Study on Photocatalytic Desulfurization and Denitrification Performance of Cu- and Cr-Modified MWCNT
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the Samples
2.3. Sample Analysis
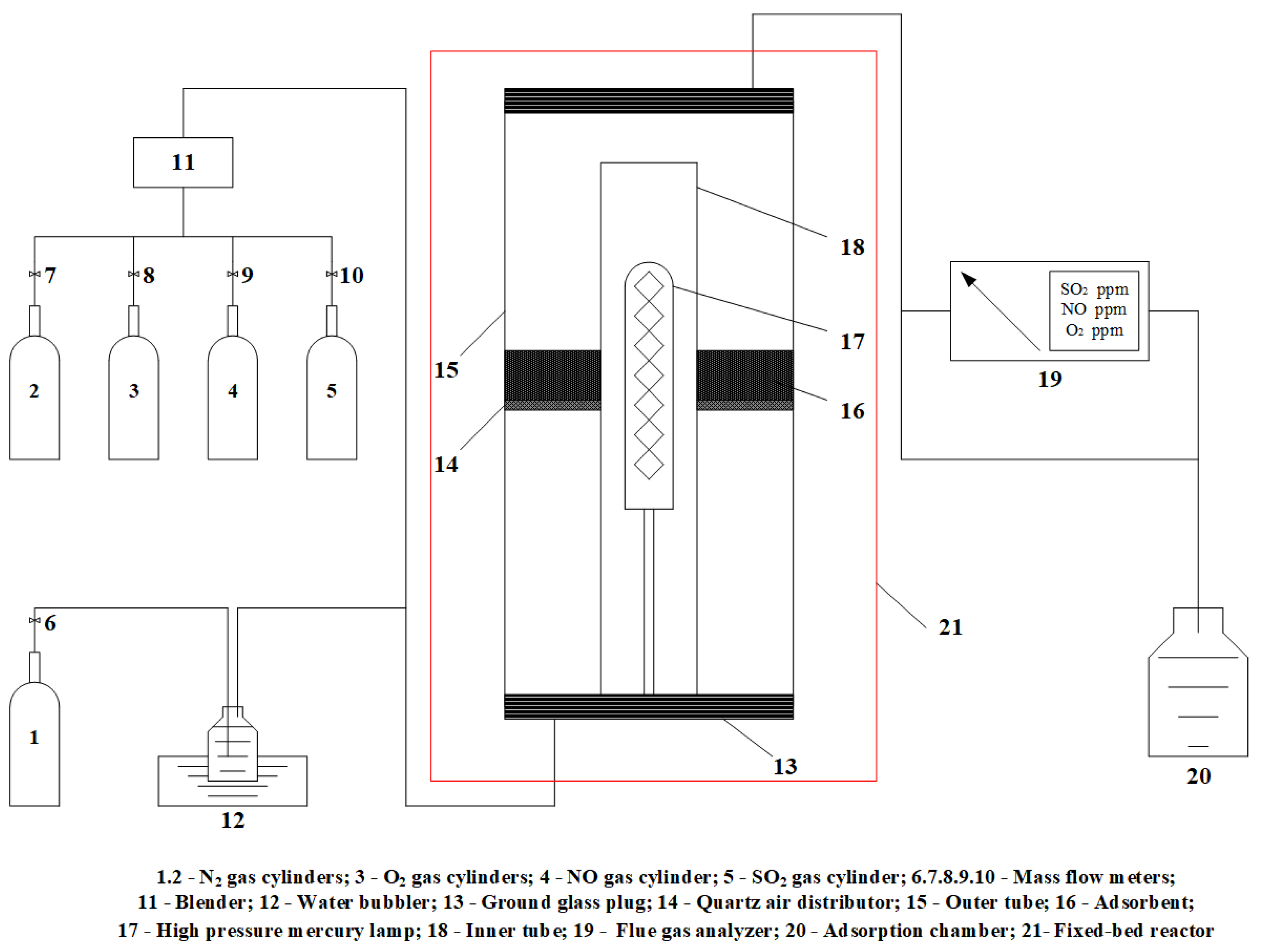
2.4. Experimental System
3. Results and Discussion
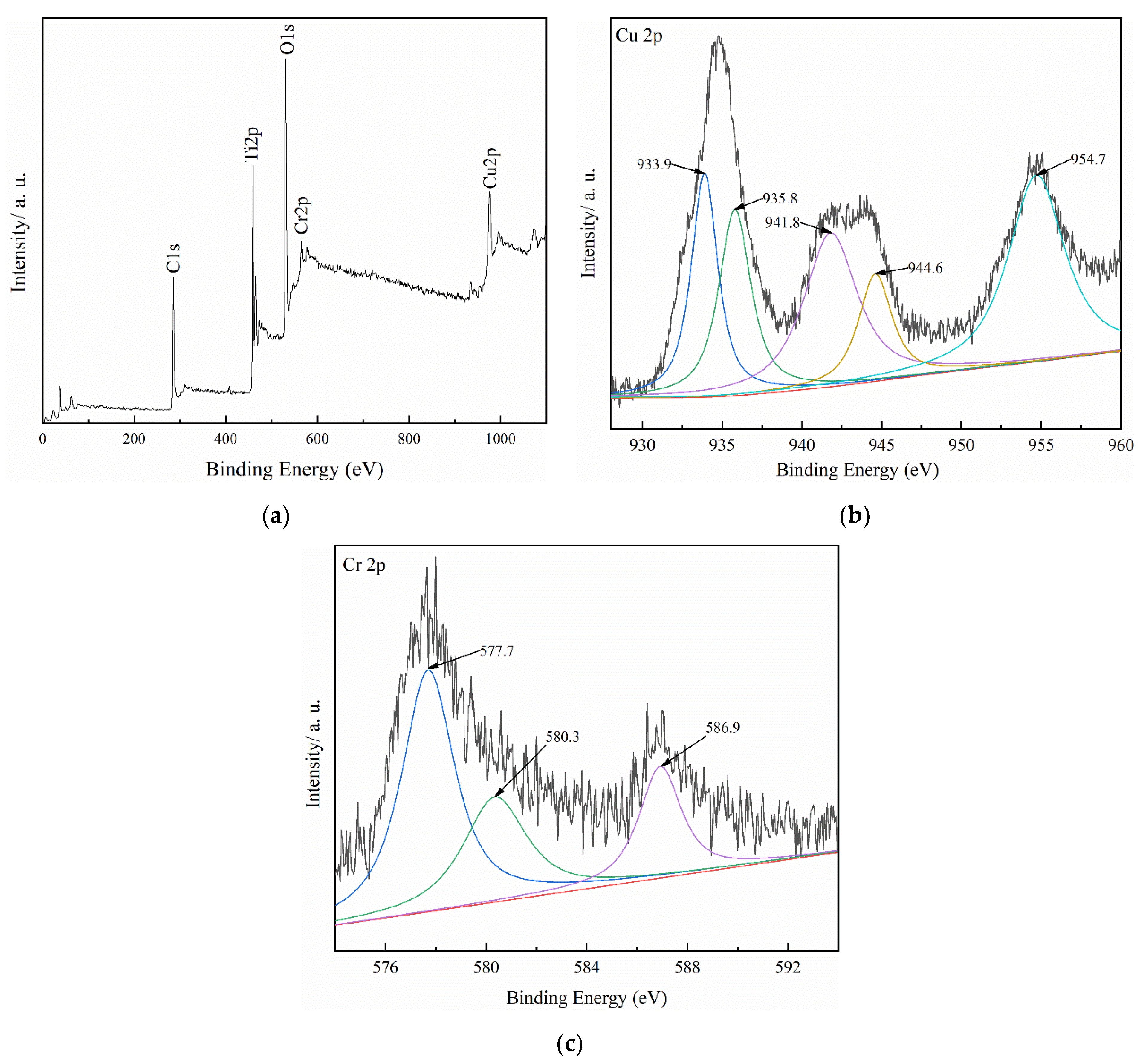
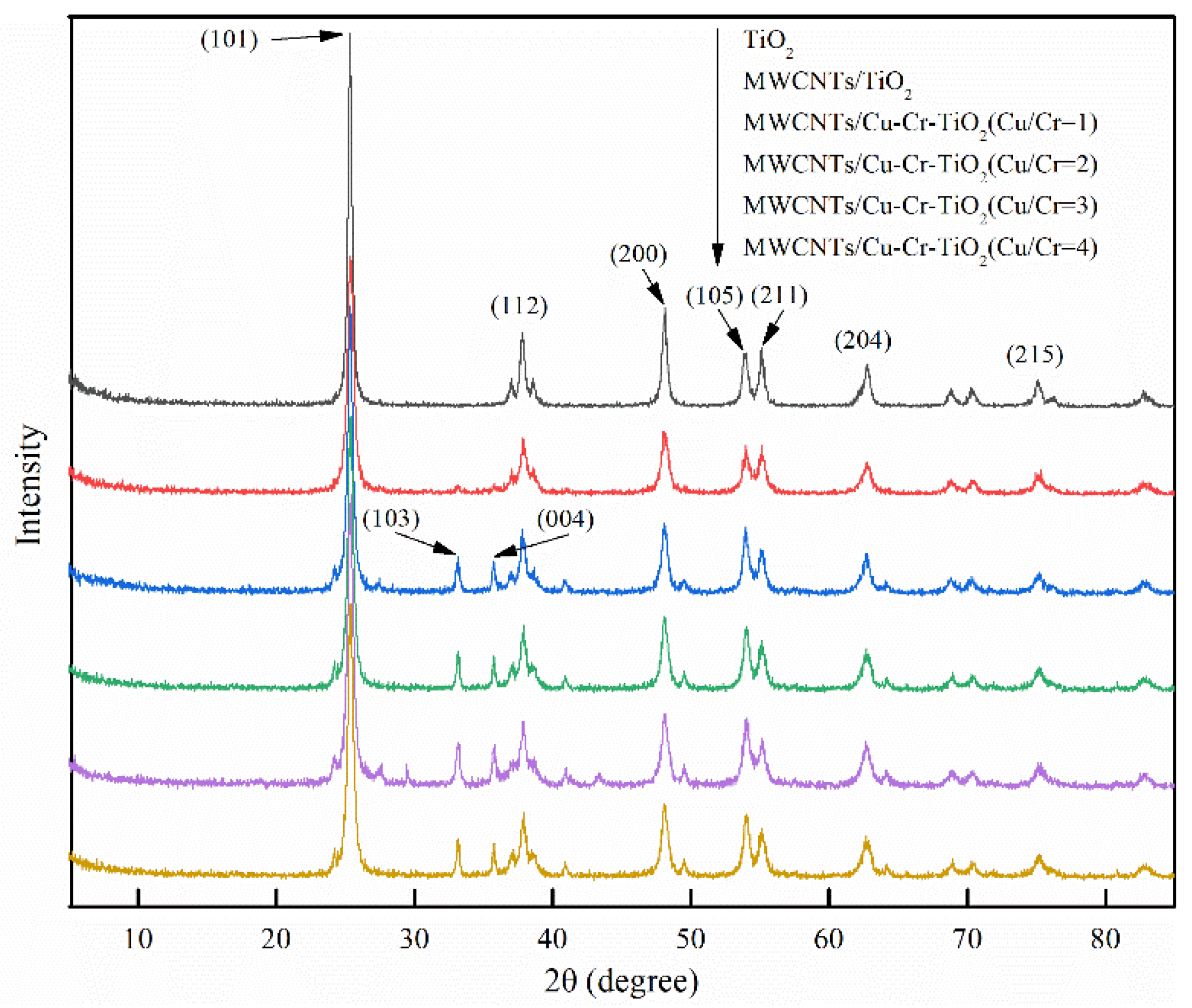
3.1. Adsorbent Characterization
3.2. Simultaneous Desulfurization and Denitrification Experiment
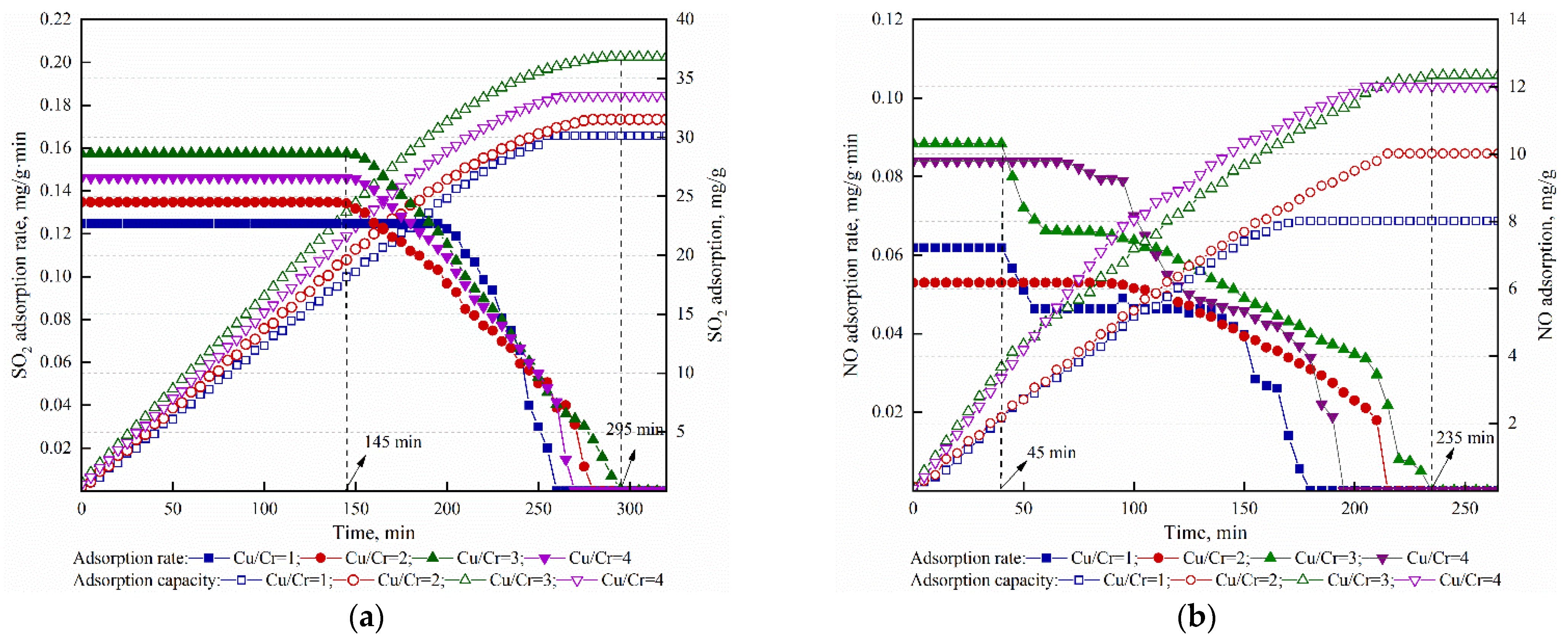
3.2.1. The Effect of the Ratio of Cu to Cr on Simultaneous Adsorption of SO2 and NO
3.2.2. The Effect of O2 on Simultaneous Adsorption of SO2 and NO
3.2.3. The Effect of H2O on Simultaneous Adsorption of SO2 and NO
3.2.4. The Effect of Space Velocity on Simultaneous Adsorption of SO2 and NO
3.2.5. Adsorption Cycles of MWCNTs/Cu-Cr-TiO2
4. Adsorption Kinetics Analysis
- qe: the adsorption amount at the adsorption equilibrium, i.e., the maximum adsorption capacity, mg/g;
- qt: the adsorption amount at time t, mg/g;
- k1: the pseudo-first-order kinetic model adsorption rate constant, min−1;
- t: the time, min.
