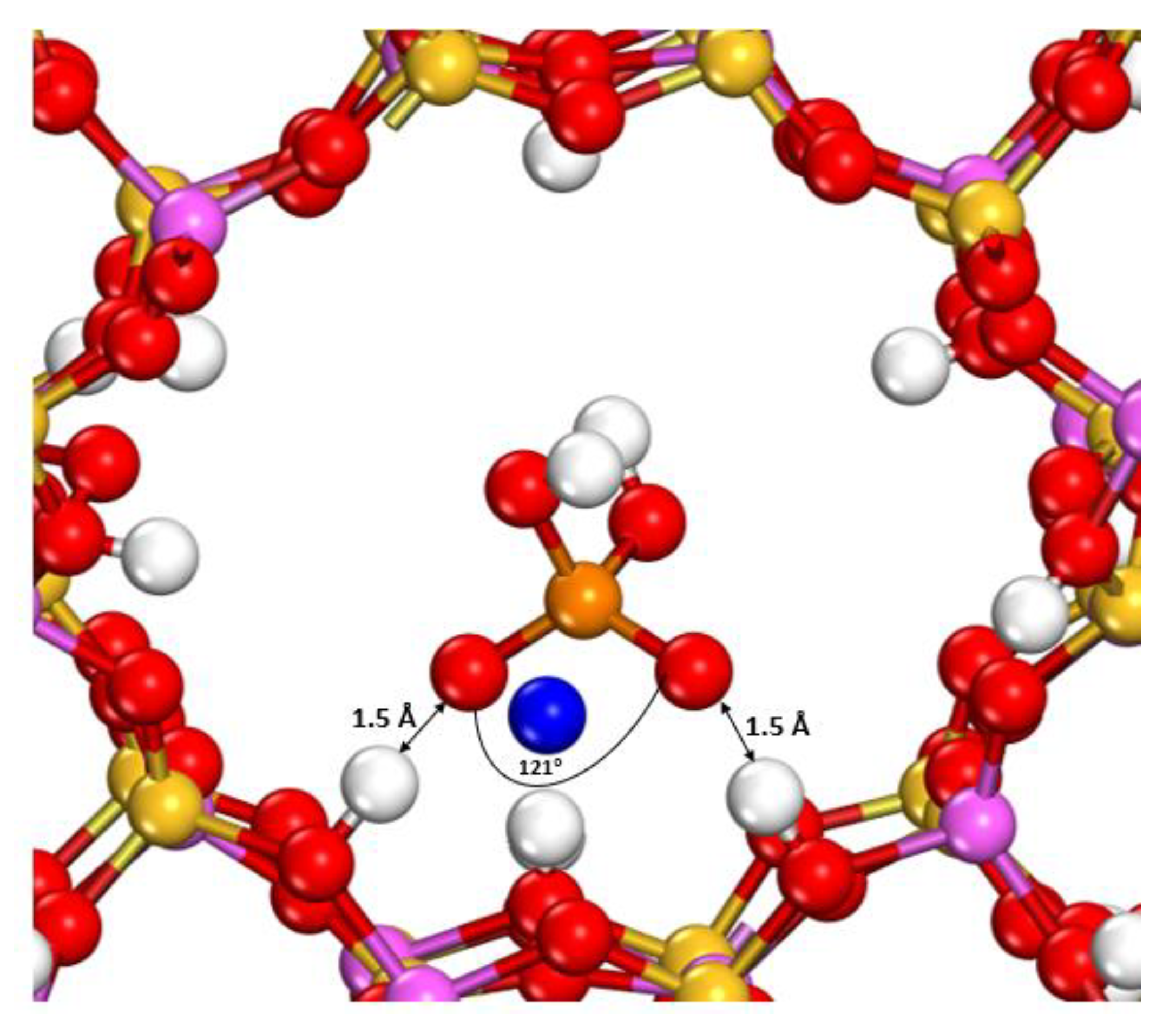
3.3. Adsorption of Phosphate on X- and Y-Type Faujasites
3.3.1. Adsorption on the FAU-X Model
3.3.2. Adsorption on FAU-Y Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Song, L.; Yang, F.; He, J. Investigation of phosphate adsorption by a polyethersulfone-type affinity membrane using experimental and DFT methods. Desalin. Water Treat. 2016, 57, 25036–25056. [Google Scholar] [CrossRef]
- Bolaños-Alfaro, J.D.; Cordero-Castro, G.; Segura-Araya, G. Determinación de nitritos, nitratos, sulfatos y fosfatos en agua potable como indicadores de contaminación ocasionada por el hombre, en dos cantones de Alajuela (Costa Rica). Rev. Tecnol. Marcha 2017, 30, 15. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Zhang, Y.; Li, D. Adsorptive removal of phosphate from water using mesoporous materials: A review. J. Environ. Manag. 2017, 193, 470–482. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, M.; Li, Y.; Li, H.; Wen, Z. Computational study of phosphate adsorption on Mg/Ca modified biochar structure in aqueous solution. Chemosphere 2021, 269, 129374. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Mainali, B.; Angove, M.J. Manganese oxides and their application to metal ion and contaminant removal from wastewater. J. Water Process Eng. 2018, 26, 264–280. [Google Scholar] [CrossRef]
- Currie, S.J.; Van Zomeren, C.M.; Berkowitz, J.F. Utilizing Wetlands for Phosphorus Reduction in Great Lakes Watersheds: A Review of Available Literature Examining Soil Properties and Phosphorus Removal Efficiency. Environ. Sci. 2017. [Google Scholar]
- Naranjo, Y. Estudio para mejorar la eliminación de fósforo en humedales artificiales empleando fangos generados en la potabilización del agua, mediante procesos de adsorción. Microporous Mesoporous Mater. 2017, 239, 111–122. [Google Scholar]
- Sierra, N.; Del Peso Díaz, M.I. Estudio del Proceso de Adsorción de Fosfatos en Hidróxidos Dobles Laminares; UPM: Madrid, Spain, 2019; pp. 18–25. [Google Scholar]