- k3: the Bangham kinetic model adsorption rate constant, min−1;
- n: constant.
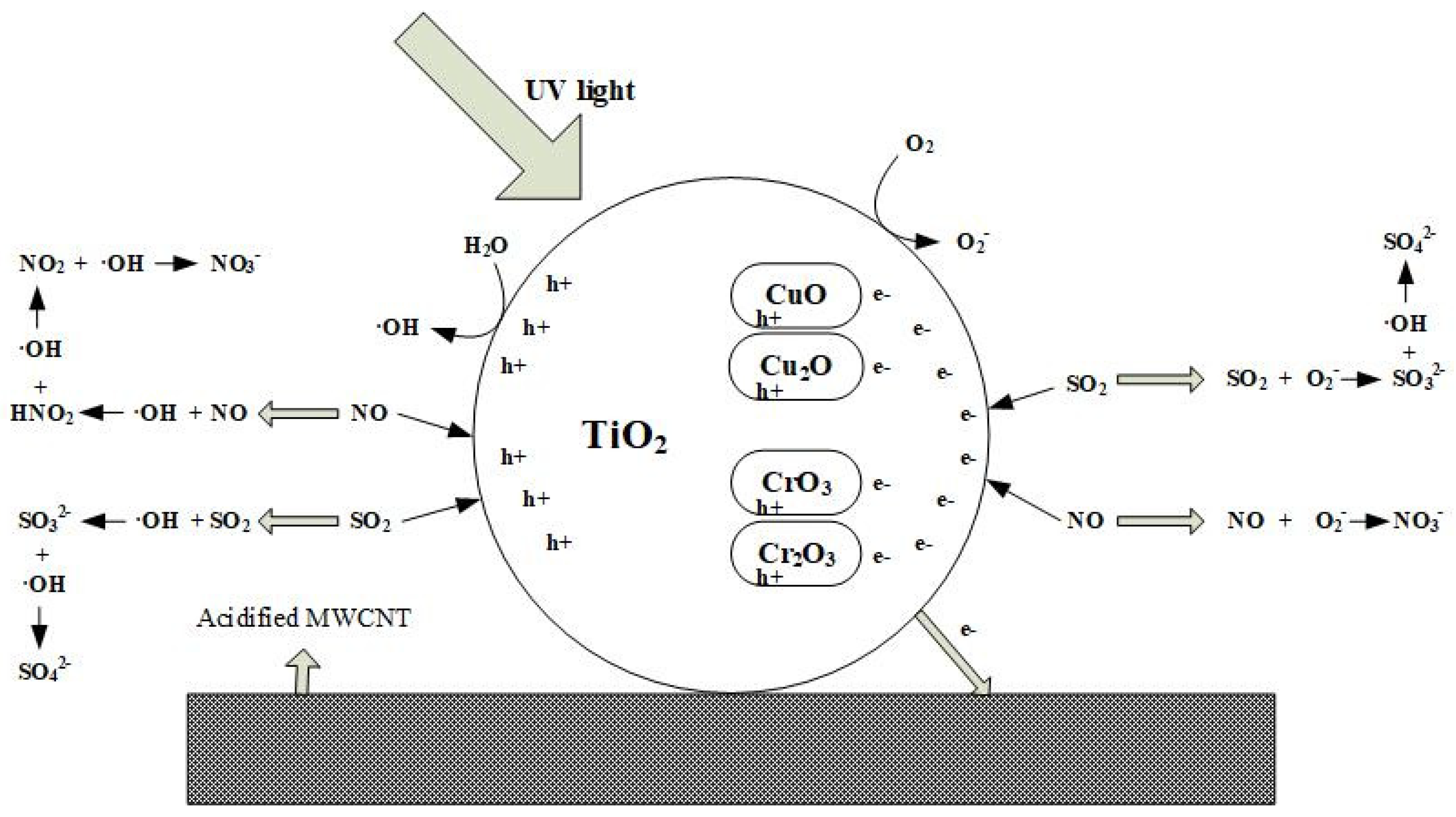
5. Reaction Mechanism
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Yuan, B.; Hao, R.; Zhao, Y.; Wang, X.J. A critical review on the method of simultaneous removal of multi-air-pollutant in flue gas. Chem. Eng. J. 2019, 378, 122–155. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhan, L.; Li, C.; Qiao, W.; Ling, L.J.I.; Research, E.C. Effect of SO2 on Activated Carbon Honeycomb Supported CeO2–MnOx Catalyst for NO Removal at Low Temperature. Ind. Eng. Chem. Res. 2015, 54, 2274–2278. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Li, C.-J. Eulerian-Eulerian numerical simulation for a flue gas desulfurization tower with perforated sieve trays. Int. J. Heat Mass Transf. 2018, 116, 329–345. [Google Scholar] [CrossRef]
- Cordoba, P.; Staicu, L.C. Flue gas desulfurization effluents: An unexploited selenium resource. Fuel 2018, 223, 268–276. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Zhu, T.; Ye, M. Investigation of SO2 and NO adsorption species on activated carbon and the mechanism of NO promotion effect on SO2. Fuel 2015, 143, 536–542. [Google Scholar] [CrossRef]
- Wu, X.; Yu, X.; He, X.; Jing, G. Insight into low-temperature catalytic NO reduction with NH3 on Ce-doped manganese oxide octahedral molecular sieves. J. Phys. Chem. Chem. 2019, 123, 10981–10990. [Google Scholar] [CrossRef]
- Sabna, V.; Thampi, S.G.; Chandrakaran, S.J.E.; Safety, E. Adsorption of crystal violet onto functionalised multi-walled carbon nanotubes: Equilibrium and kinetic studies. Ecotoxicol. Environ. Saf. 2016, 134, 390–397. [Google Scholar] [CrossRef]
- Rahimi, M.; Singh, J.K.; Muller-Plathe, F. CO2 adsorption on charged carbon nanotube arrays: A possible functional material for electric swing adsorption. J. Phys. Chem. Chem. 2015, 119, 15232–15239. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Qiu, F.; Zhang, X. Promotional effects of carbon nanotubes on V2O5/TiO2 for NOx removal. J. Hazard. Mater. 2011, 192, 915–921. [Google Scholar] [CrossRef]
- Yoosefian, M.; Zahedi, M.; Mola, A.; Naserian, S. A DFT comparative study of single and double SO2 adsorption on Pt-doped and Au-doped single-walled carbon nanotube. Appl. Surf. Sci. 2015, 349, 864–869. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Gong, H.; Wu, K.-H.; Ba, H.; Duong-Viet, C.; Jiang, C.; Pham-Huu, C.; Su, D.J. N-doped 3D mesoporous carbon/carbon nanotubes monolithic catalyst for H2S selective oxidation. ACS Appl. Nano Mater. 2019, 2, 3780–3792. [Google Scholar] [CrossRef]