- Gao, Y.; Chen, N.; Hu, W.; Feng, C.; Zhang, B.; Ning, Q.; Xu, B. Phosphate removal from aqueous solution by an effective clay composite material. J. Solut. Chem. 2013, 42, 691–704. [Google Scholar] [CrossRef]
- Bacelo, H.; Pintor, A.M.A.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 2020, 381, 122566. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Islam, A.; Asif, M.B.; Royhand, R.; Pramanik, S.K.; Shah, K.; Bhuiyan, M.; Hai, F. Emerging investigator series: Phosphorus recovery from municipal wastewater by adsorption on steelmaking slag preceding forward osmosis: An integrated process. Environ. Sci. Water Res. Technol. 2020, 6, 1559–1567. [Google Scholar] [CrossRef]
- Guaya, D.; Hermassi, M.; Valderrama, C.; Farran, A.; Cortina, J.L. Recovery of ammonium and phosphate from treated urban wastewater by using potassium clinoptilolite impregnated hydrated metal oxides as N-P-K fertilizer. J. Environ. Chem. Eng. 2016, 4, 3519–3526. [Google Scholar] [CrossRef]
- Losada, L.M.; Bonilla, C.N.; Buitrago, R.C.; Bonilla, J.H.O.; Salamanca, M. Preliminary Study of the Ability of Inorganic Ions Remotion By Synthetic Zeolite Faujasite Type. Rev. Fac. Cienc. Básicas 2016, 11, 114–123. [Google Scholar]
- Cruz, H.I.; Javier, M.S.; Luis, D.I.; Gil, M.D.J.; Pérez, A.D. Remoción De Plomo En Agua a Partir De Material Nanoestructurado, Nanotubos De Carbono Soportados En Zeolita Natural. Av. Cienc. Ing. 2017, 8, 21–27. [Google Scholar]
- Liu, C.; Li, G.; Hensen, E.J.M.; Pidko, E.A. Relationship between acidity and catalytic reactivity of faujasite zeolite: A periodic DFT study. J. Catal. 2016, 344, 570–577. [Google Scholar] [CrossRef]
- Chebbi, M.; Chibani, S.; Paul, J.F.; Cantrel, L.; Badawi, M. Evaluation of volatile iodine trapping in presence of contaminants: A periodic DFT study on cation exchanged-faujasite. Microporous Mesoporous Mater. 2017, 239, 111–122. [Google Scholar] [CrossRef]
- Serrano, D.P.; Escola, J.M.; Pizarro, P. Synthesis strategies in the search for hierarchical zeolites. Chem. Soc. Rev. 2013, 42, 4004–4035. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Kim, D.W.; Han, H.; Kim, H.; Guo, X.; Tsapatsis, M. Preparation of a graphene oxide/faujasite composite adsorbent. Microporous Mesoporous Mater. 2018, 268, 243–250. [Google Scholar] [CrossRef]
- Wongcharee, S.; Aravinthan, V.; Erdei, L. Mesoporous activated carbon-zeolite composite prepared from waste macadamia nut shell and synthetic faujasite. Chin. J. Chem. Eng. 2019, 27, 226–236. [Google Scholar] [CrossRef]
- Setthaya, N.; Chindaprasirt, P.; Yin, S.; Pimraksa, K. TiO2-zeolite photocatalysts made of metakaolin and rice husk ash for removal of methylene blue dye. Powder Technol. 2017, 313, 417–426. [Google Scholar] [CrossRef]
- Sivalingam, S.; Kella, T.; Maharana, M.; Sen, S. Efficient sono-sorptive elimination of methylene blue by fly ash-derived nano-zeolite X: Process optimization, isotherm and kinetic studies. J. Clean. Prod. 2019, 208, 1241–1254. [Google Scholar] [CrossRef]
- Frising, T.; Leflaive, P. Extraframework cation distributions in X and Y faujasite zeolites: A review. Microporous Mesoporous Mater. 2008, 114, 27–63. [Google Scholar] [CrossRef]
- Bouaziz, N.; Manaa, M.B.; Aouaini, F.; Lamine, A.B. Investigation of hydrogen adsorption on zeolites A, X and Y using statistical physics formalism. Mater. Chem. Phys. 2019, 225, 111–121. [Google Scholar] [CrossRef]
- Chiari, L.; Ohnuki, C.; Fujinami, M. A positronium-based systematic study of the physico-chemical properties of zeolite pores. Radiat. Phys. Chem. 2021, 184, 109441. [Google Scholar] [CrossRef]
- Sellaoui, L.; Hseeou, E.P.; Badawi, M.; Netto, M.S.; Dotto, G.L.; Silva, L.F.O.; Tielens, F.; Ifthikar, F.; Bonilla-Petriciolet, A.; Chen, Z. Trapping of Ag+, Cu2+, and Co2+ by faujasite zeolite Y: New interpretations of the adsorption mechanism via DFT and statistical modeling investigation. Chem. Eng. J. 2020, 127712. [Google Scholar] [CrossRef]