- Yutong, F.; Yu, S. CO2-adsorption promoted CH4-desorption onto low-rank coal vitrinite by density functional theory including dispersion correction (DFT-D3). Fuel 2018, 219, 259–269. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, R.; Nayak, A.; Saleh, T.A. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: A review. Adv. Colloid Interface Sci. 2013, 193, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Miao, J.; Yang, Z.; Xiao, F.-X.; Yang, H.B.; Liu, B.; Yang, Y. Carbon nanotube catalysts: Recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 3295–3346. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Z.; Zhao, W.; Guan, B. Photocatalytic oxidation of nitrogen oxides using TiO2 loading on woven glass fabric. Chemosphere 2007, 66, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Leary, R.; Westwood, A. Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon 2011, 49, 741–772. [Google Scholar] [CrossRef]
- Seekaew, Y.; Wisitsoraat, A.; Phokharatkul, D.; Wongchoosuk, C.J. Room temperature toluene gas sensor based on TiO2 nanoparticles decorated 3D graphene-carbon nanotube nanostructures. Sens. Actuators B Chem. 2019, 279, 69–78. [Google Scholar] [CrossRef]
- Yi, Q.; Wang, H.; Cong, S.; Cao, Y.; Wang, Y.; Sun, Y.; Lou, Y.; Zhao, J.; Wu, J.; Zou, G.J. Self-cleaning glass of photocatalytic anatase TiO 2@ carbon nanotubes thin film by polymer-assisted approach. Nanoscale Res. Lett. 2016, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Saleh, T.A.; Gupta, V.K. Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide. J. Colloid Interface Sci. 2012, 371, 101–106. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C.J. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, S.; Chang, Y.; Faria, J.L. Enhancing the photocatalytic properties of TiO2 by coupling with carbon nanotubes and supporting gold. J. Hazard. Mater. 2012, 235, 230–236. [Google Scholar] [CrossRef]
- Zhang, F.-J.; Oh, W.-C.; Choi, J.-G.; Zhang, K.; Meng, Z.-D.; Chen, M.-L. Photoelectrocatalytic degradation of methylene blue over M-CNT/TiO2 (M= Y, Ag, and Pt) composite electrodes. Fuller. Nanotub. Carbon NanoStruct. 2011, 19, 564–574. [Google Scholar] [CrossRef]
- Shaari, N.; Tan, S.; Mohamed, A.J. Synthesis and characterization of CNT/Ce-TiO2 nanocomposite for phenol degradation. J. Rare Earths 2012, 30, 651–658. [Google Scholar] [CrossRef]
- Chen, M.-L.; Oh, W.-C. Photodegradation of organic dyes over nickel distributed CNT/TiO2 composite synthesized by a simple sol-gel method. Mater. Sci. Pol. 2011, 29, 112–120. [Google Scholar] [CrossRef]
- Liu, H.; Yu, X.; Yang, H. The integrated photocatalytic removal of SO2 and NO using Cu doped titanium dioxide supported by multi-walled carbon nanotubes. Chem. Eng. J. 2014, 243, 465–472. [Google Scholar] [CrossRef]
- Bao, J.; Dai, Y.; Liu, H.; Yang, L. Photocatalytic removal of SO2 over Mn doped titanium dioxide supported by multi-walled carbon nanotubes. Int. J. Hydrog. Energy 2016, 41, 15688–15695. [Google Scholar] [CrossRef]