- Ribeiro, R.P.P.L.; Camacho, B.C.R.; Lyubchyk, A.; Esteves, I.A.A.C.; Cruz, F.J.A.L.; Mota, J.P.B. Experimental and computational study of ethane and ethylene adsorption in the MIL-53(Al) metal organic framework. Microporous Mesoporous Mater. 2016, 230, 154–165. [Google Scholar] [CrossRef]
- Piccini, G.; Alessio, M.; Sauer, J.; Zhi, Y.; Liu, Y.; Kolvenbach, R.; Jentys, A.; Lercher, J.A. Accurate adsorption thermodynamics of small alkanes in zeolites. Ab initio theory and experiment for H-chabazite. J. Phys. Chem. C 2015, 119, 6128–6137. [Google Scholar] [CrossRef]
- Sung, C.Y.; al Hashimi, S.; McCormick, A.; Cococcioni, M.; Tsapatsis, M. A DFT study on multivalent cation-exchanged y zeolites as potential selective adsorbent for H2S. Microporous Mesoporous Mater. 2013, 172, 7–12. [Google Scholar] [CrossRef]
- Göltl, F.; Grüneis, A.; Bučko, T.; Hafner, J. Van der Waals interactions between hydrocarbon molecules and zeolites: Periodic calculations at different levels of theory, from density functional theory to the random phase approximation and Moller-Plesset perturbation theory. J. Chem. Phys. 2012, 137, 114111. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B-Condens. Matter Mater. Phys. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Hu, K.; Wu, X.; Hinokuma, S.; Ohto, T.; Wakisaka, M.; Fujita, J.-C.; Ito, Y. Boosting electrochemical water splitting: Via ternary NiMoCo hybrid nanowire arrays. J. Mater. Chem. A 2019, 7, 2156–2164. [Google Scholar] [CrossRef]
- RFletcher, E.; Ling, S.; Slater, B. Violations of Löwenstein’s rule in zeolites. Chem. Sci. 2017, 8, 7483–7491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buttefey, S.; Boutin, A.; Mellot-Draznieks, C.; Fuchs, A.H. A simple model for predicting the Na+ distribution in anhydrous NaY and NaX zeolites. J. Phys. Chem. B 2001, 105, 9569–9575. [Google Scholar] [CrossRef]
- Pillai, R.S.; Khan, I.; Titus, E. C2-Hydrocarbon Adsorption in Nano-porous Faujasite: A DFT Study. Mater. Today Proc. 2015, 2, 436–445. [Google Scholar] [CrossRef]
- Nour, Z.; Berthomieu, D.; Yang, Q.; Maurin, G. A computational exploration of the CO adsorption in cation-exchanged faujasites. J. Phys. Chem. C 2012, 116, 24512–24521. [Google Scholar] [CrossRef]
- Arean, C.O.; Delgado, M.R.; Nachtigall, P.; Thang, H.V.; Rubeš, M.; Bulánek, R.; Chlubná-Eliášová, P. Measuring the Brønsted acid strength of zeolites-does it correlate with the O-H frequency shift probed by a weak base? Phys. Chem. Chem. Phys. 2014, 16, 10129–10141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaduk, J.A.; Faber, J. Crystal Structure of Zeolite Y as a Function of Ino Exchange. Rigaku J. 1995, 12, 14–34. [Google Scholar]
- Verboekend, D.; Nuttens, N.; Locus, R.; Van Aelst, J.; Groen, J.C.; Perez-Ramirez, J.; Sels, B.F. Synthesis, characterisation, and catalytic evaluation of hierarchical faujasite zeolites: Milestones, challenges, and future directions. Chem. Soc. Rev. 2016, 45, 3331–3352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Król, M.; Koleżyński, A.; Mozgawa, W. Vibrational Spectra of Zeolite Y as a Function of Ion Exchange. Molecules 2021, 26, 342. [Google Scholar] [CrossRef] [PubMed]
- Giuseppe, G.; Montes, A.; Rodríguez, G. Zeolitas: Características, propiedades y aplicaciones industriales. Ed. Por. Editor. Innovación Tecnológica 2000, 2, 46–47. [Google Scholar]
- Roussel, T.; Didion, A.; Pellenq, R.J.M.; Gadiou, R.; Bichara, C.; Vix-Guterl, C. Experimental and atomistic simulation study of the structural and adsorption properties of faujasite zeolite-templated nanostructured carbon materials. J. Phys. Chem. C 2007, 111, 15863–15876. [Google Scholar] [CrossRef]