- Yanchun, Y.; Fengrui, J.; Weiwei, J.; Shaopeng, Z.; Danzhu, M.; Guangxin, L. Influence of Cr3+ Concentration on SO2 Removal over TiO2 Based Multi-walled Carbon Nanotubes. China Pet. Process. Petrochem. Technol. 2019, 21, 23. [Google Scholar]
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Gao, B.; Chen, G.Z.; Puma, G.L. Carbon nanotubes/titanium dioxide (CNTs/TiO2) nanocomposites prepared by conventional and novel surfactant wrapping sol–gel methods exhibiting enhanced photocatalytic activity. Appl. Catal. B Environ. 2009, 89, 503–509. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Wang, D.; Gao, Y.; Shen, J.J. Cu2O/Cu/TiO2 nanotube Ohmic heterojunction arrays with enhanced photocatalytic hydrogen production activity. Int. J. Hydrog. Energy 2012, 37, 6431–6437. [Google Scholar] [CrossRef]
- Sun, M.; Chen, W.-C.; Zhao, L.; Wang, X.-L.; Su, Z.-M. A PTA@ MIL-101 (Cr)-diatomite composite as catalyst for efficient oxidative desulfurization. Inorg. Chem. Commun. 2018, 87, 30–35. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, Q.; Zhou, J.; Chen, D.; Ji, Z. Highly efficient photoelectrocatalytic removal of RhB and Cr (VI) by Cu nanoparticles sensitized TiO2 nanotube arrays. Appl. Surf. Sci. 2016, 367, 342–346. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumar, A.; Chaudhary, R.P. Structural and photocatalytic studies of Mn doped TiO2 nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 98, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Czech, B.; Buda, W.; Pasieczna-Patkowska, S.; Oleszczuk, P. MWCNT–TiO2–SiO2 nanocomposites possessing the photocatalytic activity in UVA and UVC. Appl. Catal. B Environ. 2015, 162, 564–572. [Google Scholar] [CrossRef]
- Wu, H.; Sun, J.; Qi, D.; Zhou, C.; Yang, H. Photocatalytic removal of elemental mercury from flue gas using multi-walled carbon nanotubes impregnated with titanium dioxide. Fuel 2018, 230, 218–225. [Google Scholar] [CrossRef]
- Everhart, B.M.; Baker-Fales, M.; McAuley, B.; Banning, E.; Almkhelfe, H.; Back, T.C.; Amama, P.B. Hydrothermal synthesis of carbon nanotube–titania composites for enhanced photocatalytic performance. J. Mater. Res. 2020, 35, 1451–1460. [Google Scholar] [CrossRef]
- Gui, M.M.; Chai, S.-P.; Mohamed, A.R. Modification of MWCNT@ TiO2 core–shell nanocomposites with transition metal oxide dopants for photoreduction of carbon dioxide into methane. Appl. Surf. Sci. 2014, 319, 37–43. [Google Scholar] [CrossRef]
- Doong, R.-A.; Liao, C.-Y.J.S.; Technology, P. Enhanced photocatalytic activity of Cu-deposited N-TiO2/titanate nanotubes under UV and visible light irradiations. Sep. Purif. Technol. 2017, 179, 403–411. [Google Scholar] [CrossRef]
- Kang, S.-T.; Seo, J.-Y.; Park, S.-H. The characteristics of CNT/cement composites with acid-treated MWCNTs. Adv. Mater. Sci. Eng. 2015, 2015, 308725. [Google Scholar] [CrossRef]