- Krishnamurty, S.; Pal, S.; Vetrivel, R.; Chandra, A.K.; Goursot, A.; Fajula, F. The influence of the geometric parameters on the electronic properties of faujasite cluster models as derived from density functional theory and Hartree Fock-self consistent field methods. J. Mol. Catal. A Chem. 1998, 129, 287–295. [Google Scholar] [CrossRef]
- Szyja, B.M.; Smykowski, D.; Szczygieł, J.; Hensen, E.J.M.; Pidko, E.A. A DFT Study of CO2 Hydrogenation on Faujasite-Supported Ir4 Clusters: On the Role of Water for Selectivity Control. ChemCatChem 2016, 8, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Gómez Martin, J.M. Síntesis, Caracterización y Aplicaciones Catalíticas de Zeolitas Básicas; Universidad Complutense de Madrid: Madrid, Spain, 2001. [Google Scholar]
- Davis, M.E.; Lobo, R.F. Zeolite and Molecular Sieve Synthesis. Chem. Mater. 1992, 11, 756–768. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, X.; Li, M.; Zhang, M.; Yu, Y. Density Functional Theory study of the structural and electronic properties of H3PO4/ZSM-5. RSC Adv. 2014, 4, 14573–14581. [Google Scholar] [CrossRef]
- Chaudhury, S.; Varadachari, C.; Ghosh, K. Ab Initio Studies on Hematite Surface and the Adsorption of Phosphate. J. Theor. Chem. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Acelas, N.Y.; Mejia, S.M.; Mondragón, F.; Flórez, E. Density functional theory characterization of phosphate and sulfate adsorption on Fe-(hydr)oxide: Reactivity, pH effect, estimation of Gibbs free energies, and topological analysis of hydrogen bonds. Comput. Theor. Chem. 2013, 1005, 16–24. [Google Scholar] [CrossRef]
- Ning, P.; Bart, H.J.; Li, B.; Lu, X.; Zhang, Y. Phosphate removal from wastewater by model-La(III) zeolite adsorbents. J. Environ. Sci. 2008, 20, 670–674. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Mir, J.M.; Mir, M.A.; Majid, S.A. Molecular electron density and nitrate-phosphate sorption efficiency of zeolite-A: Physico-chemical and DFT analyses. Indian J. Chem.