- Alotaibi, A.M.; Williamson, B.A.; Sathasivam, S.; Kafizas, A.; Alqahtani, M.; Sotelo-Vazquez, C.; Buckeridge, J.; Wu, J.; Nair, S.P.; Scanlon, D.O.; et al. Enhanced photocatalytic and antibacterial ability of Cu-doped anatase TiO2 thin films: Theory and experiment. ACS Appl. Mater. Interfaces 2020, 12, 15348–15361. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Mao, Y.; Leng, K.; Ye, G.; Sun, Y.; Xu, W.J.M.; Materials, M. Enhancement of oxidative desulfurization performance over amorphous titania by doping MIL-101 (Cr). Microporous Mesoporous Mater. 2017, 254, 114–120. [Google Scholar] [CrossRef]
- Bensouici, F.; Bououdina, M.; Dakhel, A.; Tala-Ighil, R.; Tounane, M.; Iratni, A.; Souier, T.; Liu, S.; Cai, W. Optical, structural and photocatalysis properties of Cu-doped TiO2 thin films. Appl. Surf. Sci. 2017, 395, 110–116. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Zhao, Z.J.; Yang, W.; Li, J.F.; Li, A.; Yang, Z.; Ozin, G.A.; Gong, J. Promoted fixation of molecular nitrogen with surface oxygen vacancies on plasmon-enhanced TiO2 photoelectrodes. Angew. Chem. Int. Ed. 2018, 57, 5278–5282. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-C.; Li, X.-Z.; Yang, Y.-H.; Sze, K.-h. Effects of dissolved oxygen, pH, and anions on the 2, 3-dichlorophenol degradation by photocatalytic reaction with anodic TiO2 nanotube films. Chemosphere 2008, 73, 805–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kansal, S.K.; Sood, S.; Umar, A.; Mehta, S.K. Photocatalytic degradation of Eriochrome Black T dye using well-crystalline anatase TiO2 nanoparticles. J. Alloys Compd. 2013, 581, 392–397. [Google Scholar] [CrossRef]
- Katal, R.; Masudy-Panah, S.; Tanhaei, M.; Farahani, M.H.D.A.; Jiangyong, H. A review on the synthesis of the various types of anatase TiO2 facets and their applications for photocatalysis. Chem. Eng. J. 2020, 384, 123384. [Google Scholar] [CrossRef]
- Wendt, S.; Matthiesen, J.; Schaub, R.; Vestergaard, E.K.; Lægsgaard, E.; Besenbacher, F.; Hammer, B. Formation and splitting of paired hydroxyl groups on reduced TiO 2 (110). Phys. Rev. Lett. 2006, 96, 066107. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, H.; Chen, Z.; Veksha, A.; Lisak, G.; You, C. Interaction between SO2 and NO in their adsorption and photocatalytic conversion on TiO2. Chemosphere 2020, 249, 126136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, T.; Xu, T.; Li, C.; Li, J.; Fu, J.; Zhang, Z.; Song, Z.; Ma, C. Comparative study on the preparation of powdered activated coke for SO2 adsorption: One-step and two-step rapid activation methods. Fuel 2021, 288, 119570. [Google Scholar] [CrossRef]
- Ammendola, P.; Raganati, F.; Chirone, R. CO2 adsorption on a fine activated carbon in a sound assisted fluidized bed: Thermodynamics and kinetics. Chem. Eng. J. 2017, 322, 302–313. [Google Scholar] [CrossRef]
- Huong, P.-T.; Lee, B.-K.J.M.; Materials, M. Improvement of selective separation of CO2 over N2 by transition metal–exchanged nano-zeolite. Microporous Mesoporous Mater. 2017, 241, 155–164. [Google Scholar] [CrossRef]
- Song, T.; Liao, J.-M.; Xiao, J.; Shen, L.-H. Effect of micropore and mesopore structure on CO2 adsorption by activated carbons from biomass. New Carbon Mater. 2015, 30, 156–166. [Google Scholar] [CrossRef]
- Coenen, K.; Gallucci, F.; Hensen, E.; van Sint Annaland, M. Kinetic model for adsorption and desorption of H2O and CO2 on hydrotalcite-based adsorbents. Chem. Eng. J. 2019, 355, 520–531. [Google Scholar] [CrossRef]
- Li, L.; Han, W.; Dong, F.; Zong, L.; Tang, Z.; Zhang, J. Controlled pore size of ordered mesoporous Al2O3-supported Mn/Cu catalysts for CO oxidation. Microporous Mesoporous Mater. 2017, 249, 1–9. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, M.-H.; Liya, E.Y. Hydrothermal synthesis and photocatalytic performance of metal-ions doped TiO2. Appl. Catal. A Gen. 2012, 413, 238–244. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Zhuang, Y.; Fu, X. New insight for enhanced photocatalytic activity of TiO2 by doping carbon nanotubes: A case study on degradation of benzene and methyl orange. J. Phys. Chem. C 2010, 114, 2669–2676. [Google Scholar] [CrossRef]
- Sun, W.; Meng, S.; Zhang, S.; Zheng, X.; Ye, X.; Fu, X.; Chen, S. Insight into the transfer mechanisms of photogenerated carriers for heterojunction photocatalysts with the analogous positions of valence band and conduction band: A case study of ZnO/TiO2. J. Phys. Chem. C 2018, 122, 15409–15420. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Lin, Y.; Wang, P.; Chen, W.; Fu, X.; Shao, Y. Evidence for the active species involved in the photodegradation process of methyl orange on TiO2. J. Phys. Chem. C 2012, 116, 3552–3560. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Jayaweera, P.M.; Grassian, V.H. Sulfur dioxide adsorption on TiO2 nanoparticles: Influence of particle size, coadsorbates, sample pretreatment, and light on surface speciation and surface coverage. J. Phys. Chem. C 2011, 115, 492–500. [Google Scholar] [CrossRef]
- Devahasdin, S.; Fan, C., Jr.; Li, K.; Chen, D.H. TiO2 photocatalytic oxidation of nitric oxide: Transient behavior and reaction kinetics. J. Photochem. Photobiol. A Chem. 2003, 156, 161–170. [Google Scholar] [CrossRef]



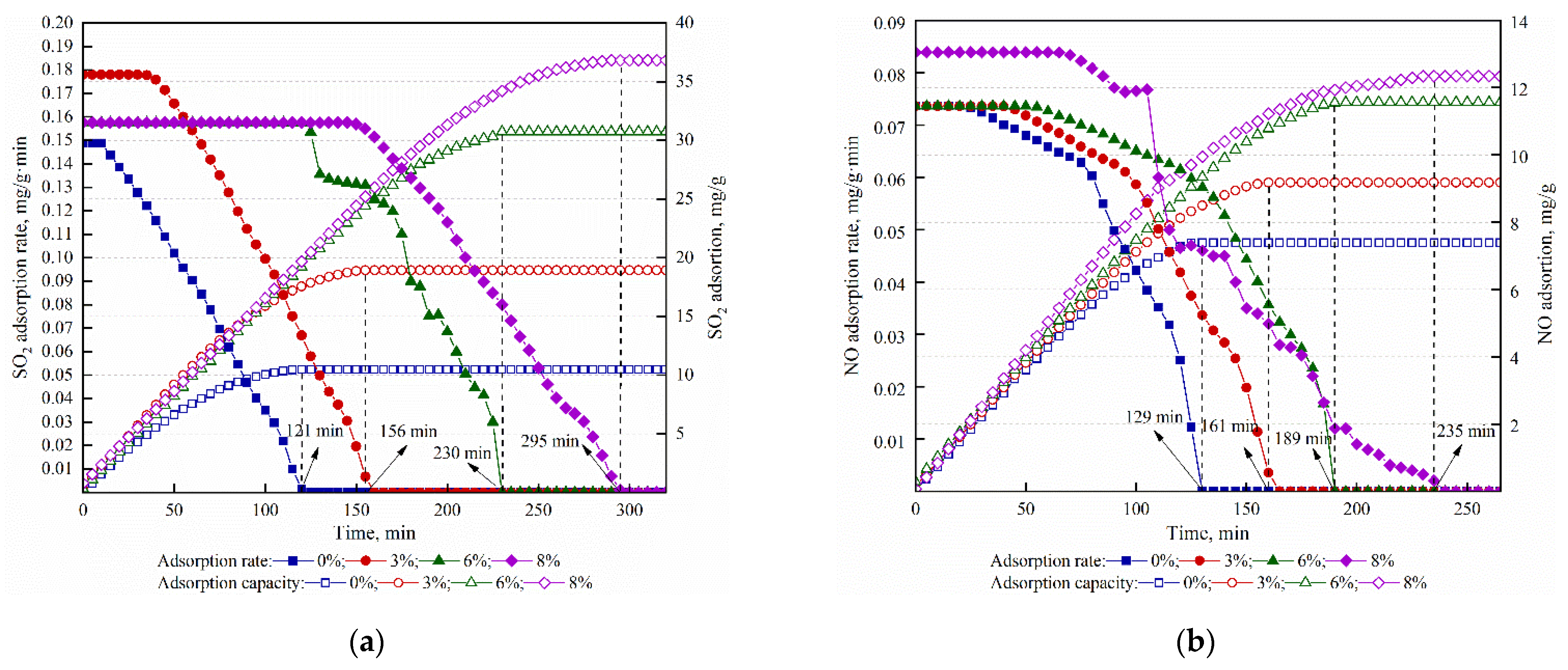


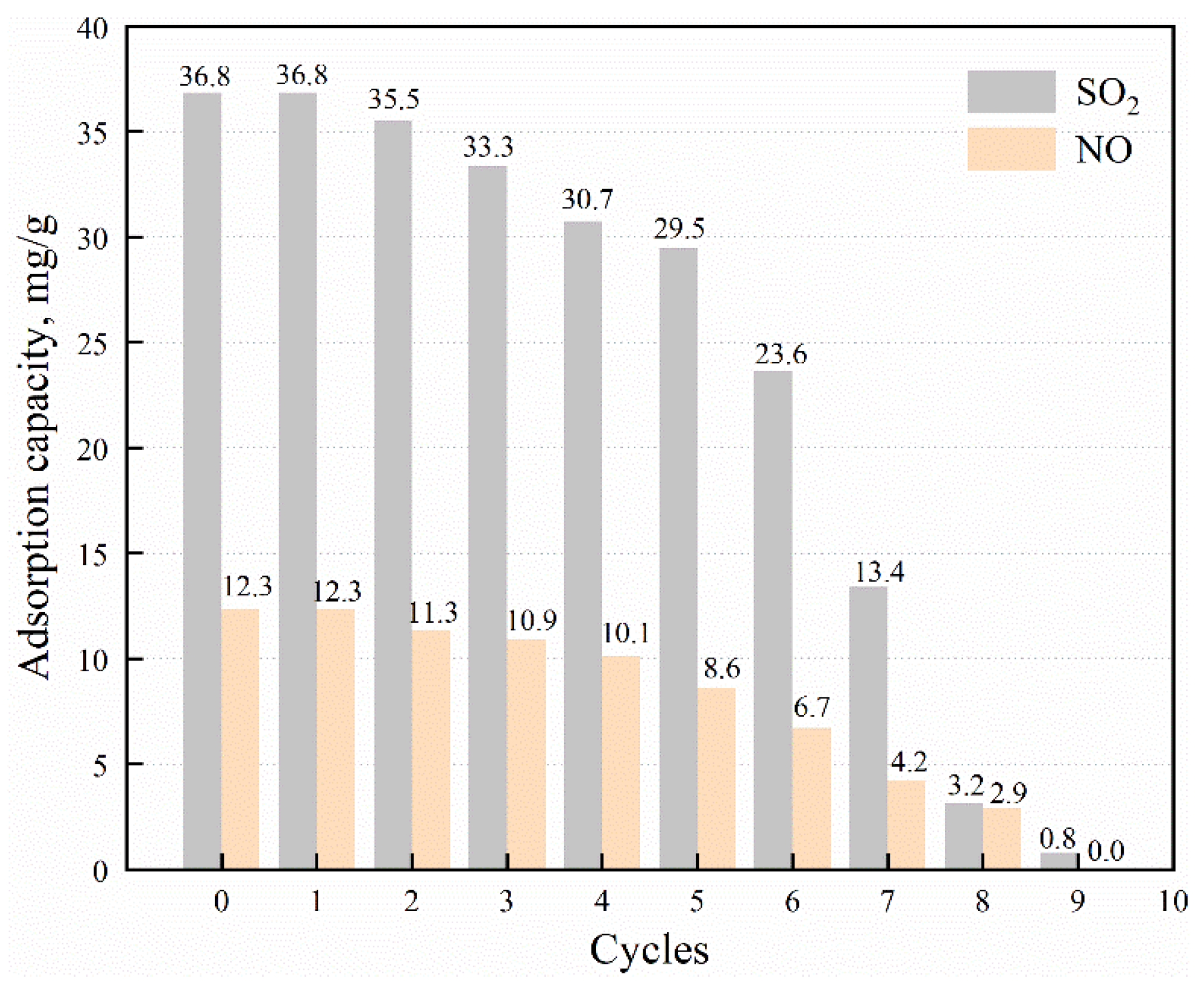
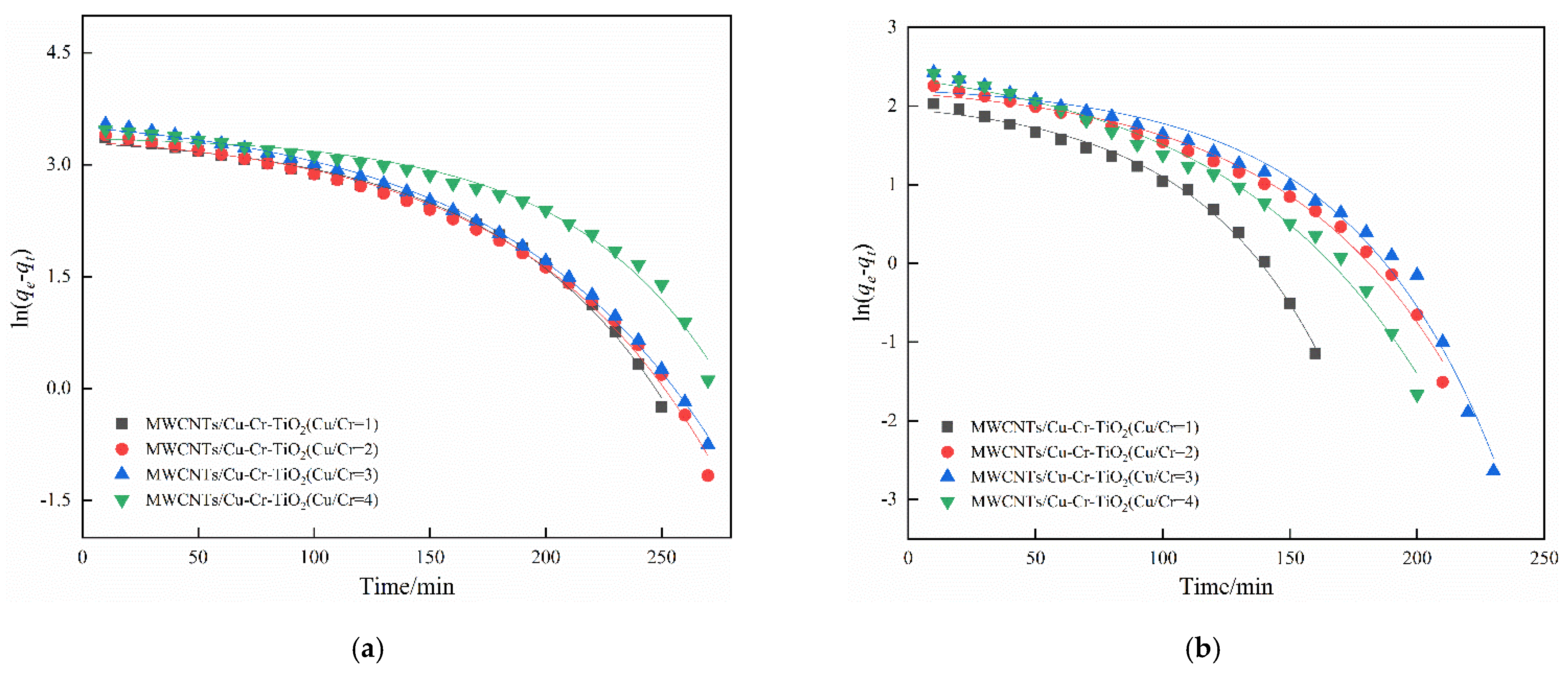
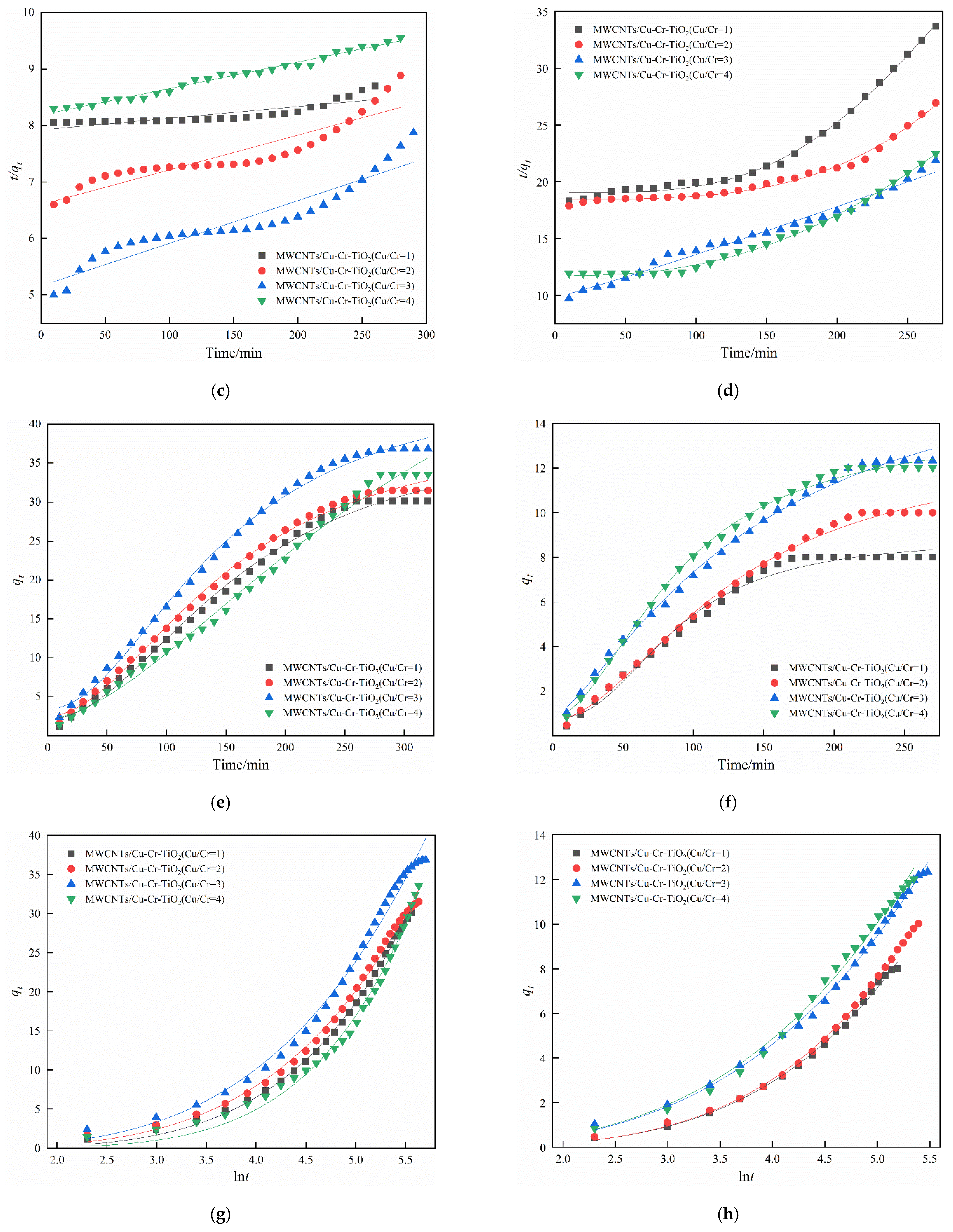
| Case | O2 (%) | H2O (%) | Space Velocity (h−1) | NO (mg·m−3) | SO2 (mg·m−3) | Samples |
|---|---|---|---|---|---|---|
| 1 | 8 | 5 | 1003 | 736 | 1575 | MWCNTs/Cu-Cr-TiO2 (Cu/Cr = 1, 2, 3, 4) |
| 2 | 0, 3, 6, 8 | 5 | 1003 | 736 | 1575 | MWCNTs/Cu-Cr-TiO2 (Cu/Cr = 3) |