-Sect. A 2020, 59, 939–947. [Google Scholar]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Pramanik, B.K.; Mainali, B.; Angove, M.J. Metal ion and contaminant sorption onto aluminium oxide-based materials: A review and future research. J. Environ. Chem. Eng. 2018, 6, 6853–6869. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Q.; Liu, J.; Chang, N.; Wan, L.; Chen, J. Phosphate adsorption on lanthanum hydroxide-doped activated carbon fiber. Chem. Eng. J. 2012, 185, 160–167. [Google Scholar] [CrossRef]
- Yaguchi, M.; Uchida, T.; Motobayashi, K.; Osawa, M. Speciation of Adsorbed Phosphate at Gold Electrodes: A Combined Surface-Enhanced Infrared Absorption Spectroscopy and DFT Study. J. Phys. Chem. Lett. 2016, 7, 3097–3102. [Google Scholar] [CrossRef]
- Li, X.; Kuang, Y.; Chen, J.; Wu, D. Competitive adsorption of phosphate and dissolved organic carbon on lanthanum modified zeolite. J. Colloid Interface Sci. 2020, 574, 197–206. [Google Scholar] [CrossRef]
- Loayza, L.; Guaya, D. Phosphate Sequestration From Aqueous Solutions Using A Zeolite in The Zinc Form. Res. Sq. 2020, 4, 10–12. [Google Scholar] [CrossRef]
- Kubicki, J.D.; Paul, K.W.; Kabalan, L.; Zhu, Q.; Mrozik, M.K.; Aryanpour, M.; Pierre-Louis, A.-M.; Strongin, D.R. ATR-FTIR and density functional theory study of the structures, energetics, and vibrational spectra of phosphate adsorbed onto goethite. Langmuir 2012, 28, 14573–14587. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Tang, Q.; Shen, Z.; Fan, M.; Li, F. The adsorption of phosphate on hydroxylated alpha-SiO2 (0 0 1) surface and influence of typical anions: A theoretical study. Appl. Surf. Sci. 2020, 501, 144233. [Google Scholar] [CrossRef]

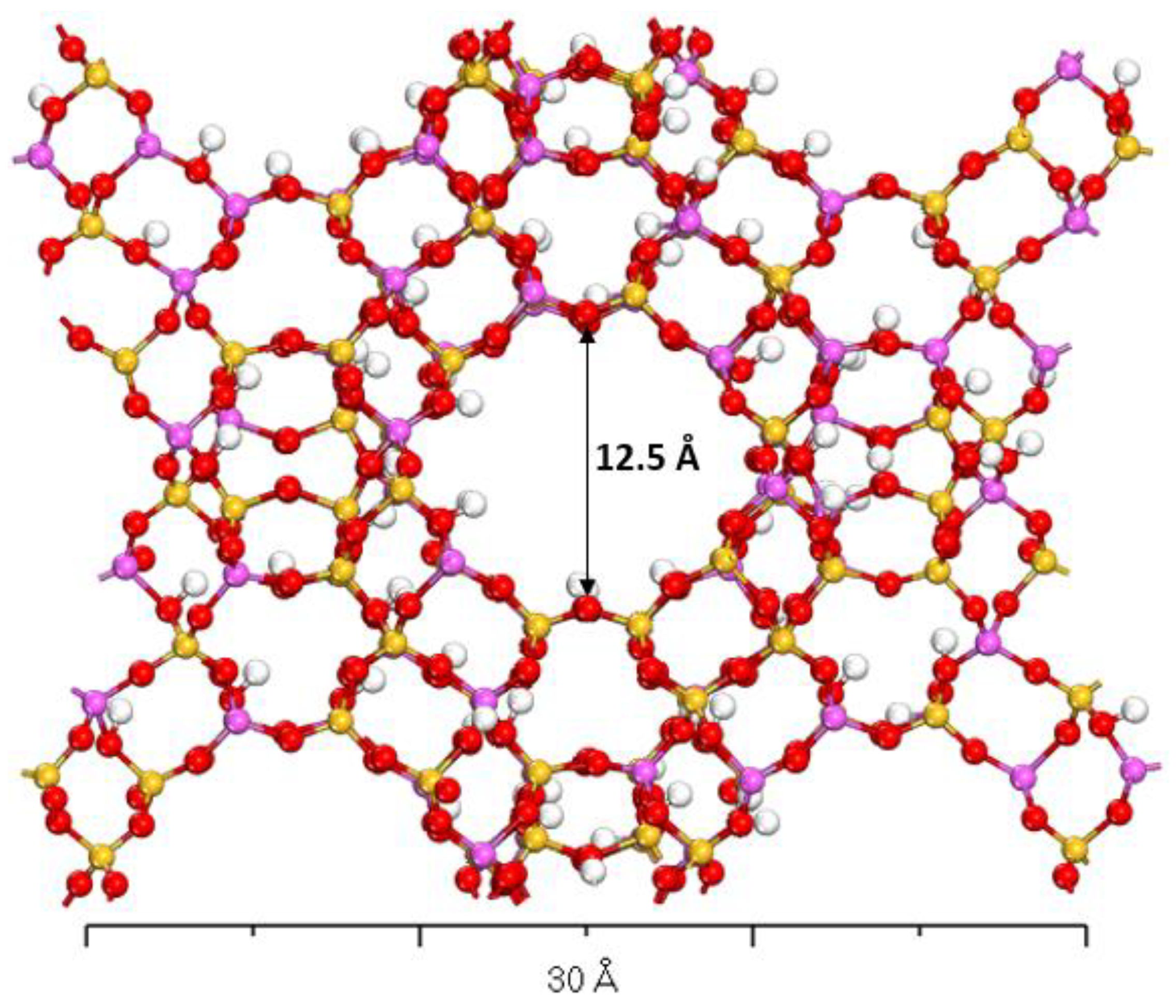
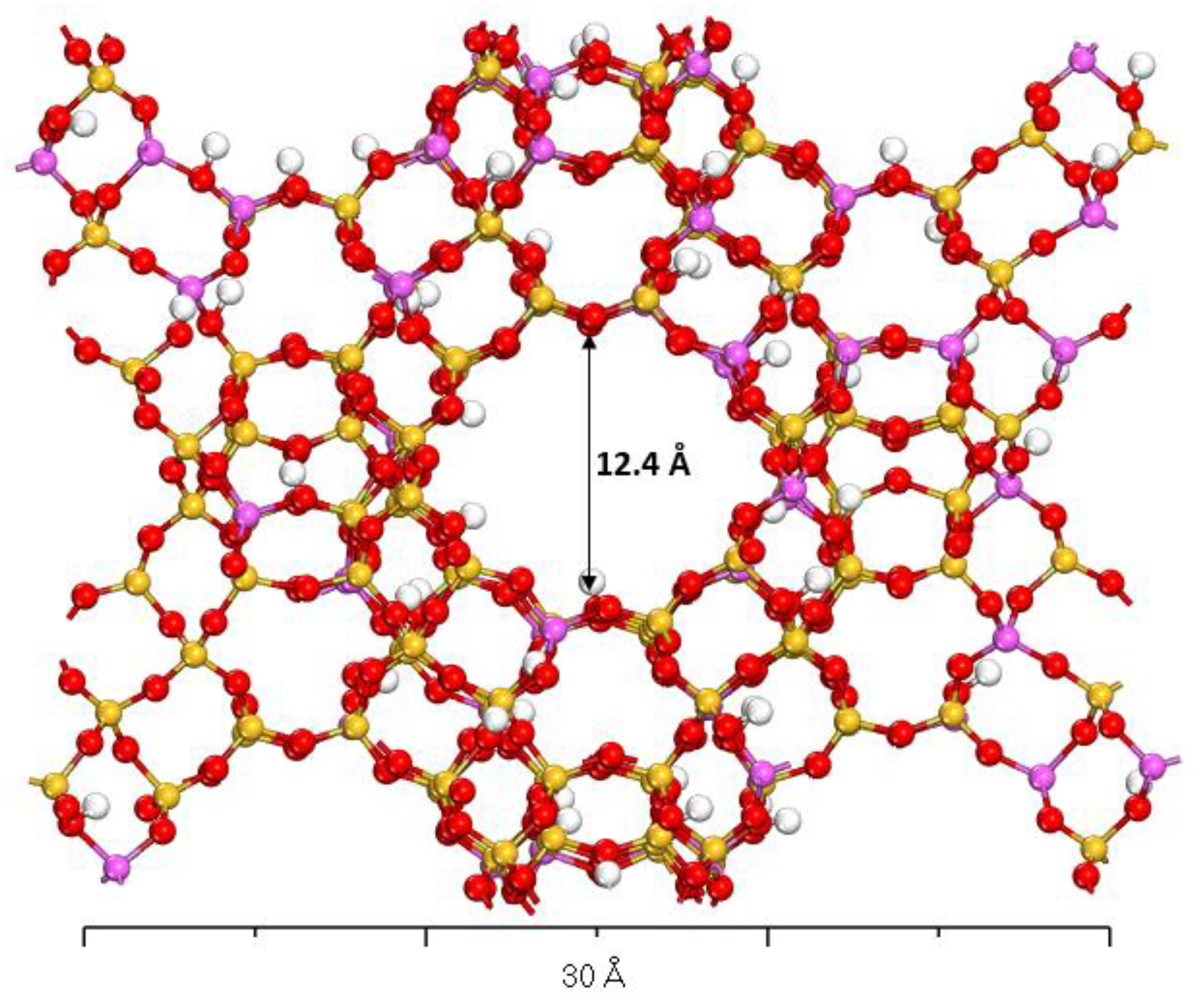
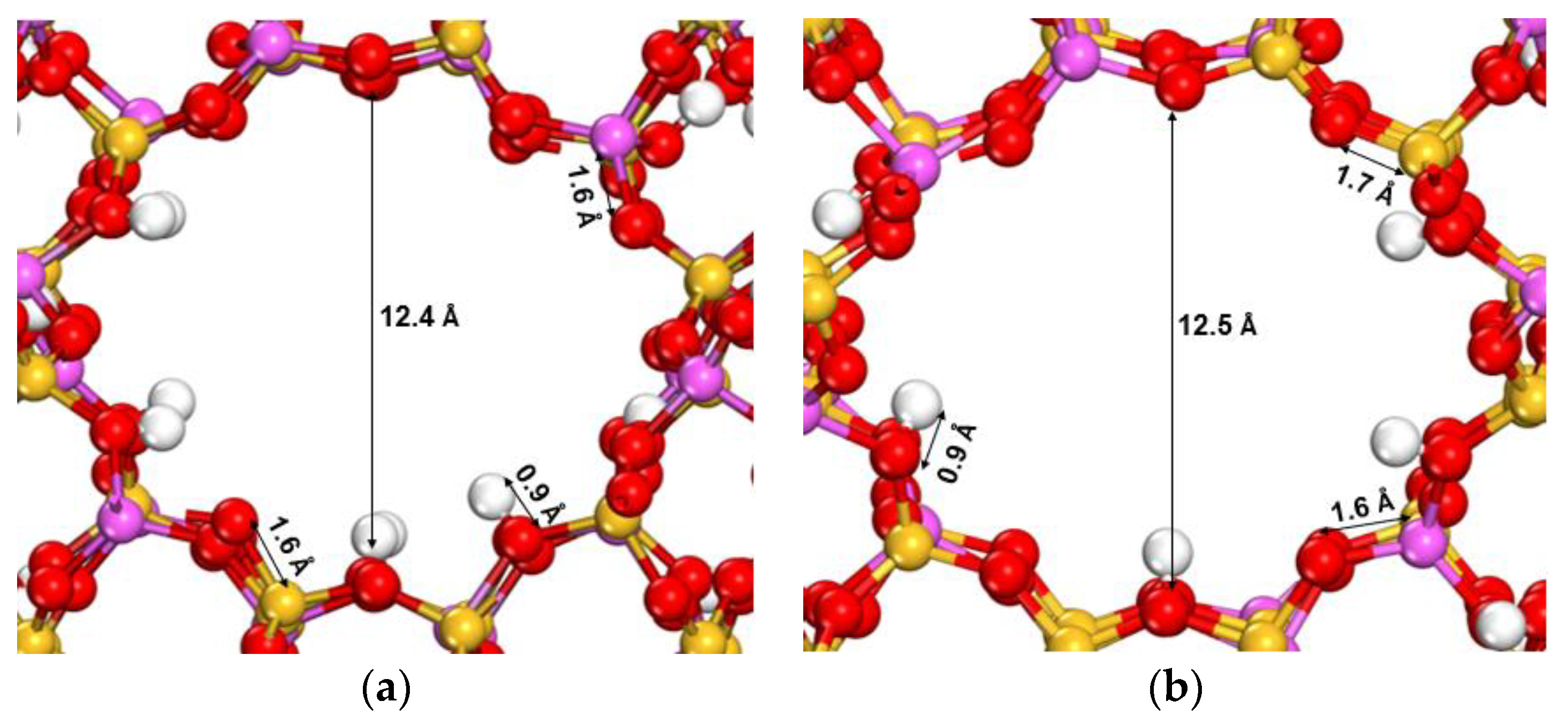

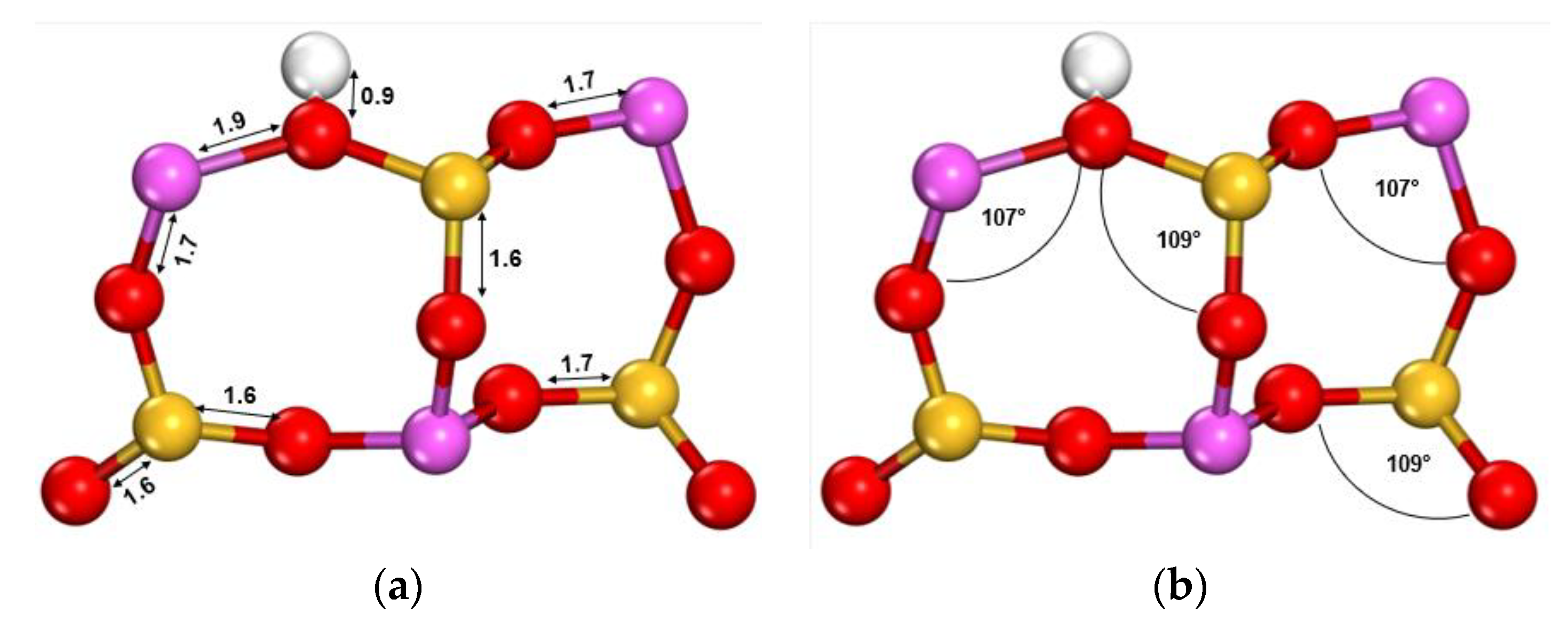

| Parameter | Faujasite X | Faujasite Y | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| a (Å) | 25.28 | 24.82 | 24.83 | 24.65 |
| b (Å) | 25.18 | 24.83 | 24.83 | 24.67 |
| c (Å) | 25.16 | 24.80 | 24.80 | 24.63 |
| α (°) | 90 | 89 | 89 | 89 |
| β (°) | 90 | 90 | 90 | 90 |
| γ (°) | 89 | 89 | 89 | 89 |
| V (Å3) | 16,012 | 15,286 | 15,286 | 14,975 |
| Parameter | Method | Si-O | Al-O |
|---|---|---|---|
| Distance (Å) | LDA | 1.6 | 1.7 |
| NDA | 1.7 | 1.7 | |
| HF-SCF | 1.6 | 1.7 | |
| Experimental This study | 1.6 1.6 | 1.7 1.7 | |
| Angle (°) | O-Si-O | O-Al-O | |
| LDA | 110 | 107 | |
| NDA | 110 | 108 | |
| HF-SCF | 109 | 107 | |
| Experimental | 109 | 109 | |
| This study | 109 | 107 |
| Structure | Adsorption Energy | |||
|---|---|---|---|---|
| EFAUX-FOS | eV | kcal/mol | kJ/mol | |
| Energy of FAUX | −4696.05 | --- | --- | |
| Energy of the isolated molecule | −44.87 | --- | --- | |
| Σ (EFAUX + ESDHP) | −4740.92 | |||
| Energy of molecule on FAUX | −4741.25 | --- | --- | |
| Eads | 0.33 | 7.6 | 32 | |
| EFAUY-FOS | Energy of FAUY | −4643.49 | --- | --- |
| Energy of the isolated molecule | −44.87 | --- | --- | |
| Σ (EFAUY + ESDHP) | −4688.36 | |||
| Energy of molecule on FAUY | −4690.03 | --- | --- | |
| Eads | 1.67 | 38.5 | 161 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capa-Cobos, L.F.; Jaramillo-Fierro, X.; González, S. Computational Study of the Adsorption of Phosphates as Wastewater Pollutant Molecules on Faujasites. Processes 2021, 9, 1821. https://doi.org/10.3390/pr9101821
Capa-Cobos LF, Jaramillo-Fierro X, González S. Computational Study of the Adsorption of Phosphates as Wastewater Pollutant Molecules on Faujasites. Processes. 2021; 9(10):1821. https://doi.org/10.3390/pr9101821
Chicago/Turabian StyleCapa-Cobos, Luis Fernando, Ximena Jaramillo-Fierro, and Silvia González. 2021. "Computational Study of the Adsorption of Phosphates as Wastewater Pollutant Molecules on Faujasites" Processes 9, no. 10: 1821. https://doi.org/10.3390/pr9101821