| 3 | 8 | 0, 1, 3, 5 | 1003 | 736 | 1575 | MWCNTs/Cu-Cr-TiO2 (Cu/Cr = 3) |
| 4 | 8 | 5 | 1003, 1671, 2462, 3342 | 736 | 1575 | MWCNTs/Cu-Cr-TiO2 (Cu/Cr = 3) |
| 5 | 8 | 5 | 1003 | 736 | 1575 | 10%MWCNTs/Cu-TiO2 |
| 6 | 8 | 5 | 1003 | 736 | 1575 | 10%MWCNTs/Cr-TiO2 |
| Adsorbents | a | b | c | d | e | f | g | h |
|---|---|---|---|---|---|---|---|---|
| Specific surface area (m2/g) | 22.9 | 80.2 | 284.9 | 243.8 | 205.8 | 188.9 | 106.2 | 403.7 |
| Pore volume (cm3/g) | 0.0541 | 0.2343 | 0.2826 | 0.2824 | 0.2988 | 0.2811 | - | - |
| Kinetic Model | Cu/Cr = 1 | Cu/Cr = 2 | Cu/Cr = 3 | Cu/Cr = 4 |
|---|---|---|---|---|
| Pseudo-first-order | ||||
| lnqe | 3.4058 | 3.4504 | 3.6062 | 3.5131 |
| k1 | 0.0146 | 0.0171 | 0.0286 | 0.0126 |
| R2 | 0.9962 | 0.9946 | 0.9987 | 0.9858 |
| Pseudo-second-order | ||||
| qe | 30.1394 | 31.5136 | 36.8277 | 33.5530 |
| k2 | 7.26 × 10−4 | 1.23 × 10−3 | 1.46 × 10−2 | 4.05 × 10−4 |
| R2 | 0.7259 | 0.8453 | 0.8910 | 0.9856 |
| Bangham | ||||
| qe | 30.1394 | 31.5136 | 36.8277 | 33.5530 |
| k3 | 5.49 × 10−3 | 4.88 × 10−3 | 5.13 × 10−3 | 2.74 × 10−3 |
| n | 0.8341 | 1.0416 | 1.1115 | 1.1975 |
| R2 | 0.9938 | 0.9965 | 0.9964 | 0.9936 |
| Elovich | ||||
| a | 1.0092 | 1.0771 | 1.2795 | 1.1172 |
| b | 0.1122 | 0.1116 | 0.0987 | 0.1037 |
| R2 | 0.9984 | 0.9958 | 0.9907 | 0.9928 |
| Kinetic Model | Cu/Cr = 1 | Cu/Cr = 2 | Cu/Cr = 3 | Cu/Cr = 4 |
|---|---|---|---|---|
| Pseudo-first-order | ||||
| lnqe | 2.0805 | 2.3044 | 2.5130 | 2.4865 |
| k1 | 1.16 × 10−2 | 8.93 × 10−3 | 1.09 × 10−2 | 1.16 × 10−2 |
| R2 | 0.9945 | 0.9888 | 0.9855 | 0.9886 |
| Pseudo-second-order | ||||
| qe | 8.0088 | 10.0187 | 12.3413 | 12.0186 |
| k2 | 9.65 × 10−3 | 2.96 × 10−3 | 5.98 × 10−3 | 3.87 × 10−3 |
| R2 | 0.9958 | 0.9888 | 0.9802 | 0.9964 |
| Bangham | ||||
| qe | 8.0088 | 10.0187 | 12.3413 | 12.0186 |
| k3 | 7.20 × 10−3 | 3.76 × 10−3 | 3.97 × 10−3 | 3.48 × 10−3 |
| n | 0.8660 | 1.1074 | 1.3417 | 1.3185 |
| R2 | 0.9874 | 0.9956 | 0.9954 | 0.9972 |
| Elovich | ||||
| a | 0.3075 | 0.3597 | 0.4754 | 0.4615 |
| b | 0.3805 | 0.3238 | 0.2810 | 0.2723 |
| R2 | 0.9976 | 0.9989 | 0.9976 | 0.9942 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Jian, W.; Tong, S.; Ma, D.; Zhuang, B.; Jia, F. Study on Photocatalytic Desulfurization and Denitrification Performance of Cu- and Cr-Modified MWCNT. Processes 2021, 9, 1823. https://doi.org/10.3390/pr9101823
Sun Y, Jian W, Tong S, Ma D, Zhuang B, Jia F. Study on Photocatalytic Desulfurization and Denitrification Performance of Cu- and Cr-Modified MWCNT. Processes. 2021; 9(10):1823. https://doi.org/10.3390/pr9101823
Chicago/Turabian StyleSun, Yi, Weiwei Jian, Siqi Tong, Danzhu Ma, Bohan Zhuang, and Fengrui Jia. 2021. "Study on Photocatalytic Desulfurization and Denitrification Performance of Cu- and Cr-Modified MWCNT" Processes 9, no. 10: 1823. https://doi.org/10.3390/pr9101823





